Abstract
Metabolic dysfunction-associated fatty liver disease (MAFLD) is characterized by a range of metabolic abnormalities, and its impact on the U.S. population requires further understanding. This study aimed to investigate the influence of each component of these metabolic abnormalities on hepatic steatosis and fibrosis in non-diabetic individuals. We analyzed data from 1711 non-diabetic participants in the National Health and Nutrition Examination Survey (NHANES) 2017–2018, which included transient elastography records. Steatosis was defined as a controlled attenuation parameter (CAP) value above 285 dB/m, and significant fibrosis was defined as a liver stiffness measurement greater than 8.0 kPa. Our results revealed a strong correlation between body mass index and waist circumference, with waist circumference being a better predictor of liver disease progression. All seven metabolic abnormalities were associated with increased severity of steatosis, and hypertension (OR = 2.43, 95% CI 1.55–3.82), elevated C-reactive protein levels (OR = 2.26, 95% CI 1.53–3.47), and waist circumference (OR = 2.19, 95% CI 1.39–3.45) were linked to significant fibrosis. These findings suggest that metabolic risk factors are associated with an increased risk of steatosis in non-diabetic subjects, while hypertension, central obesity, and chronic inflammation are related to significant fibrosis and may lead to worse prognosis.
Similar content being viewed by others
Introduction
Chronic liver disease is responsible for approximately 2 million deaths annually worldwide, with cirrhosis and liver cancer being the 11th and 16th leading causes of mortality, respectively1,2. Although hepatitis B vaccination and hepatitis C antiviral treatment have greatly reduced the number of liver disorders, a rising population of metabolic dysfunction-associated fatty liver diseases (MAFLD) poses a considerable health and economic burden on communities3.
MAFLD is a multisystemic condition that affects a quarter of the adult population and encompasses a broad spectrum of disease severity and natural history4. Although the majority of patients display simple steatosis with minimal symptoms, up to 20% of patients can develop steatohepatitis, which can lead to cirrhosis, liver cancer, and death5. Substantial interpatient variation suggests that MAFLD has a heterogenous phenotype, and the individual susceptibility to progressive and complicated liver disorders is still unknown. As no medical treatment apart from lifestyle modification has been identified for steatohepatitis and fibrosis, preventing the advancement of MAFLD in its early stages is essential. Consequently, identifying a reliable metabolic factor to predict the progression of steatosis and fibrosis in MAFLD patients is critical.
Individuals with steatosis are diagnosed with MAFLD if one of the following three criteria is met: overweight or obesity, type 2 diabetes mellitus (T2DM), and a state of metabolic dysregulation, defined as the presence of more than two metabolic risk abnormalities6. The severity of hepatic fibrosis is a crucial prognostic determinant for patients with fatty liver diseases7,8. Since the National Cholesterol Education Program—Adult Treatment Panel III (ATP III) adopted the minimal criteria for metabolic syndrome9, it has been shown that metabolic syndrome and its individual components, particularly T2DM, promote fibrosis progression10,11,12. Although some studies have found a correlation between fibrosis severity and the number of metabolic abnormalities13, the majority of previous research has focused on the effect of a single metabolic risk factor, as opposed to their combination, on the course of liver diseases. Substantial evidence has illustrated that T2DM is prevalent in MAFLD and is closely associated with greater risks of steatohepatitis, fibrosis, and advanced fibrosis14. Non-diabetes patients may overlook their underlying metabolic dysfunctions, which have the potential to increase the risk of steatosis and fibrosis.In fact, although non-diabetic populations are not generally considered to be at high risk, existing research suggests that metabolic abnormalities in these populations can also significantly increase the risk of developing liver diseases. For example, non-alcoholic fatty liver disease (NAFLD) has been widely recognized to exist in individuals without diabetes, and its association with liver fibrosis is increasingly recognized. However, studies on the impact of metabolic abnormalities on the risk of liver fibrosis in non-diabetic patients are still lacking. Most of the existing studies have not comprehensively assessed the role of metabolic disorders in the non-diabetic population, and this gap limits our understanding of how metabolic abnormalities independently affect the progression of liver diseases15.
We hypothesized that the existence of metabolic aberrations included in the MAFLD diagnostic criteria is related to the severity of liver disorders in patients without diabetes. Using liver elastography data from NHANES 2017–2018, we assessed the impact of each metabolic dysregulation component on the risk of steatosis and significant fibrosis in non-diabetic populations.
Results
Patient features
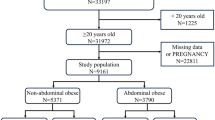
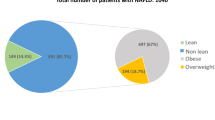
A total of 2149 patients with transient elastography data included, classified into BMI ≥ 25 kg/m2, T2DM, and metabolic dysfunction groups according to the diagnostic criteria for MAFLD (Supplementary Fig. 1). Our analysis shows a significant difference in the risk of fibrosis between individuals with and without diabetes. Specifically, we found that diabetic patients have a markedly higher risk of liver fibrosis progression compared to non-diabetic patients (Supplementary Table 1). These results suggest that although both groups exhibit metabolic abnormalities, the presence of diabetes further exacerbates the risk of fibrosis. This may lead to an increased focus on diabetes management, potentially overlooking the health management of non-diabetic individuals. Then the analysis was performed on 1711 non-diabetic patients, who were categorized by their CAP and LSM levels (Table 1). 47.1% of participants were male, with an average age of 46.71 years. Steatosis (CAP ≥ 285 dB/m) and significant fibrosis (LSM ≥ 8.0 kPa) were present in 519 and 113 patients, respectively, with substantially worse metabolic panels. The values of metabolic parameters such as BMI, waist circumference, TG, fasting glucose, fasting insulin, HbA1c, and HOMA-IR were significantly higher in steatosis and fibrosis patients. 13.9% of patients with fatty liver diseases exhibited significant fibrosis, while 4.1% of the patients had advanced fibrosis. A sex difference was also seen, with more male than female patients affected.
Effect of metabolic abnormalities on steatosis and fibrosis
As previously described, the MAFLD criteria defined seven metabolic risk abnormalities, including central obesity, hypertension, hyperlipidemia, low HDL-C levels, pre-diabetes, insulin resistance, and chronic inflammation. The most prevalent metabolic abnormalities in non-diabetic individuals with steatosis and fibrosis were dysregulation of triglycerides (50.9%) and blood pressure (10.3%) (Fig. 1). Overall, the probabilities of steatosis significantly increased when participants had more than two anomalies, whereas the association between metabolic dysfunction and fibrosis was modest (Fig. 2).
Univariate and multivariate analyses were performed on each metabolic aberration in the non-diabetic population. Univariate analysis (adjusted to age, sex, and race) showed a rise in all seven metabolic abnormalities for steatosis participants; the risk for substantial fibrosis was elevated in patients with central obesity, hypertension, pre-diabetes, insulin resistance, and higher hsCRP levels. In multiple regression, steatosis was significantly related to waist circumference (OR = 3.81 [95% CI 2.74–5.30]), HOMA-IR score (OR = 2.26 [95% CI 1.70–3.01]), elevated hsCRP levels (OR = 1.73 [95% CI 1.30–2.29]), and hypertension (OR = 1.68 [95% CI 1.25–2.25]) (Table 2). Moreover, hypertension (OR = 2.43 [95% CI 1.55–3.82]), elevated hsCRP levels (OR = 2.26 [95% CI 1.53–3.47]), and waist circumference (OR = 2.19 [95% CI 1.39–3.45]) were associated with fibrosis (Table 3). In addition, based on the latest guidelines, we used different thresholds to differentiate fatty degeneration and cirrhosis16. The results showed no significant differences (Supplementary Table 2 and Supplementary Table 3). This suggests that changes in the diagnostic thresholds have a minimal impact on the main results and conclusions of the study. Therefore, regardless of the specific thresholds used for diagnosing fatty degeneration and cirrhosis, our findings remain reliable.
Note that the waist circumference and the BMI showed a significant collinearity in our analysis (r = 0.90, Fig. 3). 95.3% of 856 patients with high waist circumference had a BMI ≥ 25 kg/m2, while central obesity was found in 70.6% of the overweight/obese patients. We compared the effects of waist circumference and BMI on the progression of liver diseases. Waist circumference was a more relevant predictor with a greater coefficiency than BMI in regression models. When incorporating BMI into our analysis, it would be excluded from the model by stepwise regression when analyzing LSM and fibrosis.
Discussion
Multiple factors influenced the occurrence and progression of MAFLD, such as metabolic disorders, physical activities, and diet. In this study, we analyzed the effect of metabolic abnormalities from the MAFLD criteria on liver steatosis and fibrosis after eliminating the effect of diabetes. All aberrant abnormal metabolic parameters may contribute to the severity of steatosis, whereas elevated blood pressure, hsCRP levels, and waist circumference indicate an increased risk of fibrosis progression.
MAFLD is currently the most common liver disease globally and has long been seen as a liver manifestation of the metabolic syndrome, with obesity, diabetes, and hyperlipidemia recognized as risk factors17. However, the connection between fatty liver diseases and components of the metabolic syndrome is more complicated than previously assumed18,19, and the roles of metabolic abnormalities require a deeper understanding20. Comparable to other chronic liver diseases, MAFLD presented with a broad pathophysiological spectrum extending from simple steatosis through steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma21. Multiple factors, including hereditary factors, extrinsic environmental factors, and internal microbial factors, contributed to the complexity of fibrosis progression in patients with MAFLD14,22.
Hypertension and even pre-hypertension may increase the incidence of MAFLD23. However, few studies have evaluated the association between HTN and the progression of liver diseases, which is not negligible, even though its effect is smaller than that of obesity and dyslipidemia. An Italian cohort demonstrated that a baseline HTN may predict fibrosis progression by serial liver biopsies, and a meta-analysis of 11 cohort studies validated these findings, showing that HTN at baseline almost doubled the risk (OR = 1.94, [95% CI 1.00–3.74]) of fibrosis progression throughout the observation period24. Our analysis also revealed a link between hypertension and the development of fibrosis, necessitating closer monitoring of blood pressure and liver stiffness in high-risk individuals.
HsCRP is a prominent acute-phase protein that serves as a sensitive marker of systemic inflammation, which has been reported to be a powerful predictor of cardiovascular events25,26, insulin resistance, and visceral obesity27,28. A profound elevation in hsCRP was shown in the course of MAFLD progression29. Dogru et al. found that in obese people, increased hsCRP indicated the presence of steatosis rather than the severity of MAFLD, despite that the metabolic background profiles such as diabetes, hypertension, and dyslipidemia were absent from the models in this research30. Our research confirmed an independent connection between hsCRP and the degree of steatosis and fibrosis, suggesting that hsCRP may be a valuable complementary predictor for monitoring fibrosis in individuals with metabolic dysfunction.
The obesity epidemic has been consistently associated with the rising prevalence of MAFLD, which is not only linked to simple steatosis, but also with progressive fibrosis31. The MAFLD criteria revealed a considerable overlap between waist circumference and BMI in the definition of overweight/obesity32. In our study, we witnessed a substantial correlation between BMI and waist circumference, with waist circumference had superior performance in predicting steatosis and fibrosis. BMI is a well-established cardiometabolic-related index that requires simple measurement of weight and height, whereas waist circumference was widely acknowledged as a superior clinical index to BMI for describing abnormal fat distribution. Using waist circumference in clinical practice is important for predicting steatosis and subsequent fibrosis throughout the MAFLD courses.
According to population studies, patients with established T2DM had a higher prevalence of MAFLD33,34,35, ranging from 30% to 70%36. As determined by repeated liver biopsies, T2DM was regarded as the strongest precursor for the fibrosis progression37. Due to the strong bidirectional pathophysiological connections between insulin resistance, diabetes, and the progression of fatty liver diseases, numerous studies have sought licensed anti-diabetic medications for the treatment of fatty liver diseases38. By examining the relationship between metabolic profiles and the risk of liver fibrosis in non-diabetic individuals, our study highlights that metabolic disorders remain a key factor in the development of liver disease, even in the absence of diabetes. Therefore, our research focused on non-diabetic populations and revealed the impact of metabolic abnormalities on hepatic fibrosis across a broad population, providing new perspectives for early intervention and personalized treatment.
The nomenclature of MAFLD closely reflects the link between metabolic disorders and fatty liver diseases, casting a wider net and allowing for an earlier diagnosis of the systemic disorder. The seven metabolic abnormalities were largely derived from the diagnostic criteria for metabolic syndrome, with similar events (waist circumference, blood pressure, diabetes, dyslipidemia, and low HDL-C levels) and cut-off values. To increase the specificity of MAFLD criteria, it is necessary to evaluate the effect of each component. In this study, we found that in non-diabetic patients, BMI and seven other metabolic anomalies might partially predict the severity of steatosis, with waist circumference, hypertension, and hsCRP levels being related to significant fibrosis. Despite the modifications in nomenclature, MAFLD requires more precise definitions and classifications for improved individual care. In addition, the multi-society consensus introduced the concept of MASLD (Metabolic Dysfunction-associated Steatotic Liver Disease), and both terms reflect the trend of advancing fatty liver disease research towards precision medicine. Compared to MAFLD, MASLD places more emphasis on the role of metabolic cardiovascular risk factors in the disease. For fatty liver disease patients with a normal BMI and no diabetes, MASLD can be diagnosed with the presence of just one metabolic cardiovascular risk factor, while MAFLD requires at least two metabolic risk factors for diagnosis. We conducted a comparison between the two and found no significant differences in terms of steatosis and fibrosis risk (Supplementary Table 4).
This study had several strengths and limitations. It is the first study to analyze the effect of each metabolic abnormality based on MAFLD criteria. The data from NHANES datasets were credible and nationally representative, in contrast to previous studies that recruited patients from liver clinics. Transient elastography provided a reliable and quantitative noninvasive assessment of the severity of steatosis and fibrosis. However, the liver transient elastography may overestimate older and obese individuals, and no commonly accepted cutoff values for CAP and LSM for steatosis and fibrosis have been determined. Liver biopsies, the gold-standard diagnostic criteria for steatosis and fibrosis, were absent from the survey. Lastly, we did not analyze the effect of diet and physical activity, which may affect the progression of liver diseases.
In conclusion, we found positive associations between metabolic anomalies and the severity of liver diseases in the non-diabetic population. Among all components, the risk of significant liver fibrosis went up when central obesity, hypertension, and chronic inflammation were present.
Methods
Subjects
National Health and Nutrition Examination Surveys (NHANES) was a series of national surveys directed by the National Center for Health Statistics (NCHS) and comprised a representative sample of the non-institutionalized United States population. We conducted a cross-sectional analysis of NHANES data from 2017 to 2018. All data was downloaded in November 2022. All individuals provided written informed consent, and the NCHS Research Ethics Board has approved the NHANES protocol (Protocols #2011–17 and #2018–01). More information about the study design are provided elsewhere39.However, as this study solely utilizes publicly available data for research and publication purposes, it was exempted from ethical review and approval.
NHANES 2017–2018 contained 9254 participants, 4493 of whom were non-pregnant adults underwent liver transient elastography. 2344 subjects were excluded on the basis of the following criteria: (1) positive serologic markers for hepatitis B (n = 28) or hepatitis C (n = 48) virus; (2) current insulin users (n = 193); and (3) missing data for fasting insulin (n = 2575). After applying the aforementioned exclusion criteria, a total of 2149 subjects, of which 1711 did not have diabetes, were eligible for the analysis (Fig. 4).
Liver ultrasound transient elastography
Vibration-Controlled Transient Elastography (VCTE) was performed on NHANES 2017–2018 subjects, utilizing the FibroScan 502 V2 Touch (Echosens, Paris, France) with a medium (M) or extra-large (XL) probe according to the skin–liver capsule distance. Although liver biopsy is still the gold standard for diagnosing fibrosis, the liver elastography is cost-effective and sensitive, and simple to implement in clinical settings. By FibroScan (Echosens), controlled attenuation parameter (CAP) scores reported in decibels per meter (dB/m) and liver stiffness measurement (LSM) expressed in kilopascals (kPa) have been established as reliable noninvasive markers for liver steatosis and fibrosis. The stiffness of the liver is determined by the velocity of a 50-Hz shear wave traveling through it. Prior to testing, participants were instructed to fast for at least three hours. Multiple CAP and LSM were taken from the right lobe of the liver via the intercostal space located at the junction of the mid-axillary line and a transverse line at the level of the xiphoid process, and their median values were recorded as the representative CAP and LSM values. All NHANES health technicians were trained and certified, performing the elastography exam in accordance with the manufacturer’s guidelines39. Here, we defined steatosis as CAP ≥ 285 dB/m, significant fibrosis as LSM ≥ 8.0 kPa (≥ F2), and suspected cirrhosis as LSM ≥ 13.1 kPa33.
Measurements
Demographic information and health-related histories were collected in household interviews by trained interviewers using a computer-assisted personal interview system. All body measurements were recorded by experienced health technicians. Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared and rounding to one decimal place; a BMI ≥ 25 kg/m2 was considered as overweight or obese. The participants fasted for nine hours before the blood sample was collected, from which the concentrations of glycated hemoglobin (HbA1c), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured. Plasma fasting glucose and insulin levels were determined by hexokinase and two-site immunoenzymometric assays. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = fasting insulin (μIU/mL) × fasting glucose (mg/dL) / 40540. According to the MAFLD definition, metabolic risk abnormalities included the following: (1) waist circumference ≥ 102 cm in men and ≥ 88 cm in women; (2) blood pressure ≥ 130/85 mmHg or specific drug treatment; (3) TG ≥ 150 mg/dL or specific drug treatment; (4) HDL-C < 40 mg/dL for men and < 50 mg/dL for women; (5) prediabetes (fasting plasma glucose = 100–125 mg/dL or HbA1c = 5.7%–6.4%); (6) HOMA-IR score ≥ 2.5; and (7) CRP ≥ 2 mg/L. Diabetes is defined as a self-reported history of diabetes, fasting plasma glucose (FPG) ≥ 126 mg/dL, or HbA1c ≥ 6.5%. In non-diabetic patients, MAFLD was diagnosed if any of the following two conditions were present: overweight/obesity or the existence of at least two metabolic risk abnormalities6. Measurement details can be found at https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) and interquartile ranges (IQR); categorical variables were presented as frequency and percentage. Differences between groups in baseline characteristics underwent a Student’s t-test for continuous variables and a chi-square test for categorical variables. Variables related to metabolic dysfunction components, together with age, sex, race, and education level were included in a forward stepwise logistic regression procedure to identify the association with steatosis and fibrosis. All tests were two-tailed, and a P value of less than 0.05 was considered statistically significant. R 4.2.0 (https://www.r-project.org/) was used for our analyses.
Data availability
The dataset supporting the conclusions of this article is available in the Nhanes, https://wwwn.cdc.gov/nchs/nhanes/default.aspx, which can be accessed by everyone through the links provided in this study.
References
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. Burden of liver diseases in the world. J. Hepatol. 70, 151–171 (2019).
Tsochatzis, E. A., Bosch, J. & Burroughs, A. K. Liver cirrhosis. Lancet 383, 1749–1761 (2014).
Younossi, Z. M. Non-alcoholic fatty liver disease - A global public health perspective. J. Hepatol. 70, 531–544 (2019).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 (2013).
Lazarus, J. V. et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 19, 60–78 (2022).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Ekstedt, M. et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015).
Vilar-Gomez, E. et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology 155, 443–457.e417 (2018).
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285, 2486-2497, https://doi.org/10.1001/jama.285.19.2486 (2001).
Shimada, M., Hashimoto, E., Kaneda, H., Noguchi, S. & Hayashi, N. Nonalcoholic steatohepatitis: risk factors for liver fibrosis. Hepatol. Res 24, 429–438 (2002).
Pelusi, S. et al. Prevalence and risk factors of significant fibrosis in patients with nonalcoholic fatty liver without Steatohepatitis. Clin. Gastroenterol. Hepatol. 17, 2310–2319.e2316 (2019).
Ratziu, V. et al. Liver fibrosis in overweight patients. Gastroenterology 118, 1117–1123 (2000).
Marchesini, G. et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37, 917–923 (2003).
Wree, A., Broderick, L., Canbay, A., Hoffman, H. M. & Feldstein, A. E. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 10, 627–636 (2013).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018).
Sterling, R. K. et al. AASLD Practice Guideline on imaging-based noninvasive liver disease assessment of hepatic fibrosis and steatosis. Hepatology 81, 672–724 (2025).
Sorrentino, P. et al. Predicting fibrosis worsening in obese patients with NASH through parenchymal fibronectin, HOMA-IR, and hypertension. Am. J. Gastroenterol. 105, 336–344 (2010).
Lonardo, A., Nascimbeni, F., Mantovani, A. & Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 68, 335–352 (2018).
Eslam, M., Sanyal, A. J. & George, J. MAFLD: A Consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158, 1999–2014.e1991 (2020).
Petta, S. et al. Metabolic syndrome and severity of fibrosis in nonalcoholic fatty liver disease: An age-dependent risk profiling study. Liver Int 37, 1389–1396 (2017).
Cotter, T. G. & Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 158, 1851–1864 (2020).
Singh, S. et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 13, 643–654 (2015).
Wu, S. J. et al. Increased levels of systolic blood pressure within the normal range are associated with significantly elevated risks of nonalcoholic fatty liver disease. Medicine 94, e842 (2015).
Zhang, T. et al. Metabolic syndrome and its components as predictors of nonalcoholic fatty liver disease in a northern urban Han Chinese population: a prospective cohort study. Atherosclerosis 240, 144–148 (2015).
Ridker, P. M., Rifai, N., Rose, L., Buring, J. E. & Cook, N. R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 347, 1557–1565 (2002).
Lloyd-Jones, D. M. & Levy, D. C-reactive protein in the prediction of cardiovascular events. N. Engl. J. Med. 348, 1059–1061 (2003).
Kim, K., Valentine, R. J., Shin, Y. & Gong, K. Associations of visceral adiposity and exercise participation with C-reactive protein, insulin resistance, and endothelial dysfunction in Korean healthy adults. Metabolism 57, 1181–1189 (2008).
Hak, A. E. et al. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler. Thromb. Vasc. Biol. 19, 1986–1991 (1999).
Yoneda, M. et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J. Gastroenterol. 42, 573–582 (2007).
Dogru, T., Genc, H. & Bagci, S. C reactive protein levels in non-alcoholic fatty liver disease. J. Hepatol. 56, 507–508 (2012).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 92, 82–97 (2019).
Polyzos, S. A. et al. Commentary: Nonalcoholic or metabolic dysfunction-associated fatty liver disease? The epidemic of the 21st century in search of the most appropriate name. Metabolism 113, 154413 (2020).
Kim, D., Cholankeril, G., Loomba, R. & Ahmed, A. Prevalence of fatty liver disease and fibrosis detected by transient elastography in Adults in the United States, 2017-2018. Clin. Gastroenterol. Hepatol. 19, 1499–1501.e1492 (2021).
Stepanova, M. et al. Patients with diabetes and chronic liver disease are at increased risk for overall mortality: a population study from the United States. Clin. Diab. 35, 79–83 (2017).
Ciardullo, S., Monti, T. & Perseghin, G. High prevalence of advanced liver fibrosis assessed by Transient Elastography Among U.S. adults with Type 2 Diabetes. Diab. Care 44, 519–525 (2021).
Lonardo, A. et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig. Liver Dis. 47, 997–1006 (2015).
McPherson, S. et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J. Hepatol. 62, 1148–1155 (2015).
Ferguson, D. & Finck, B. N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 17, 484–495 (2021).
Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey. Atlanta: CDC;2022. [cited 2022 November 18]. Available from: https://www.cdc.gov/nchs/nhanes/index.htm.
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Acknowledgements
The authors would like to thank the National Health and Nutrition Examination Survey for contributing the data used in this study.The National Natural Science Foundation of China (No. 82172983) provided research funding for this study. The data that supports the study's conclusions is available upon request from the authors.
Author information
Authors and Affiliations
Contributions
JF: Article Conception and Design. YY, LL, and XS: Data collection, data management, and formal statistical analysis. JF: manuscript writing. YC: manuscript revision. All the authors were involved in writing the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fu, J., Yi, Y., Li, L. et al. Association of metabolic abnormalities and the risk of hepatic fibrosis. npj Gut Liver 2, 13 (2025). https://doi.org/10.1038/s44355-025-00025-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44355-025-00025-z