Abstract
Experimental evidence supports the role of cellular senescence in the pathophysiology of atrial fibrillation (AF) and suggests that Insulin-like Growth Factor Binding Protein-7 (IGFBP7) is an important senescence-inducing factor. Whether elevated IGFBP7 levels precede the development of AF remains unknown. We measured IGFBP7 levels in plasma of 5884 adult participants without prevalent AF (mean age 53.6 ± 12.1 years; 51.5% women) from the PREVEND community-based cohort (2001–2004). Incident AF was ascertained from hospital and study electrocardiograms. During a median follow-up of 6.4 (5.9–6.9) years, 154 participants (2.6%) developed AF. In Cox proportional hazards models, IGFBP7 was associated with increased risk for incident AF (unadjusted hazard ratio [HR]: 1.85 per 1-SD increase in log-IGFBP7; 95% confidence interval [CI]: 1.65–2.07) which remained significant after adjustment for clinical variables (HR: 1.28; 95% CI: 1.07–1.52). Based on these data, we conclude that IGFBP7, a senescence-inducing factor, is associated with the risk of developing AF in community-dwelling adults.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a major contributor to cardiovascular morbidity and mortality1. Key risk factors for AF include age, genetics, lifestyle, and pre-existing heart disease, with age being the most significant2. As the global population continues to age3, the prevalence and incidence of AF are also expected to increase sharply2. This is because AF disproportionately affects the elderly, with its incidence rising steeply from ~0.9 per 1000 person-years at ages 40–49 years to over 17 per 1000 person-years in those over 70 years4.
Cellular senescence plays a crucial role in the development of AF by accelerating cardiovascular ageing and promoting myocardial fibrosis5. These changes cause adverse myocardial and atrial remodeling, which can directly influence structural as well as electrical properties of the cardiac tissue6,7—contributing to the development of AF. However, clinical studies exploring the link between senescence and AF remain limited, which may, in part, be due to the lack of reliable circulating markers reflecting senescence.
Insulin-like growth factor binding protein-7 (IGFBP7) is a senescence-related marker that can be reliably measured in circulation8,9. Recent studies suggest that IGFBP7 contributes to cardiovascular senescence by stimulating IGF-1 receptor-dependent suppression of Forkhead Box O3a (FOXO3a)10, a transcription factor involved in DNA repair and oxidative stress defense11,12,13. Whereas increased circulating IGFBP7 levels are known to be associated with heart failure (HF) development and progression14,15,16, large population-based studies examining its relationship with AF are lacking.
Given the role of cellular senescence in AF pathogenesis, we hypothesized that higher circulating IGFB7 levels, reflecting increased cellular senescence, would be associated with a greater risk of developing AF. Therefore, the objective of our study was to measure circulating IGFBP7 in community-dwelling adults and to examine its association with incident AF.
Results
Plasma IGFBP7 levels
The study included 5884 individuals who were free of AF at baseline. The mean (SD) age of the overall population was 53.6 (12.1) years, and 51.5% were females. The distribution of IGFBP7 in the PREVEND general population is shown in Supplementary Fig. 1 and Supplementary Table 1. Median IGFBP7 level in the total population was 84.7 µg/L, and the range varied from 14.0 to 320.4 µg/L. The distribution of IGFBP7 was broadly similar in both sexes, but median IGFBP7 levels were significantly higher in males than in females (89.1 vs 79.7 µg/L, P < 0.001).
Participant characteristics
We divided the study population according to incident AF status, and results are presented in Table 1. Compared to participants not developing AF, those developing AF had a higher age (53.3 ± 12.0 vs 65.4 ± 8.4 years, P < 0.001), were less often females (52.1% vs 27.9%, P < 0.001), and had higher levels of IGFBP7 (84.3 [74.8, 95.4] vs 98.1 [97.3, 111.0] µg/L, P < 0.001).
Associations of plasma IGFBP7 with incident AF
During a median (P25–P75) follow-up of 6.4 (5.9–6.9) years, 154 participants (2.6%) developed AF, corresponding to an incidence rate of 4.2 new AF events per 1000 person-years.
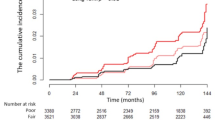
Population characteristics according to sex-pooled IGFBP7 tertiles are presented in Table 2. The cumulative incidence were significantly different across the sex-pooled IGFBP7 tertiles (P < 0.001), with participants with higher IGFBP7 levels having the highest cumulative incidence of AF (Fig. 1). Incidence rates per 1000 person-years of AF also increased progressively across IGFBP7 tertiles with 1.6 events in the first tertile; 3.4 in the second tertile; and 7.9 in the third tertile.
This figure illustrates the cumulative incidence of atrial fibrillation (AF) across tertiles of insulin-like growth factor binding protein-7 (IGFBP7). The y-axis represents the cumulative incidence of AF, while the x-axis shows the time from study inclusion to AF event or censoring. The cumulative incidence curves are color-coded: tertile 1 is shown in black, tertile 2 in cranberry, and tertile 3 in red. The table below (number at risk) indicates the number of participants at risk at different time points for each IGFBP7 tertile.
In unadjusted Cox regression models, IGFBP7 levels were significantly associated with increased risk for incident AF (hazard ratio (HR): 1.85 per 1-SD increase in log IGFBP7; 95% confidence interval (CI): 1.65–2.07). This association remained statistically significant after adjustment for age and sex (HR: 1.34; 95% CI: 1.15–1.56), and after further adjustment for weight, height, systolic blood pressure (BP), antihypertensive medication, smoking, type 2 diabetes, history of myocardial infarction or stroke, history of HF and estimated glomerular filtration rate (eGFR) (HR: 1.28; 95% CI: 1.07–1.52) (Table 3, Fig. 2). Results did not materially change when we additionally adjusted for NT-proBNP (HR: 1.25; 95% CI: 1.04–1.50), interim HF (HR: 1.28; 95% CI: 1.07–1.53) or competing risk of death (HR: 1.26; 95% CI: 1.05–1.53).
This figure illustrates the association between insulin-like growth factor binding protein-7 (IGFBP7) levels and the hazard of incident atrial fibrillation (AF) using a restricted cubic spline model. The x-axis represents IGFBP7 concentrations (µg/L), and the y-axis represents the hazard ratio for AF. The solid red line denotes the hazard ratio, with light-red shading indicating the 95% confidence intervals. Knots were placed at the 5th, 35th, 65th, and 95th percentiles of IGFBP7. The model was adjusted for age, sex, weight, height, systolic blood pressure, antihypertensive medication, smoking, type 2 diabetes, history of myocardial infarction or stroke, history of heart failure, and estimated glomerular filtration rate. The gray-shaded background (histogram) represents the distribution of IGFBP7 in the study population.
For clinical relatability, we also provided HRs per 20 µg/L increase in IGFP7 levels, which approximately corresponds to a 1-SD increase of IGFBP7 (18.3 µg/L) in the PREVEND population (Supplementary Table 2). Finally, in post-hoc analysis, we found no significant interaction between IGFBP7 levels and age, sex, obesity, prevalent HF, prevalent cardiovascular disease (CVD), renal dysfunction, or microalbuminuria—with regard to AF incidence (Supplementary Table 3).
Discussion
In the current study, including 5884 community-dwelling adults free of prevalent AF, we report for the first time that IGFBP7, a senescence-inducing factor, is associated with incident AF. Our findings expand on observations from smaller studies examining cross-sectional associations of IGFBP7 and AF. Specifically, IGFBP7 levels were associated with prevalent AF in an elderly community-based cohort17 and also with prevalent AF in HF patients with reduced ejection fraction18. Additionally, in a cohort of patients diagnosed with AF, higher IGFBP7 levels were associated with future HF-related hospitalization19.
These observations suggest that IGFBP7 may have some predictive value in the development and progression of AF. However, considering that IGFBP7 has a ubiquitous expression (Supplementary Fig. 2), and only a fraction of the circulating IGFBP7 pool might arise from the cardiac tissue20, its potential to predict/diagnose cardiac-specific disorders such as AF, when used as a single marker, may be limited. In this regard, a recent study examining the value of biomarker clusters found that a combination of cardiac-specific proteins (NT-proBNP and bone morphogenetic protein (BMP)-10) along with non-cardiac proteins (IGFBP7, angiopoietin 2, and growth differentiation factor-15) effectively identified high risk in AF patients21.
Besides its suggestive value in AF risk estimation, IGFBP7 should also be considered from a pathophysiologic perspective—as a cardiovascular geroprotein amenable to therapy. Indeed, there is experimental evidence showing that cardiovascular ageing/senescence can be modified by inhibiting IGFBP7. Specifically, in a pressure-overload mouse model, IGFBP7 accelerated HF progression by promoting cardiac senescence, whereas antibody-mediated IGFBP7 neutralization (in vivo) attenuated HF progression10. Additionally, in a similar mouse model of HF, IGFBP7 produced by senescent endothelial cells promoted cardiac dysfunction, and a vaccine targeting IGFB7 ameliorated cardiac dysfunction22.
Cardiovascular senescence and fibrosis are involved not only in the pathogenesis of HF23,24, but also in the pathogenesis of AF5,6,7. As higher IGFBP7 levels, reflecting increased senescence, precede the development of AF in community-dwelling adults, we hypothesize that IGFBP7 inhibition may also emerge as a potential treatment for AF. To this end, validation of our results in other population-based cohorts (including AF patients), along with carefully designed mechanistic studies investigating the role of IGFBP7 inhibition in experimental AF, is first needed.
This is one of the first studies investigating the association of IGFBP7 with incident AF in community-dwelling adults. Other strengths include a detailed clinical assessment, an almost 1:1 sex ratio, and adjudicated AF outcomes25. Notwithstanding, we acknowledge the following limitations. First, due to the observational design of our study, residual confounding cannot be ruled out, and causal relations between IGFBP7 and AF development cannot be established. Second, the PREVEND study by design enrolled a higher proportion of individuals with mildly elevated urinary albumin excretion (UAE); however, this is unlikely to affect the interpretation, as previous research has demonstrated that findings from the PREVEND cohort align well with those from broader population cohorts like the Framingham Heart Study26. Third, we acknowledge that the PREVEND cohort was established several years ago, and changes in population demographics and risk profiles over time could potentially impact the external validity of our results. Finally, this study was conducted in a predominantly White population, which may limit the generalizability of our findings to other ethnicities/population groups. Therefore, our results should be validated in more recent cohorts with contemporary follow-up and in different ethnicities/population groups.
In conclusion, we report that IGFBP7, a senescence-inducing factor, is associated with increased risk for incident AF among community-dwelling adults. Future studies should explore IGFBP7 inhibition as a potential treatment for AF.
Methods
Study population
The Prevention of Renal and Vascular End-stage Disease (PREVEND) study was founded in 1997 as a prospective community-based study25,27. In brief, all inhabitants (between 28 and 75 years) of the city of Groningen, the Netherlands, were invited (n = 85,421), and 47.8% (n = 40,856) responded. Individuals with UAE > 10 mg/L (n = 7768) in their morning urine, as well as a randomly selected control group with UAE < 10 mg/L (n = 3394), were selected to attend the PREVEND outpatient clinic28. After excluding participants with insulin-dependent diabetes, pregnant women, and individuals unable/unwilling to participate, a final total of 8592 individuals completed the (first) PREVEND screening program (1997–1998)28, during which multiple demographic, anthropometric, and health-related factors were assessed. Fasting venous blood samples and two 24-h urine samples per person were collected and stored at −80 °C. Participants were then regularly screened at ~3-year intervals at the PREVEND outpatient clinic. During each follow-up visit, a similar screening protocol was followed, including the collection and storage of fasting venous blood samples and two 24-h urine samples per person; additionally, a 12-lead ECG was also taken. IGFBP7 was measured in samples from the second visit, which was attended by 6894 participants. Therefore, the second PREVEND visit was considered as the baseline for the present analyses.
From the 6894 participants attending the baseline visit (2001–2004), we excluded those with prevalent AF (n = 85), unknown rhythm status (n = 178), and unavailable plasma samples/IGFBP7 measurements (n = 733). As IGFBP7 displayed a strong correlation with renal function14, we additionally excluded individuals with eGFR less than 30 mL/min/1.73 m2 (n = 14), resulting in 5884 participants for analyses. Participant characteristics of the baseline sample (n = 6894) and the sample used for the current analyses (n = 5884) are shown in Supplementary Table 4. Ethical approval was obtained from the local medical ethics committee of the University Medical Center Groningen (MEC96/01/022); all participants signed informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Baseline assessment
All anthropometric measurements were performed in a standing position. Waist circumference (WC) was measured midway between the lowest rib and the iliac crest at the end of expiration. Relative fat mass was calculated using height and WC with the following equation: 64−(20 × height/WC) + (12 × sex), with sex = 0 (men) and sex = 1 (women)28. Body mass index was calculated as weight/height2 (kg/m2). BP was calculated as the average of two seated measurements. Diabetes was defined as fasting glucose 126 mg/dL (7.0 mmol/L) or higher, a non-fasting glucose of 200 mg/dL (11.1 mmol/L) or higher, or hypoglycaemic medication usage. Prevalent CVD, defined as a history of myocardial infarction or stroke, was obtained from a structured questionnaire, which included criteria such as hospitalization lasting 3 days or more due to the specified condition. This data collection was supplemented by an examination of medical records. The history of HF was obtained from hospital charts. Smoking behavior was self-reported and was defined as current smoking (smoking at present or smoking cessation within the previous year) or past smoking (smoking cessation over 1 year), or never smoking. Plasma glucose was measured by dry chemistry (Eastman Kodak, Rochester, NY, USA). Plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) was measured using an electro-chemiluminescence sandwich immunoassay (Elecsys proBNP, Roche Diagnostics, Mannheim, Germany). Urinary albumin concentration was determined by nephelometry (BNII, Dade Behring Diagnostic, Marburg, Germany)27. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) combined creatinine-cystatin C equation.
IGFBP7 measurements
IGFBP7 was measured in plasma samples using a high-precision precommercial COBAS Elecsys assay (Roche Diagnostics GmbH, Penzberg, Germany)14. The detection method for IGFBP7 was a sandwich immunoassay developed on the Elecsys® platform utilizing electro-chemiluminescence detection by Roche Diagnostics GmbH (Mannheim, Germany). Mouse monoclonal antibodies were produced and tested to specifically identify IGFBP7. The precision within-run coefficient of variation for IGFBP7 was 2%, and the limit of detection was 0.01 µg/L.
Incidence of atrial fibrillation
Ascertainment of incident AF has been described in detail previously25,29,30. In brief, incident AF was diagnosed if AF was present on a 12-lead ECG obtained during scheduled PREVEND visits (ie, visit 3 or 4), or during an outpatient visit or hospital admission in either of the two hospitals of the city of Groningen. All ECGs were digitally stored and were first screened electronically for atrial flutter, ectopic atrial rhythm, or the absence of PR interval. Then, two independent observers (ie, physicians with experience in evaluating ECGs) reviewed all suspected AF cases as determined by electronic screening. When there was an inconsistency between the observers or when both observers agreed on the diagnosis of atrial flutter or AF, the ECGs were validated by 2 independent cardiologists. For the date of the incident AF, the date of the first ECG, with a definite diagnosis of AF, was used30. The follow-up duration was calculated as the time between the baseline (ie, visit 2) and incident AF, death, or 31 December 2008.
Statistical analyses
Continuous variables are presented as means (standard distributions) for normally distributed data or as medians (25th–75th percentiles) for skewed data. Categorical variables are presented as counts (percentages). Differences in continuous variables between groups were assessed using Student’s t-test or the Mann–Whitney U test for two groups, and ANOVA or the Kruskal–Wallis test for more than two groups. Categorical variables were compared using chi-square tests and Fisher’s exact tests.
We first examined the association between IGFBP7 and incident AF using Cox proportional hazards regression (after checking the proportional hazard assumption) using four models. The first model was unadjusted; the second model was adjusted for age and sex; the third model was additionally adjusted for components of CHARGE-AF model (weight, height, systolic BP, antihypertensive medication, smoking, type 2 diabetes, history of myocardial infarction or stroke, history of HF)31; and the fourth model was additionally adjusted for eGFR. For these analyses, IGFBP7 was log-transformed and standardized separately for males and females.
Additionally, we examined associations of IGFBP7 and incident AF per 20 µg/L increase in IGFBP7 levels. In post-hoc subgroup analyses, we explored age-adjusted and/or sex-adjusted associations of IGFBP7 with incident AF according to age, sex, obesity, prevalent HF, prevalent CVD, renal dysfunction (eGFR < 60 mL/min/1.63 m2), and microalbuminuria (UAE ≥ 30 mg/24 h).
We report our findings as (HRs) and their corresponding 95% CIs. All analyses were performed using STATA version 14.0 (Stata Corp., College Station, TX, USA). All tests were two-tailed, and p values < 0.05 were considered statistically significant.
Data availability
The dataset analyzed during the current study is available in the PREVEND repository https://umcgresearch.org/w/prevend and can be obtained after an application is approved by the PREVEND board.
Code availability
Codes of Stata version 14 (Stata Corp., College Station, TX, USA) for statistical analysis of this study are available upon request from Navin Suthahar.
References
Brundel, B. J. J. M. et al. Atrial fibrillation. Nat. Rev. Dis. Prim. 8, 21 (2022).
Kornej, J., Börschel, C. S., Benjamin, E. J. & Schnabel, R. B. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ. Res. 127, 4–20 (2020).
Skirbekk, V. et al. The health-adjusted dependency ratio as a new global measure of the burden of ageing: a population-based study. Lancet Healthy Longev. 3, e332–e338 (2022).
Morseth, B. et al. Age-specific atrial fibrillation incidence, attributable risk factors and risk of stroke and mortality: results from the MORGAM Consortium. Open Heart 8, e001624 (2021).
Mehdizadeh, M. et al. The role of cellular senescence in profibrillatory atrial remodelling associated with cardiac pathology. Cardiovasc. Res. 120, 506–518 (2024).
Krul, S. P. J. et al. Atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation. Circ Arrhythmia Electrophysiol. 8, 288–295 (2015).
Xintarakou, A., Tzeis, S., Psarras, S., Asvestas, D. & Vardas, P. Atrial fibrosis as a dominant factor for the development of atrial fibrillation: facts and gaps. EP Eur. 22, 342–351 (2020).
Severino, V. et al. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 4, e911 (2013).
Chugh, S. et al. Pilot study identifying myosin heavy chain 7, desmin, insulin-like growth factor 7, and annexin A2 as circulating biomarkers of human heart failure. Proteomics 13, 2324–2334 (2013).
Zhang, L. et al. Insulin-like growth factor-binding protein-7 (IGFBP7) links senescence to heart failure. Nat. Cardiovasc. Res. 1, 1195–1214 (2022).
Chung, Y. M. et al. FOXO3 signalling links ATM to the p53 apoptotic pathway following DNA damage. Nat. Commun. 3, 1000 (2012).
Ferber, E. C. et al. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 19, 968–979 (2012).
Bigarella, C. L. et al. FOXO3 Transcription Factor Is Essential for Protecting Hematopoietic Stem and Progenitor Cells from Oxidative DNA Damage. J. Biol. Chem. 292, 3005–3015 (2017).
Abou Kamar, S. et al. Association of baseline and longitudinal changes in insulin-like growth factor-binding protein-7 with the risk of incident heart failure: data from the PREVEND study. Eur. J. Heart Fail. https://doi.org/10.1002/ejhf.3328 (2024).
Ferreira, J. P. et al. Insulin-like growth factor binding protein-7 concentrations in chronic heart failure: results from the EMPEROR programme. Eur. J. Heart Fail. 26, 806–816 (2024).
Ghasempour, P., Khanmohammadi, S. & Rezaei, N. Association between insulin-like growth factor binding protein-7 and heart failure. Curr. Med. Chem. https://doi.org/10.2174/0109298673346933241223063559 (2025).
Meessen, J. M. T. A. et al. IGFBP7 and GDF-15, but not P1NP, are associated with cardiac alterations and 10-year outcome in an elderly community-based study. BMC Cardiovasc. Disord. 21, 328 (2021).
Hage, C. et al. Comparison of prognostic usefulness of serum insulin-like growth factor-binding protein 7 in patients with heart failure and preserved versus reduced left ventricular ejection fraction. Am. J. Cardiol. 121, 1558–1566 (2018).
Blum, S. et al. Insulin-like growth factor-binding protein 7 and risk of congestive heart failure hospitalization in patients with atrial fibrillation. Heart Rhythm 18, 512–519 (2021).
Du, W. et al. Plasma levels of heart failure biomarkers are primarily a reflection of extracardiac production. Theranostics 8, 4155–4169 (2018).
Fabritz, L. et al. Blood-based cardiometabolic phenotypes in atrial fibrillation and their associated risk: EAST-AFNET 4 biomolecule study. Cardiovasc. Res. 120, 855–868 (2024).
Katoh, M. et al. Vaccine therapy for heart failure targeting the inflammatory cytokine Igfbp7. Circulation 150, 374–389 (2024).
Triposkiadis, F., Xanthopoulos, A. & Butler, J. Cardiovascular aging and heart failure. J. Am. Coll. Cardiol. 74, 804–813 (2019).
De Boer, R. A. et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur. J. Heart Fail. 21, 272–285 (2019).
Vermond, R. A. et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J. Am. Coll. Cardiol. 66, 1000–1007 (2015).
Suthahar, N. et al. Association of initial and longitudinal changes in C-reactive protein with the risk of cardiovascular disease, cancer, and mortality. Mayo Clin. Proc. 98, 549–558 (2023).
Hillege, H. L. et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106, 1777–1782 (2002).
Suthahar, N. et al. Relative fat mass, a new index of adiposity, is strongly associated with incident heart failure: data from PREVEND. Sci. Rep. 12, 147 (2022).
Rienstra, M. et al. Cluster individuals based on phenotype and determine the risk for atrial fibrillation in the PREVEND and Framingham Heart Study populations. PLoS ONE 11, e0165828 (2016).
Al-Mubarak, A. A. et al. Micronutrient deficiencies and new-onset atrial fibrillation in a community-based cohort: data from PREVEND. Clin. Res. Cardiol. 114, 41–52 (2025).
Alonso, A. et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J. Am. Heart Assoc. 2, e000102 (2013).
Acknowledgements
N.S. and R.A.d.B. are supported by the Netherlands Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek), co-funded by ERA4Health through the CARDINNOV 2023 call, as part of the EnerLIGHT project (Grant Agreement N. 101095426 of the EU Horizon Europe Research and Innovation Programme); by the Netherlands Heart Foundation (Nederlandse Hartstichting) through grants 2020B005 (DOUBLE DOSE) and 01-003-2022-0358 (CARMA); and by the European Research Council (ERC CoG 818715; SECRETE-HF). M.R. is supported by an unrestricted research grant from the Dutch Heart Foundation and Dutch CardioVascular Alliance (01-002-2022-0118 EmbRACE); by an unrestricted research grant from ZonMW and the Dutch Heart Foundation (DECISION project 848090001); by unrestricted research grants from the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation (RACE V, CVON 2014–9 and RED-CVD, CVON2017-11); by an unrestricted research grant from Top Sector Life Sciences & Health to the Dutch Heart Foundation (PPP Allowance; CVON-AI (2018B017)); and by an unrestricted research grant from the European Union’s Horizon 2020 research and innovation program under grant agreement; EHRA-PATHS (945260). We thank Yiqian Yang for her assistance in statistical modeling during manuscript revision.
Author information
Authors and Affiliations
Contributions
N.S. conceptualized the study, developed the research question, performed the main data analysis, prepared the table, interpreted the data, and wrote the paper. R.T.G. and M.R. assisted with the development of the research question and methodology. R.T.G., I.K., E.B., T.F.K., T.P., and M.E.Q. contributed to the revision of methodology and assisted in the statistical modeling. R.T.G., S.J.L.B., and K.D. were also involved in the critical review of the project proposal, methodology and in the approval of the project proposal on behalf of the PREVEND board. R.A.d.B. was involved in conceptualization, supervision, resources, and funding acquisition. All authors reviewed the manuscript drafts, critically revised the manuscript, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Outside the current work, R.A.d.B. has had speaker engagements with and/or received fees from and/or served on an advisory board for Abbott, AstraZeneca, Bristol-Myers Squibb, NovoNordisk, Roche and Zoll. R.A.d.B. received travel support from Abbott and NovoNordisk. The institution where R.A.d.B. is employed has received research grants and/or fees from Alnylam, AstraZeneca, Abbott, Bristol-Myers Squibb, Novo Nordisk and Roche. M.R. received consultancy fees from Bayer (OCEANIC-AF national PI), InCarda Therapeutics (RESTORE-SR national PI) to the institution. I.K. received travel reimbursement from Olink and SomaLogic. The remaining authors do not have anything to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suthahar, N., Gansevoort, R.T., Kok, T.F. et al. The senescence-inducing factor IGFBP7 and risk of atrial fibrillation: findings from the PREVEND study. npj Cardiovasc Health 2, 42 (2025). https://doi.org/10.1038/s44325-025-00079-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44325-025-00079-1