Abstract
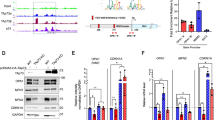
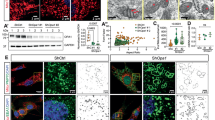
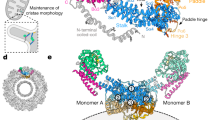
Mitochondrial complex I dysfunction has long been associated with Parkinson’s disease (PD). Recent evidence suggests that mitochondrial involvement in PD may extend beyond a sole respiratory deficit and also include perturbations in mitochondrial fusion/fission or ultrastructure. Whether and how alterations in mitochondrial dynamics may relate to the known complex I defects in PD is unclear. Optic atrophy 1 (OPA1), a dynamin-related GTPase of the inner mitochondrial membrane, participates in mitochondrial fusion and apoptotic mitochondrial cristae remodeling. Here we show that complex I inhibition by parkinsonian neurotoxins leads to an oxidative-dependent disruption of OPA1 oligomeric complexes that normally keep mitochondrial cristae junctions tight. As a consequence, affected mitochondria exhibit major structural abnormalities, including cristae disintegration, loss of matrix density and swelling. These changes are not accompanied by mitochondrial fission but a mobilization of cytochrome c from cristae to intermembrane space, thereby lowering the threshold for activation of mitochondria-dependent apoptosis by cell death agonists in compromised neurons. All these pathogenic changes, including mitochondrial structural remodeling and dopaminergic neurodegeneration, are abrogated by OPA1 overexpression, both in vitro and in vivo. Our results identify OPA1 as molecular link between complex I deficiency and alterations in mitochondrial dynamics machinery and point to OPA1 as a novel therapeutic target for complex I cytopathies, such as PD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AAV:
-
adenoassociated viral
- Drp1:
-
dynamin-related protein 1
- EDC:
-
ethyl-3-(3-dimethylaminopropyl) carbodiimide
- GFP:
-
green fluorescent protein
- IMM:
-
inner mitochondrial membrane
- IMS:
-
mitochondria intermembrane space
- Mfn1/2:
-
Mitofusins 1 and 2
- MPP+:
-
1-methyl-4-phenylpyridinium
- MPTP:
-
1-methyl-4-pheny-1,2,3,6-tetrahydropyridine
- OMM:
-
outer mitochondrial membrane
- OPA1:
-
Optic atrophy 1
- PARL:
-
presenilin-associated rhomboid-like protease
- PD:
-
Parkinson’s disease
- PINK1:
-
PTEN-induced putative kinase-1
- ROS:
-
reactive oxygen species
- SNpc:
-
substantia nigra pars compacta
- TH:
-
tyrosine hydroxylase
- TMPD:
-
N,N,N′,N′-tetramethyl-p-phenylenediamine
- TOM:
-
translocase of the mitochondrial outer membrane
- UT:
-
untreated
References
Dauer W, Przedborski S . Parkinson’s disease: mechanisms and models. Neuron 2003; 39: 889–909.
Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D et al. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 2001; 98: 2837–2842.
Vila M, Przedborski S . Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci 2003; 4: 365–375.
Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V et al. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci USA 2005; 102: 19126–19131.
Perier C, Bove J, Wu DC, Dehay B, Choi DK, Jackson-Lewis V et al. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc Natl Acad Sci USA 2007; 104: 8161–8166.
Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH et al. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 2003; 23: 10756–10764.
Schon EA, Przedborski S . Mitochondria: the next (neurode)generation. Neuron 2011; 70: 1033–1053.
Chen H, Chan DC . Mitochondrial dynamics – fusion, fission, movement, and mitophagy – in neurodegenerative diseases. Hum Mol Genet 2009; 18: R169–R176.
Frezza C, Cipolat S, Martins DB, Micaroni M, Beznoussenko GV, Rudka T et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 2006; 126: 177–189.
Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ . Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 2004; 15: 5001–5011.
Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 2003; 278: 7743–7746.
Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 2000; 26: 207–210.
Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 2004; 36: 449–451.
Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP, Miller SW et al. Abnormal mitochondrial morphology in sporadic Parkinson’s and Alzheimer’s disease cybrid cell lines. Exp Neurol 2000; 162: 37–50.
Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem 2011; 286: 20710–20726.
Jahani-Asl A, Pilon-Larose K, Xu W, MacLaurin JG, Park DS, McBride HM et al. The mitochondrial inner membrane GTPase, optic atrophy 1 (Opa1), restores mitochondrial morphology and promotes neuronal survival following excitotoxicity. J Biol Chem 2011; 286: 4772–4782.
Perier C, Bove J, Dehay B, Jackson-Lewis V, Rabinovitch PS, Przedborski S et al. Apoptosis-inducing factor deficiency sensitizes dopaminergic neurons to parkinsonian neurotoxins. Ann Neurol 2010; 68: 184–192.
Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Developmental Cell 2002; 2: 55–67.
Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 2006; 126: 163–175.
Jackson-Lewis V, Przedborski S . Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc 2007; 2: 141–151.
Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol 1997; 12: 25–31.
Sekiya S, Tanaka M, Hayashi S, Oyanagi S . Light- and electron-microscopic studies of intracytoplasmic acidophilic granules in the human locus ceruleus and substantia nigra. Acta Neuropathol 1982; 56: 78–80.
Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 2006; 441: 1162–1166.
Park J, Lee SB, Lee S, Kim Y, Song S, Kim S et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 2006; 441: 1157–1161.
Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA 2006; 103: 10793–10798.
Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ . Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA 2003; 100: 4078–4083.
Wang C, Lu R, Ouyang X, Ho MW, Chia W, Yu F et al. Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities. J Neurosci 2007; 27: 8563–8570.
Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 2007; 27: 12413–12418.
Fernandes C, Rao Y . Genome-wide screen for modifiers of Parkinson’s disease genes in Drosophila. Mol Brain 2011; 4: 17.
Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 2006; 25: 3900–3911.
Meuer K, Suppanz IE, Lingor P, Planchamp V, Goricke B, Fichtner L et al. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ 2007; 14: 651–661.
Gomez-Lazaro M, Bonekamp NA, Galindo MF, Jordan J, Schrader M . 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic Biol Med 2008; 44: 1960–1969.
Kim-Han JS, Antenor-Dorsey JA, O’Malley KL . The Parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci 2011; 31: 7212–7221.
Sheridan C, Delivani P, Cullen SP, Martin SJ . Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell 2008; 31: 570–585.
Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D et al. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington’s disease to apoptotic stimuli. EMBO Mol Med 2010; 2: 490–503.
Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem 2009; 284: 22938–22951.
Cui M, Tang X, Christian WV, Yoon Y, Tieu K . Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem 2010; 285: 11740–11752.
Germain M, Mathai JP, McBride HM, Shore GC . Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J 2005; 24: 1546–1556.
Mopert K, Hajek P, Frank S, Chen C, Kaufmann J, Santel A . Loss of Drp1 function alters OPA1 processing and changes mitochondrial membrane organization. Exp Cell Res 2009; 315: 2165–2180.
Shi G, Lee JR, Grimes DA, Racacho L, Ye D, Yang H et al. Functional alteration of PARL contributes to mitochondrial dysregulation in Parkinson’s disease. Hum Mol Genet 2011; 20: 1966–1974.
Deng H, Dodson MW, Huang H, Guo M . The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA 2008; 105: 14503–14508.
Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ . The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA 2008; 105: 1638–1643.
Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 2010; 8: e1000298.
Martinou JC, Youle RJ . Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 2011; 21: 92–101.
Acknowledgements
We thank A Parent and E Pérez for their technical assistance, T Lindsten (University of Pennsylvania, USA) for providing Bak-deficient mice and A Strasser (The Walter and Eliza Hall Institute of Medical Research, Australia) for providing Bid-deficient mice. This work was supported by European Commission’s Marie Curie Excellence Grant and Marie Curie International Reintegration Grant (MV), Fundació la Caixa, Spain (MV), FIS-ISCIII, Spain (MV and CP), MICINN, Spain (MV), Ramón y Cajal Program (MICINN, to CP) and Miguel Servet Program (FIS-ISCIII, to JB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by R Youle
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Ramonet, D., Perier, C., Recasens, A. et al. Optic atrophy 1 mediates mitochondria remodeling and dopaminergic neurodegeneration linked to complex I deficiency. Cell Death Differ 20, 77–85 (2013). https://doi.org/10.1038/cdd.2012.95
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.95
Keywords
This article is cited by
-
Establishment of an in vitro model for analyzing mitochondrial ultrastructure in PRKN-mutated patient iPSC-derived dopaminergic neurons
Molecular Brain (2021)
-
The cristae modulator Optic atrophy 1 requires mitochondrial ATP synthase oligomers to safeguard mitochondrial function
Nature Communications (2018)
-
Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson’s disease
Cell Death & Disease (2018)
-
MIF/CD74 axis is a target for novel therapies in colon carcinomatosis
Journal of Experimental & Clinical Cancer Research (2017)
-
Fusion or Fission: The Destiny of Mitochondria In Traumatic Brain Injury of Different Severities
Scientific Reports (2017)