Abstract
In the Drosophila wing anterior margin, the dendrites of gustatory neurons occupy the interior of thin and long bristles that present tiny pores at their extremities. Many attempts to measure ligand-evoked currents in insect wing gustatory neurons have been unsuccessful for technical reasons. The functions of this gustatory activity therefore remain elusive and controversial. To advance our knowledge on this understudied tissue, we investigated the architecture of the wing chemosensory bristles and wing trachea using Raman spectroscopy and fluorescence microscopy. We hypothesized that the wing gustatory hair, an open-ended capillary tube and the wing trachea constitute biological systems similar to nano-porous materials. We present evidence that argues in favour of the existence of a layer or a bubble of air beneath the pore inside the gustatory hair. We demonstrate that these hollow hairs and wing tracheal tubes fulfil conditions for which the physics of fluids applied to open-ended capillaries and porous materials are relevant. We also document that the wing gustatory hair and tracheal architectures are capable of trapping volatile molecules from the environment, which might increase the efficiency of their spatial detection by way of wing vibrations or during flight.
Similar content being viewed by others
Introduction
Insect wings are an evagination of the exoskeleton and their membrane is formed by two closely apposed layers of integument1,2,3. The two integument layers remain separate in the veins, where they usually thicken and sclerotize1,2,3. The veins create a network of pipes that shelter air-filled tracheal tubes surrounded by a haemolymph layer1,2,3. The haemolymph flows into the wing veins as an extension of the body haemocoel1,2,3. In parallel, the environmental air diffuses into the wing tracheal system via the body tracheal pipe network3. Importantly, a tracheal tube and a nerve coexist within the dorsal anterior margin vein where the wing mechano- and gustatory sensilla are closely associated4,5,6,7,8,9. This generates a sophisticated exchange structure between the haemocoel, air and living cells of the sensilla. The evolutionary success of the insect wing neuroanatomy results in a sophisticated flight guidance partly by providing volatile and hydrophilic compounds to the sensilla cells1,2,3.
Two types of bristles exist along the anterior Drosophila wing margin vein7,10. The short and robust bristles are part of the mechanoreceptor sensilla, whose main role consists of sensing mechanical information such as touch, vibration and air turbulence. These mechanical bristles alternate with long and thin hairs that shelter the dendrites of the gustatory neurons for which the functions and transduction pathways are poorly documented7,8,10,11,12. In Drosophila, the wing gustatory chemosensory sensilla are each composed of 2–4 neurons and one mechanosensory neuron7,8. Three accessory sheath cells surround these neurons: tormogen, trichogen and thecogen cells10,11,12. Surprisingly, in the anterior wing margin, the chemosensory sensilla harbour gustatory instead of olfactory receptors.
Until now, little has been reported regarding the physiology of neurosensory cells in the insect wing. Most electrophysiology studies failed to provide consistent and repeatable results in this tissue, although a priori conditions seem ideal in terms of the accessibility of external ligands to the receptors through the pore at the extremity of the chemosensory hair. Many studies using powerful genetic tools in the Drosophila model11,12 along with ultrastructural analysis using scanning electron microscopy4,7 have abundantly documented the insect wing neuroanatomy and morphology. In contrast, the complex physiology of the insect wing remains understudied. One explanation might be that the chemosensory neurons of wings should be protected against the mechanical stress and heat generated by wing vibrations during flight13,14. This does not apply to the neurons that are sheltered in the contractile proboscis or in the legs, for which many electrophysiology data have been reported12. The wing vibrations during flight would likely expel the lymph through the pore and dry the dendrites if evolution had not engineered a structural architecture to prevent this. In other words, if we assume that the vibrational component of the insect wing is part of the process of wing chemodetection, the air vortex created by the flapping wings is likely required to facilitate the accessibility of environmental compounds to the wing neuronal receptors. In such a case, a partially air-filled bristle would be a more efficient system to trap environmental molecules such as volatile compounds and/or hydrophilic molecules solubilized in water microdroplets than a hair filled with lymph up to its extremity.
The absorption/desorption of gas or liquids and the transition vapour to liquid in a porous material is a well-documented phenomenon15,16,17,18,19,20,21 that should apply to open-ended insect hairs along with tracheal pipes. Water vapour condenses in a liquid state inside open carbon nanotubes15,16,17,18,19,20,21. An increased and high number of van der Waals interactions among water molecules occurring inside the confined space of a capillary provoke the vapour to liquid transition and this occurs when the vapour density in the atmosphere is lower than the saturation point15,16,17,18,19,20,21. This phenomenon leads to molecule trapping in a confined space of porous material, which cannot be achieved on smooth surfaces15,16,17,18,19,20,21. The vapour to liquid phase transition in confined geometries has many applications in chemical sensing, although the basic mechanisms of capillary condensation and adsorption are still poorly understood22,23,24,25. We hypothesize that an insect sensorial hair made of chitin with an apical pore and hollow inside (even though it is partially filled with lymph) corresponds to the physical attributes of an empty, open capillary. In the same way, the insect tracheal system made up of a network of microtubes is similar to a porous material obeying to the same physical principles.
Here, we investigated the process through which volatile molecules found in nature such as ethanol, organic acids (propionic and acetic acids) or hydrophilic molecules dissolved in water microdroplets enter and condense inside the chemosensory hair space of the Drosophila wing. This should also apply to the overall network of air-filled tubes that constitutes the tracheal system. The first goal of this study is therefore to probe the content of the hollow hairs that shelter the taste receptors in the Drosophila wing. We used Raman spectroscopy methods for two reasons. First, the fingerprints of vibrational frequencies are well known and are characteristic of chemical bonds or molecules26,27,28,29. Second, these fingerprints can be found directly in complex biological tissues without destructive extraction procedures26,27,28,29. Raman spectroscopy is a powerful tool to identify molecules or chemical groups by analysing their vibrational modes26,27,28,29. The lateral resolution of Raman spectroscopy imaging (approximately 0.5 micron) provides not only the identification of compounds but also their spatial distribution below the dimension of the hair diameter26,27,28,29. We analysed the Drosophila wing gustatory hairs by tracking the vibrational mode(s) of molecule(s) associated with lymph compounds. For example, the amide I and II groups of proteins and also aromatic amino acids such tryptophan, phenylalanine and tyrosine show characteristic Raman modes in the 1,200–2,000 cm−1 region26,27,28,29. We investigated this spectral region to establish whether the interior beneath the pore is empty or filled with lymph. We reasoned that Raman analysis using a focused laser beam with a diameter below 0.5 μm, smaller than the hairs, could be used to probe whether the air/lymph meniscus is close to the pore or inside the hair. The penetration depth of the light probe, sensitivity, photo-degradation, emitted fluorescence and to a lesser extent spatial resolution strongly depend on the excitation wavelength26,27,28,29. For these reasons, we used a green line of an argon laser at 514 nm and a near infrared diode at 785 nm. Raman spectral imaging is performed directly on intact chemosensory hairs through the cuticular barrier, which allows us to qualify and quantitate the signatures of chemical bonds as amide and amino acid residues in lymph proteins at the different levels of the hairs. The envelope of these hairs is composed of a rigid matrix of polysaccharides (chitin)30,31,32, which provides the advantages of minimizing the interferences with proteins in Raman spectrometry analysis and greatly improving the signal-to-noise ratio. In addition, we investigated whether the wing tracheal system condenses volatile molecules present in the environment. To investigate this, we used benzaldehyde, which has three properties. It is volatile and aromatic, its aldehyde group forms covalent bonds with the lysine or amine groups of proteins and its benzyl group auto fluoresces when attached to proteins. The increased autofluorescence generated in the wing was analysed as an index of the benzaldehyde accumulation in the trachea and sensory hairs.
The wing beat produces air turbulence called the “leading edge vortex” that spirals off along the anterior wing margin, precisely where the chemo- and mechanosensory hairs are located13,14. All these elements (air vortex and capillary fluid dynamics) fit a model for which the insect wing chemosensory hairs along with the wing tracheal pipes are structurally designed for an optimal efficiency of chemical detection. Our data support the hypothesis that the wing gustatory hairs are filled with air beneath the pore and that the wing trachea condenses environmental organic volatile molecules. Together, these elements suggest that the wing gustatory sensilla might obtain environmental information from both the tracheal pipes and the hollow hairs with an opened pore at their extremity.
Results
Morphological structure of the gustatory sensilla along the anterior margin nerve of the Drosophila wing
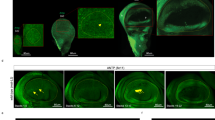
The photograph in Fig. 1A shows the border of the anterior wing margin where the stout bristles alternate with thin and long hairs (thin red arrows). The former are associated with the mechanoreceptor sensilla, while the latter host gustatory sensilla. These thin hairs are hollow tubes opened to the environment by a unique pore at their extremity7. The photographs in Fig. 1B show the fluorescence emitted by a calcium sensor (G-Camp), which is a hybrid of a modified GFP linked to a calcium binding motif that emits fluorescence upon Ca2+ binding33. We observed that the bitter molecule denatonium and the sweet molecule glucose produced significant signals in the sensilla. Consecutive snapshots (1 to 5) over a period of twenty seconds after glucose stimulation are shown (Fig. 1B). These flashes oscillated during the time course of activation. A time course of emitted fluorescence (ΔF/F°) is presented on a large number of sensilla recruited in a panel of 10 individual flies. This assay and the genetic construct based on a ubiquitous strong promoter (tub-Gal4) to drive the calcium sensor allowed us to unambiguously demonstrate the functionality of taste receptors in Drosophila wings. Figure 2 illustrates the morphology of these bristles revealed by electron microscopy. Damage caused by a razor (arrows) highlights that the interior is empty, which confirms that these bristles are hollow tubes.
Distribution of gustatory sensilla and calcium imaging in Drosophila wings.
(A) The photograph shows a wing subset corresponding to the anterior wing margin. The stout bristles are mechanosensilla and the thin hairs (red arrows) are chemosensilla that shelter gustatory neurons. The dark line along the anterior margin is the vein of the anterior wing margin that contains haemolymph, trachea and the nerve made of bundles of axons of the mechanosensory and chemosensory (gustatory) neurons (see the schematic of a cross section in the box at the right). (B) The photographs display calcium imaging by a fluorescent probe. The gustatory sensilla light up after denatonium (1 mM) or glucose (50 mM) binding to their respective receptors. The wing corresponds to the F1 progeny of a cross between homozygous UAS-GCamp and heterozygous Tub-GAL4 flies. The photographs (1 to 4) represent successive sequences over a total period of 20 seconds after glucose stimulation (1 is the time 0 before induction). (C) Time course of the fluorescence after denatonium (1 mM) or glucose (50 mM) stimulation. The graphs represent the average of the determination (delta F/F0) for 10 wings and 3 sensilla per wing (n = 30, mean + /−SE, p < 0.0005 between the 15 and 30 sec., Student’s t-test).
Electron microscopy photographs of the Drosophila wing bristles of the anterior margin.
(A) The wing anterior margin has two types of bristles. The stout and short ones are linked to the mechanosensilla, while the long hairs are associated with the gustatory sensilla. (B) Sensorial bristle made of chitin with a nano-pore at the top. (C) An opening made with a razor shows that the thin hairs are hollow tubes. These interior spaces shelter the lymph and the dendrites of sensilla neurons. (D) Collapsed nano-pore located at the apex of the bristle (scale bar = 300 nm).
Probing the hollow thin hair by Raman spectroscopy
The anterior wing margin contains a trachea (an air-filled tube) and a nerve both bathed by a haemolymph layer to feed the sensilla cells (box in Fig. 1A). However, the opened thin hairs shelter dendrites that are bathed with lymph secreted by the apical side of sensilla cells, but are separated from the haemolymph vein7. The comparative Raman spectra along the hair from the base to the extremity are shown in Fig. 3 for two excitation wavelengths (514 and 785 nm). Under 514 nm excitation (Fig. 3, middle), the diameter of the probed volume was approximately 0.28 μm, which is smaller than the hair diameter. The spectra showed a strong fluorescence background and two broad bands at approximately 1,360 and 1,580 cm−1. These bands, respectively assigned in the literature to the D-band and G-band of carbon compounds26,27,28,29, are explained by the carbon residues resulting from the thermal breakdown of proteins induced by the laser beam, even under a very low power density (<12 μW). However, the Raman spectra varied along the hair, with low responses near the tip (Fig. 3, position C), suggesting a strong position-dependence of the intensity of the signals.
Raman spectra of Drosophila chemosensory wing hairs under 514 nm and 785 nm laser excitation.
Each chemosensory wing hair shows a diameter little changed from the base to the extremity, where a unique pore is located. The Raman beam was focused on three places: at the base of the bristle (A), at 2/3rd of the length (B) and at 9/10th of the length (near the extremity) (C). On the left, microphotographs represent one hair of a wing with the impact of the laser beam. Excitation lengths were 514 nm (middle) and 785 nm (right). The three corresponding spectra are represented on each graph. We noted a large main peak between 1,500 and 1,650 cm−1. Each individual line in the three categories (A–C) was obtained with different individual wings. Strong differences in the intensity of Raman signals are shown among the three locations of the laser beam.
To avoid the fluorescence induced by the excitation wavelength and the degradation of organic molecules by the laser beam, we performed Raman experiments under the 785 nm excitation (Fig. 3, right). As a consequence, this led to a larger probed volume and a lower spatial resolution (approximately 0.45 μm) compared to those obtained under the 514 nm excitation. The spectra showed several vibrational features. A mode at 1,540 cm−1 corresponds to the position of the amide II bands resulting from the combination of N-H bending and C-N stretching. Another mode at 1,610 cm−1 could be assigned to the C=O stretching of amide I. Finally, the 1,350 cm−1 band fits with the position of C-N stretching and N-H bending of aromatic amino acids, which is consistent with the expected presence of tryptophan, phenylalanine and tyrosine. As previously observed under 514 nm excitation, we obtained strong differences in Raman intensity depending on the laser beam position along the hair, with a loss of signal close to the pore (Fig. 3, position C). Although the Raman vibrational frequencies are sensitive to the local environment, the obtained spectra are “signatures” of molecular motifs such as amide I and II and also aromatic amino acids such as tryptophan, phenylalanine and tyrosine. The band positions revealed the conjugated presence of these molecular motifs and the overlap of the corresponding peaks. For both excitation wavelengths, the Raman intensity along the hair drastically changed when crossing a “frontier” position B (Fig. 3). We noticed high and almost constant Raman signals before this frontier (position A) and very low signals after this position towards the apical pore (position C). Considering these data and the fact that the hair diameter appeared unchanged from its base to its extremity, we can state that a lymph meniscus existed approximately one third of the hair length beneath the pore. The frontier position migrated slightly towards the base of the hair after a period of one hour, suggesting that the lymph dried slowly after wing dissection (all Raman spectra reported in Fig. 3 were obtained within a few minutes after dissection). Finally, a statistical analysis was performed on ten determinations at the base or the tip and each measure was performed on one arbitrary gustatory hair. Ten wings were examined for each set (Fig. 4). We observed significant differences in Raman intensity depending on the position of the laser beam. In contrast with high intensity signals at the basis of the hair, very low signals at the tip of the hair are systematically obtained, suggesting an air layer beneath the pore.
Statistical analysis of the Raman spectra under 514 nm and 785 nm laser excitation.
Ten determinations each on the tip and the base of arbitrary gustatory wing hairs were analysed for each excitation wavelength. Each of these measures was performed on different wings. The statistical analysis was carried out using Student’s t-test and is represented at the right (bars are the mean + /−SE, n = 10, *p value < 0.0005 between the measurements at the tip and the base of the hairs).
Probing benzaldehyde condensation in the trachea and in the chemosensory hairs of Drosophila wings
The insect respiratory system presents a complex network of tracheae (large tubes) and tracheoles (micro/nanometer diameter tubes) that irrigate the organs and the wing veins. To address whether the volatile molecules present in the air are condensed in tracheae/tracheoles, we tested the dynamics of benzaldehyde in the wing veins. The aldehyde domain of the molecule covalently binds to the amine groups of proteins. When attached to proteins, the benzyl domain autofluoresces with the excitation/fluorescence wavelength of FITC. In Fig. 5, these two chemical properties were used to analyse the concentration time course in the wing tracheal veins. We observed that the autofluorescence in living wings increased with the time of exposure to the environmental benzaldehyde (Fig. 5A). This experiment required that the flies be alive, as this phenomenon is abolished in dissected wings (Fig. 5B). Thus, an intact respiratory system of living animals was crucial for the condensation of chemical compounds in the wing trachea. Furthermore, the lymph that baths the dendrites in the thin hairs of the anterior wing margin is physically separated from the wing haemolymph circuit. Based on our Raman analysis that documents the probable existence of an air layer beneath the pore, we investigated whether this facilitates the diffusion/condensation of volatile molecules inside the hair. The thin hairs containing chemosensilla exhibited fluorescence after benzaldehyde exposure (Fig. 6A), unlike the stout bristles harbouring mechanosensilla (Fig. 6B). Again, fluorescence was present in the wing gustatory hairs of living animals but not in the dissected wings (Fig. 6C), suggesting that wing vibration and/or grooming contribute to the adsorption. More importantly, the fluorescence generated by benzaldehyde adsorption in the wing anterior margin vein correlated with the benzaldehyde concentration. With living animals, a strong fluorescence appeared in this vein proportional to the concentration of benzaldehyde and this effect was abolished in cut wings (Fig. 6C,D). This strongly suggests that the trachea/tracheole networks concentrate/condense environmental volatile molecules in living animals. Our data also show that wing sensory hairs with a capillary-like structure should allow the concentration/condensation of many other environmental volatile molecules, including organic acids (acetic acid and propionic acid), amphoteric molecules (aldehyde compounds), or small molecules (CO2 or ethanol). This implies that the partially empty capillary structure of gustatory hairs likely influences wing chemoperception by modifying parameters such as local pH, local ionic forces and chemical concentrations. Figure 7 proposes a model inspired by the presented experiments. In summary, the tracheal system likely concentrates organic volatile compounds in the body and this phenomenon is presently ignored by entomologists. The stimulation of wing sensilla neurons appears to be regulated by environmental molecules entering both through the pore of the gustatory hair and the trachea of the wing veins, creating a more complex system than previously thought.
Autofluorescence generated by benzaldehyde in the veins of a Drosophila wing.
(A) The four photographs represent exposure to benzaldehyde (10 μl on a paper towel soaked in water and placed in a 200 ml Drosophila vial) of living seven day old Drosophila females. 1–4 represent the time of exposure at 0, 1, 2 and 4 hours, respectively. At the right of each photograph is shown the relative intensity of the autofluorescence corresponding to the two white cross traits from a range of 0 to 1. The asterisks correspond to the average of five experiments and represent the mean + /−SE. 0 h: 0.15 + /−0.05 for both stars; 1 h: 0.3 + /− 0.1 for both stars; photo 2 h: 0.45 + /−015 and 0.55 + /−0.25, respectively; 4 h: 0.75 + /− 0.2 and 0.85 + /−0.5, respectively. A Student’s t-test analysis gives a p < 0.001 between the corresponding determinations at 2 and 4 hours (photos 3 and 4). (B) The same experiment was conducted in identical conditions, but cut wings were used instead of living animals. The relative fluorescence intensity corresponding to white traits in the photographs are represented in the graphs at the right (average of n = 5 experiments). In cut wings, no significant differences were observed between 1 and 2 that correspond to the time of exposure at 0 and 4 hours, respectively. We observed that the benzaldehyde covalent binding marks the tracheal network in wings and this labelling requires living animals with an intact respiratory system.
Autofluorescence generated by benzaldehyde in the chemosensory hairs and the anterior margin vein of the Drosophila wing.
Flies were exposed to a high dose of benzaldehyde (50 μL in a 300 ml vial for two hours). (A) The anterior wing vein is fluorescent and the thin hairs corresponding to the chemosensory sensilla (thin arrows) are also labelled. (B) The photograph shows fluorescence in the chemosensory hairs but not in the stout bristles corresponding to the mechanoreceptors (thick arrows). The vein is intensely labelled. (C) Fluorescence obtained with a cut wing treated in the same conditions. The margin vein is weakly labelled, but the thin chemosensory hairs are not labelled at all. (D) Dose response of emitted fluorescence obtained with living flies (blue stars) after two hours of exposure to increasing concentrations of benzaldehyde. The fluorescence was measured in the anterior wing margin region delimited by the red oval in the box. Each determination is the mean + /− SE, n = 10. A relative measure of fluorescence obtained within the anterior wing margin of cut wings treated in the same conditions (red stars) is reported for two concentrations of benzaldehyde (mean + /−SE, n = 5). For 10 and 50 μl benzaldehyde, p < 0.01 and < 0.001, respectively, between the cut and living wings (Student’s t-test).
Schematic that summarizes the microphysics of the Drosophila wing chemosensory hair.
(A) Schematic of a wing chemosensory hair structure showing a dendrite (black) surrounded by lymph (red). The top third of the hair from the base does not contain lymph and allows the exchange with the surrounding air environment. The sub-micronic diameter of this capillary structure exhibits the conditions for the physics of adsorption of volatile molecules. Letters represent the positions of the laser beam that generated the Raman spectra. (B) Schematic of the putative mechanism of a chemosensory hair showing an opened capillary structure. Inside the apical pore, the environmental volatile compounds are adsorbed, condensed and transferred by dissolution to the lymph (red) to access the dendrite (black). VOC denotes “volatile organic compounds”.
Discussion
The insect cardiac tube is a longitudinal dorsal tube running along the whole thorax to the extremity of the abdomen34,35,36,37,38. The abdominal region of the cardiac tube (the heart) contains a succession of small bulbs with two symmetric holes at their bases called ostia34,35,36,37,38. A circular flow of haemolymph is pushed from the abdomen to the head by the contraction of the heart tube, then returns to the abdominal organs and re-enters the dorsal heart tube through the ostia39. Furthermore, insects have several accessory hearts located at the bases of appendages such as the antennae and wings, perfusing those structures39,40,41,42,43,44. These pulsatile accessory hearts alleviate the efforts of the main abdominal heart and are autonomously regulated34,35,36,37,38,39. Consequently, the wing accessory hearts might have a stronger pulsatile rhythmicity when wings vibrate in flight compared to when they rest and might increase the concentration of volatile environmental molecules in the wing haemolymph via the tracheal system of pipes. This constitutes a sophisticated interphase between the respiratory and circulatory systems36,42,45 and suggests that environmental compounds access the wing gustatory receptors via the pores of the hairs and via the tracheal tubes embedded in the wing veins. Chemically, the wing is made of chitin, a polymer of N-acetylglucosamine30,46. The hairs are made of alkanes on the outer surface layer and a matrix of intertwined chitin chains on the inner layer30,46. The fact that the rigid structure of the hairs made of polymers of N-glucosamine and n-alkanes does not contain detectable proteins is an ideal system to probe the interior via Raman spectroscopy imaging with minimal interference. The Raman imaging technology successfully established the level of the air/lymph meniscus inside the hair. The Raman signals of water molecules were weak, which prompted us to focus on the strong signals of the amide bonds I and II and the aromatic amino acids tyrosine, tryptophan and phenylalanine between 1,200 and 2,000 cm−1. Although the green laser (514 nm) penetration was lower than the infrared laser (785 nm) penetration and the fluorescence was higher, both spectra are in accordance with each other. In parallel, a strong signal of carbon residues resulting from the breakdown of proteins under green laser irradiation was recorded. Altogether, the Raman experiments clearly indicated that the detected lymph was strongly dependent on the position of the laser beam along the bristle. We recorded high signals from the base up to half of the bristle and very low signals from two thirds along the bristle towards the apex. Considering the obtained Raman spectra of proteins and the drastic changes in signal intensity for both laser excitations, we can state that the hairs were partially filled with air. Rarely have electrophysiology studies been successfully performed in the wing tissue12. This strongly supports our conclusions for the wing nanoarchitecture of an air layer that exists beneath the pore of the hair, preventing the contact between dendrites and electrodes. In addition, we observed that autofluorescence generated by benzaldehyde was strong in the wing veins of living animals but was abolished in dissected wings. This indicates that the entire tracheal pipeline network is necessary to concentrate volatile molecules in wing veins in a dose-dependent manner and this might rely on wing vibrations. These data also strongly support the argument that the physiology of gustatory sensilla in the wing is controlled by environmental molecules coming from the opened bristles and the trachea of the veins acting in synergy, both realizing the microphysics of porous materials and carbon nanotubes.
More importantly, we might question why evolution has selected gustatory cells in insect wings instead of olfactory ones. In mammals, the common paradigm is that volatile/ hydrophobic molecules activate olfactory receptors and hydrophilic molecules activate gustatory receptors. In fact, the boundaries between these two sensory modalities based on the physic-chemistry of stimuli do not apply in insects47. In insects, taste cells detect CO2 (in carbonate form)48, water (through osmosensitive ion channels)49 and pheromones47. The carboxylic acids such as acetic, propionic and citric acids amplify the bitter detection and inhibit the sweet perception50. All these molecules are volatile and convey taste cell signalling in insects. As for any porous material, the condensation of volatile molecules such as ethanol, water and carboxylic acids in the wing tracheal system and the hollow wing taste hairs is likely a component of the process of the wing chemosensory detection. Vibrations of the wing creating vortexes and mixing molecules in the lymph/hemolymph are likely the other important component. To date, although the presence of taste sensilla in the Drosophila wing is fully accepted, we report here that these cells are functional and respond to sweet and bitter molecules as do proboscis taste cells. The wing taste receptors stimulated by hydrophilic tastants such as glucose and denatonium induce an intracellular calcium peak as they do in the proboscis. The access of tastants to wing receptors is likely provoked by leg grooming for Drosophila or by nebulization of microdroplets due to wing vibrations over flowers for insect pollinators. Altogether, these elements highlight the complex modality of taste in insects, which seems to result from the integration of coincidental signals triggered by volatile organic molecules, water vapour or CO2 along with classic hydrophilic tastants.
Our data suggest strongly that the nano architecture of the tracheal pipes and hollow hairs of the wing are intimately linked to the efficiency of chemical detection by wing sensilla. For this purpose, the emerging physics of carbon nanotubes and/or porous materials that highlight their impressive properties to concentrate or condense molecules present in the air15,16,17,18,19,20,21 can be used as a model to understand chemosensory physiology in insects. The functions of wing chemosensory neurons in insects and how they probe the complexity of a given chemical environment is currently unknown and understudied. We suggest that the Drosophila wing combines molecular condensation in submicron hollow tubes and trachea with wing vibrations acting as a vortex to solubilize molecules in the lymph/hemolymph. The presented data support the concept that wing sensilla receive chemical information from two channels: the pore of the hollow hair and the trachea pipes along the anterior wing margin at the base of the taste sensilla. In the future, we anticipate that this result could lead to a new generation of bio-inspired chemical sensors using high frequency bending nanotubes.
Methods
Drosophila strains and genetic constructs
G-CaMP is an engineered hybrid protein that is an efficient in vivo calcium sensor. Its gene combined with a yeast promoter (UAS) was introduced in Drosophila. This transgenic fly is publicly available from the Bloomington Drosophila Stock Center (stock n. 42037). G-CaMP is the fusion of calmodulin (CaM) and a modified GFP (N- and C-domains are fused to make an inverse and permuted protein33). In the absence of calcium release, this molecule emits a weak fluorescent signal. GAL4 is an exogenous yeast transcription factor that recognizes the UAS motif and transcribes the downstream gene. For our experiments, we used the Tubulin-GAL4 (Tub-GAL4) driver (Bloomington stock n. 5138) because no other driver, including the gustatory receptor drivers, produced strong enough expression in our calcium imaging procedure. Confocal microscopy observations were carried out in the dissected wings of Tub-GAL4/ + ; UAS-G-CaMP/ + flies ubiquitously expressing UAS-G-CaMP. The wings of five day old Tub-GAL4/ + ; UAS-G-CaMP/ + flies were cut with a razor blade, mounted in water between a glass slide and a cover slip and observed with a confocal ZEISS LSM 510 META microscope (20× objective). A drop of the sugar/bitter solution (50 mM glucose and 1 mM denatonium in water) was deposited at the edge of the cover slip and the time course of the fluorescence variation was recorded in real time.
Raman spectroscopy analysis
A Labram HR800 Horiba Jobin-Yvon spectrophotometer was used with a 514 nm line of a tuneable air-cooled Melles-Griot Ar ion laser for excitation. The light intensity measured on the sample was maintained below 12 μW during the 30 s acquisition time. A near infrared laser diode at 785 nm was also used to avoid damaging the sample. The measured power at this wavelength was below 100 μW, with an acquisition time of 60 s. The analysis (excitation and collection) was performed for the excitation wavelength using a 100x objective with numerical aperture (NA) equal to 0.9. Under these conditions, the corresponding analysed area was below 1 μm2. Living Drosophila wings were cut with a razor at the wing/thoracic muscle junctions and immediately placed on a glass slide. The excitation Raman laser beam was focused on the bristles of the anterior wing margin. The Raman spectra are reported as the intensity of the scattered light on the y-axis and the Raman shift on the x-axis.
Scanning electron microscopy imaging
SEM images were performed using an SU-70 Hitachi ultra-high resolution analytical field effect scanning electron microscope (FE-SEM) with a low operating voltage (5 kV), current of 49 mA and chamber pressure of 7 × 10−4 Pa. Drosophila wings were cut, fixed on an aluminium holder with carbon tape and metalized with a 20-nm osmium coating by sputtering techniques.
Benzaldehyde exposure and wing autofluorescence
The flies (25, males and females, five days old) were placed in Drosophila vials (300 ml volume) in which 1 to 50 μl of benzaldehyde were deposed on a piece of paper soaked in water. The wings were cut according to the times indicated in the figure legends and the fluorescence corresponding to the spectral properties of FITC (fluorescein isothiocyanate, excitation at 490 and emission at 520 nm) was analysed using the Zeiss Axiocam MRm ApoTome.2 fluorescence microscope. These experiments were designed based on the model of porous material in which the atmospheric gas condenses in the liquid phase inside the capillaries according the physical laws as described by the Kelvin equation: ln Pv/Psat = −(2HγVl)/RT where: Pv = equilibrium vapour pressure; Psat = saturation vapour pressure; H = mean curvature of meniscus; γ = liquid/vapour surface tension; Vl = liquid molar volume; R = ideal gas constant; T = temperature22,23,25.
Additional Information
How to cite this article: Valmalette, J. C. et al. Nano-architecture of gustatory chemosensory bristles and trachea in Drosophila wings. Sci. Rep. 5, 14198; doi: 10.1038/srep14198 (2015).
References
Arnold, J. W. Blood circulation in insect wings. Memoirs of the Entomological Society of Canada 96 (Supplement S38), 5–60 (1964).
Wasserthal, L. T. Haemolymph flows in the wings of pierid butterflies visualized by vital staining (Insecta, Lepidoptera). Zoomorphology 103, 177–192 (1983).
Wasserthal, L. T. Interaction of circulation and tracheal ventilation in holometabolous insects. Adv Insect Physiol 26, 297–351 (1996).
Zacharuk, R. Y. Ultrastructure and Function of Insect Chemosensilla. Annual Review of Entomology 25, 27–47 (1980).
Lawrence, P. A. Development and determination of hairs and bristles in the milkweed bug, Oncopeltus fasciatus (Lygaeidae, Hemiptera). J Cell Sci 1, 475–498 (1966).
Huang, F., Dambly-Chaudière, C. & Ghysen, A. The emergence of sense organs in the wing disc of Drosophila. Development 111, 1087–1095 (1991).
Stocker, R. F. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 275, 3–26 (1994).
Pollack, G. S. & Balakrishnan, R. Taste sensilla of flies: function, central neuronal projections and development. Microsc Res Tech 39, 532–546 (1997).
Hallberg, E. & Hansson, B. S. Arthropod sensilla: morphology and phylogenetic considerations. Microsc Res Tech 47, 428–439 (1999).
Hartenstein, V. & Posakony, J. W. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 107, 389–405 (1989).
Vosshall, L. B. & Stocker, R. F. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci 30, 505–533 (2007).
Montell, C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol 19, 345–353 (2009).
Dickinson, M. H., Lehmann, F. O. & Sane, S. P. Wing rotation and the aerodynamic basis of insect flight. Science 284, 1954–1960 (1999).
Dickinson, M. Insect flight. Curr Biol 16, R309–14 (2006).
Gelb, L. D., Gubbins, K. E. & Radhakrishnan, R. Phase separation in confined systems. Rep Prog Phys 62, 1573–1659 (1999).
Alvine, K. J., Shpyrko, O. G., Pershan, P. S., Shin, K. & Russell, T. P. Capillary filling of anodized alumina nanopore arrays. Phys Rev Lett 97, 175503 (2006).
Díaz, E., Ordóñez, S. & Vega, A. Adsorption of volatile organic compounds onto carbon nanotubes, carbon nanofibers and high-surface-area graphites. J Colloid Interface Sci 305, 7–16 (2007).
Valiullin, R. et al. Exploration of molecular dynamics during transient sorption of fluids in mesoporous materials. Nature 443, 965–968 (2006).
Casanova, F. et al. Gas adsorption and capillary condensation in nanoporous alumina films. Nanotechnology 19, 315709 (2008).
Shahraeeni, E. & Or, D. Pore-scale evaporation-condensation dynamics resolved bysynchrotron x-ray tomography. Phys Rev E 85, 016317 (2012).
Yzeiri, I., Patra, N. & Král, P. Porous carbon nanotubes: molecular absorption, transport and separation. J Chem Phys 140, 104704 (2014).
Gao, J., Gao, T., Li, Y. Y. & Sailor, M. J. Vapor sensors based on optical interferometry from oxidized microporous silicon films. Langmuir 18, 2229–2233 (2002).
Butt, H. J., Graf, K. & Kappl, M. in Physics and Chemistry of Interface 2nd ed. 2006 (Wiley-VCH).
Pacholski, C., Sartor, M., Sailor, M. J., Cunin, F. & Miskelly, G. M. Biosensing using porous silicon double-layer interferometers: reflective interferometric Fourier transform spectroscopy. J Am Chem Soc 127, 11636–11645 (2005).
Butt, H. J. & Kappl, M. in Surface and Interfacial Forces 2010 (Wiley-VCH).
Swain, R. J. & Stevens, M. M. Raman microspectroscopy for non-invasive biochemical analysis of single cells. Biochem Soc Trans 35, 544–549 (2007).
Evans, C. L. & Xie, X. S. Coherent Anti-Stokes Raman Scattering Microscopy: Chemical Imaging for Biology and Medicine. Annual Review of Analytical Chemistry 1, 883–909 (2008).
Zhang, Y., Hong, H. & Cai, W. Imaging with Raman spectroscopy. Current pharmaceutical biotechnology 11, 654–661 (2010).
Nallala, J. et al. Infrared and Raman imaging for characterizing complex biological materials: a comparative morpho-spectroscopic study of colon tissue. Appl Spectrosc 68, 57–68 (2014).
Andersen, S. O. Biochemistry of insect cuticle. Annu Rev Entomol 24, 29–61 (1979).
Nagaraj, R. & Adler, P. N. Dusky-like functions as a Rab11 effector for the deposition of cuticle during Drosophila bristle development. Development 139, 906–916 (2012).
Ben Rokia-Mille, S. et al. Continued neurogenesis in adult Drosophila as a mechanism for recruiting environmental cue-dependent variants. PLoS ONE 3, e2395 (2008).
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol 19, 137–141 (2001).
Gereben-Krenn, B. A. & Pass, G. Circulatory organs of Diplura (Hexapoda): the basic design in Hexapoda? Journal of Insect Morphology and Embryology 28, 71–79 (1999).
Markou, T. & Theophilidis, G. The pacemaker activity generating the intrinsic myogenic contraction of the dorsal vessel of Tenebrio molitor (Coleoptera). J Exp Biol 203, 3471–3483 (2000).
Hertel, W. & Pass, G. An evolutionary treatment of the morphology and physiology of circulatory organs in insects. Comp Biochem Physiol, Part A: Mol Integr Physiol 133, 555–575 (2002).
Tartes, U., Vanatoa, A. & Kuusik, A. The insect abdomen—a heartbeat manager in insects? Comp Biochem Physiol Part A: Mol Integr physiol 133, 661–623 (2002).
Andereck, J. W., King, J. G. & Hillyer, J. F. Contraction of the ventral abdomen potentiates extracardiac retrograde hemolymph propulsion in the mosquito hemocoel. PLoS ONE 5, e12943 (2010).
Hessel, J. H. The Comparative Morphology of the Dorsal Vessel and Accessory. Entomological Society of America 62, 353–370 (1969).
Wasserthal, L. T. Oscillating haemolymph “circulation” in the butterflyPapilio machaon L. revealed by contact thermography and photocell measurements. J Comp Physiol B 139, 145–163 (1980).
Hustert, R. Accessory hemolymph pump in the mesothoracic legs of locusts, (Schistocerca gregaria forskal) (Orthoptera, Acrididae). International Journal of Insect Morphology and Embryology 28, 91–96 (1999).
Wasserthal, L. T. Functional morphology of the heart and of a new cephalic pulsatile organ in the blowfly Calliphora vicina (Diptera: Calliphoridae) and their roles in hemolymph tracheal transport and ventilation. International Journal of Insect Morphology and embryology 28, 111–129 (1999).
Pass, G. Accessory pulsatile organs: evolutionary innovations in insects. Annu Rev Entomol 45, 495–518 (2000).
Tögel, M., Pass, G. & Paululat, A. The Drosophila wing hearts originate from pericardial cells and are essential for wing maturation. Dev Biol 318, 29–37 (2008).
Wasserthal, L. T. Antagonism between haemolymph transport and tracheal ventilation in an insect wing (Attacus atlas L.). Journal of comparative physiology 147, 27–40 (1982).
Ivanova, E. P. et al. Molecular organization of the nanoscale surface structures of the dragonfly Hemianax papuensis wing epicuticle. PLoS ONE 8, e67893 (2013).
Lee, Y. & Poudel, S. Taste Sensation in Drosophila melanoganster. Hanyang Med Rev 34, 130–136 (2014).
Fischler, W., Kong, P., Marella, S. & Scott, K. The detection of carbonation by the Drosophila gustatory system. Nature 448, 1054–1058 (2007).
Cameron, P., Hiroi, M., Ngai, J. & Scott, K. The molecular basis for water taste in Drosophila. Nature 465, 91–95 (2010).
Charlu, S., Wisosky, Z., Medina, A. & Dahanukar, A. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nature Communications 4, article number 2042 (2013).
Acknowledgements
We thank the Bloomington Drosophila Stock Center for fly stocks. The authors acknowledge Dr. Yoshiko Takahashi and Dr. Takamasa Hanaichi (Ultrastructure Research Institute, Hanaichi Co. Ltd) for their help in SEM measurements. We are grateful to Jean Jacques Remy, Andrea Fernandes and Mathilde Clement for fruitful discussions and to Angela Algeri for her comments on the manuscript.
Author information
Authors and Affiliations
Contributions
J.C.V. and A.R. conceived the experimental design, performed the experiments, analysed the data and wrote the manuscript. N.Q. and S.O. contributed to the electron microscopy photographs. H.R. and M.C. performed the cell biology experiments with fluorescent markers. M.C. participated in the manuscript writing.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Valmalette, J., Raad, H., Qiu, N. et al. Nano-architecture of gustatory chemosensory bristles and trachea in Drosophila wings. Sci Rep 5, 14198 (2015). https://doi.org/10.1038/srep14198
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14198
This article is cited by
-
Transcriptome Profiling of Neurosensory Perception Genes in Wing Tissue of Two Evolutionary Distant Insect Orders: Diptera (Drosophila melanogaster) and Hemiptera (Acyrthosiphon pisum)
Journal of Molecular Evolution (2017)
-
A simple method for imaging axonal transport in aging neurons using the adult Drosophila wing
Nature Protocols (2016)