Abstract
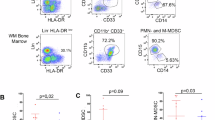
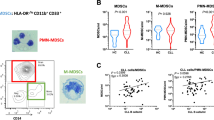
Extracorporeal photopheresis (ECP) is beneficial in patients with T-cell-mediated disorders, including GvHD, but the underlying immunological mechanisms are incompletely understood. Myeloid-derived suppressor cells (MDSCs) are innate immune cells characterized by their capacity to suppress T-cell proliferation. We quantified MDSCs by flow cytometry in peripheral blood from patients after BMT with GvHD before and after ECP treatment, patients after BMT but without GvHD and age-matched healthy controls. MDSC functionality was analyzed using T-cell proliferation, cytokine release and arginase activity. GvHD patients showed increased baseline percentages of neutrophilic MDSCs (PMN-MDSCs) compared with healthy controls and patients after BMT without GvHD. ECP treatment in GvHD patients rapidly increased circulating percentages of PMN-MDSCs. Functionally, PMN-MDSCs efficiently dampened Th1 and Th17 responses and were paralleled by an increase of cellular and extracellular arginase activity. Following ECP longitudinally over 16 weeks, two GvHD responder subgroups were identified, with group one continuously increasing PMN-MDSCs and group two with stable or decreasing PMN-MDSCs over time. This study demonstrates for the first time that ECP increases T-cell-dampening PMN-MDSCs in GvHD patients, a finding that should be confirmed in larger series of GvHD patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Edelson R, Berger C, Gasparro F, Jegasothy B, Heald P, Wintroub B et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy—preliminary-results. New Engl J Med 1987; 316: 297–303.
Hautmann AH, Wolff D, Hahn J, Edinger M, Schirmer N, Ammer J et al. Extracorporeal photopheresis in 62 patients with acute and chronic GVHD: results of treatment with the COBE Spectra System. Bone Marrow Transplant 2013; 48: 439–445.
Flowers ME, Apperley JF, van Besien K, Elmaagacli A, Grigg A, Reddy V et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood 2008; 112: 2667–2674.
Seaton ED, Szydlo RM, Kanfer E, Apperley JF, Russell-Jones R . Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood 2003; 102: 1217–1223.
Knobler R, Barr ML, Couriel DR, Ferrara JL, French LE, Jaksch P et al. Extracorporeal photopheresis: past, present, and future. J Am Acad Dermatol 2009; 61: 652–665.
Bladon J, Taylor PC . Extracorporeal photopheresis: a focus on apoptosis and cytokines. J Dermatol Sci 2006; 43: 85–94.
Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood 2008; 112: 1515–1521.
George JF, Gooden CW, Guo L, Kirklin JK . Role for CD4(+)CD25(+) T cells in inhibition of graft rejection by extracorporeal photopheresis. J Heart Lung Transplant 2008; 27: 616–622.
Meloni F, Cascina A, Miserere S, Perotti C, Vitulo P, Fietta AM . Peripheral CD4(+)CD25(+) TREG cell counts and the response to extracorporeal photopheresis in lung transplant recipients. Transplant Proc 2007; 39: 213–217.
Schmitt S, Johnson T, Karakhanova S, Naher H, Mahnke K, Enk A . Extracorporeal photopheresis augments the number and increases the function of regulatory T cells by triggering Adenosine production. Exp Dermatol 2009; 18: 306–306.
Gerner M, Holig K, Wehner R, Zhao S, Schakel K, Bachmann MP et al. Extracorporeal photopheresis efficiently impairs the proinflammatory capacity of human 6-sulfo LacNAc dendritic cells. Transplantation 2009; 87: 1134–1139.
Gorgun G, Miller KB, Foss FM . Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood 2002; 100: 941–947.
Goussetis E, Varela I, Tsirigotis P . Update on the mechanism of action and on clinical efficacy of extracorporeal photopheresis in the treatment of acute and chronic graft versus host disease in children. Transfus Apheresis Sci 2012; 46: 203–209.
Lamioni A, Parisi F, Isacchi G, Giorda E, Di Cesare S, Landolfo A et al. The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation 2005; 79: 846–850.
Kuzmina Z, Greinix HT, Knobler R, Worel N, Kouba M, Weigl R et al. Proportions of immature CD19+CD21- B lymphocytes predict the response to extracorporeal photopheresis in patients with chronic graft-versus-host disease. Blood 2009; 114: 744–746.
Whittle R, Taylor PC . Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host disease to extracorporeal photopheresis. Blood 2011; 118: 6446–6449.
Xia CQ, Campbell KA, Clare-Salzler MJ . Extracorporeal photopheresis-induced immune tolerance: a focus on modulation of antigen-presenting cells and induction of regulatory T cells by apoptotic cells. Current Opin Organ Transplant 2009; 14: 338–343.
Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S . Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immun 2012; 61: 1155–1167.
Youn JI, Gabrilovich DI . The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 2010; 40: 2969–2975.
Mantovani A . The growing diversity and spectrum of action of myeloid-derived suppressor cells. Eur J Immunol 2010; 40: 3317–3320.
Highfill SL, Rodriguez P, Zhou Q, Goetz CA, Veenstra R, Taylor PA et al. Bone marrow myeloid-derived suppressor cells (MDSC) inhibit graft versus host disease (GVHD) via an Arginase-1 dependant mechanism that is upregulated by IL-13. Blood 2010; 116: 111–112.
Capitini CM, Davis JP, Larabee SM, Herby S, Nasholm NM, Fry TJ . Extracorporeal photopheresis attenuates murine graft-versus-host disease via bone marrow-derived interleukin-10 and preserves responses to dendritic cell vaccination. Biol Blood Marrow Transplant 2011; 17: 790–799.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Lee SE, Cho BS, Kim JH, Yoon JH, Shin SH, Yahng SA et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant 2013; 48: 587–592.
Vigorito AC, Campregher PV, Storer BE, Carpenter PA, Moravec CK, Kiem HP et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood 2009; 114: 702–708.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Bisaccia E, Vonderheid EC, Geskin L . Safety of a new, single, integrated, closed photopheresis system in patients with cutaneous T-cell lymphoma. Br J Dermatol 2009; 161: 167–169.
Brandau S, Trellakis S, Dumitru CA, Bruderek K, Lang S . Neutrophils in head and neck cancer: identification of putative immature neutrophilic myeloid-derived suppressor cells in the peripheral blood and pathophysiological relevance of intratumoral granulocytes. Eur J Clin Invest 2011; 41: 36–36.
Rieber N, Brand A, Hector A, Graepler-Mainka U, Ost M, Schafer I et al. Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. J Immunol 2013; 190: 1276–1284.
Gabrilovich DI, Nagaraj S . Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9: 162–174.
Merlin E, Goncalves-Mendes N, Hannani D, de la Torre A, Farges MC, Laroye H et al. Extracorporeal photochemotherapy induces arginase 1 in patients with graft versus host disease. Transplant Immunol 2011; 24: 100–106.
Di Biaso I, Di Maio L, Bugarin C, Gaipa G, Dander E, Balduzzi A et al. Regulatory T cells and extracorporeal photochemotherapy: correlation with clinical response and decreased frequency of proinflammatory T cells. Transplantation 2009; 87: 1422–1425.
Biagi E, Di Biaso I, Leoni V, Gaipa G, Rossi V, Bugarin C et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation 2007; 84: 31–39.
Dani T, Knobler R . Extracorporeal photoimmunotherapy-photopheresis. Front Biosci 2009; 14: 4769–4777.
Oliven A, Shechter Y . Extracorporeal photopheresis: a review. Blood Rev 2001; 15: 103–108.
Scarisbrick J . Extracorporeal photopheresis: what is it and when should it be used? Clin Exp Dermatol 2009; 34: 757–760.
Ward DM . Extracorporeal photopheresis: how, when, and why. J Clin Apheresis 2011; 26: 276–285.
Rodriguez PC, Ernstoff MS, Hernandez CP, Atkins M, Zabaleta J, Sierra RA et al. Arginase I producing myeloid derived suppressor cells (MDSC) in renal cell carcinoma are a sub population of activated granulocytes. J Immunother 2008; 31: 931–931.
Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI . Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol 2012; 91: 167–181.
Liu Z, Lu Y, Lebwohl M, Wei H . PUVA (8-methoxy-psoralen plus ultraviolet A) induces the formation of 8-hydroxy-2'-deoxyguanosine and DNA fragmentation in calf thymus DNA and human epidermoid carcinoma cells. Free Radic Biol Med 1999; 27: 127–133.
Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med 2010; 207: 1453–1464.
Sakaguchi S, Vignali DAA, Rudensky AY, Niec RE, Waldmann H . The plasticity and stability of regulatory T cells. Nat Rev Immunol 2013; 13: 461–467.
Centuori SM, Trad M, LaCasse CJ, Alizadeh D, Larmonier CB, Hanke NT et al. Myeloid-derived suppressor cells from tumor-bearing mice impair TGF-beta-induced differentiation of CD4+CD25+FoxP3+ Tregs from CD4+CD25-FoxP3- T cells. J Leukoc Biol 2012; 92: 987–997.
Acknowledgements
We thank all study nurses involved in ECP in our department.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
This work was supported by the German Research Foundation (DFG, Emmy Noether Programme HA 5274/3-1 to DH), the CRC685 at Tübingen (Project A14 to DH) and a scientific grant by Therakos, Inc (scientific grant to DH). Fundings had no influence on the study.
Additional information
Author contributions
NR and DH designed the research, analyzed data and wrote the paper. IW performed experiments and analyzed data. DN, AB and IS performed FACS experiments. KF performed the Arginase assays. OA supervised the ECP procedure. WB, PL, MP and RH contributed to patient stratification and clinical parameter assessment.
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Rieber, N., Wecker, I., Neri, D. et al. Extracorporeal photopheresis increases neutrophilic myeloid-derived suppressor cells in patients with GvHD. Bone Marrow Transplant 49, 545–552 (2014). https://doi.org/10.1038/bmt.2013.236
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/bmt.2013.236
Keywords
This article is cited by
-
Uncovering the multifaceted roles played by neutrophils in allogeneic hematopoietic stem cell transplantation
Cellular & Molecular Immunology (2021)
-
Heterogeneity Among Neutrophils
Archivum Immunologiae et Therapiae Experimentalis (2018)