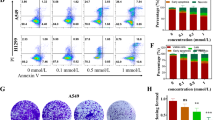
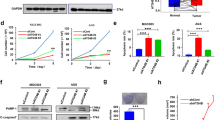
Abstract
Obatoclax (GX15-070), a small-molecule inhibitor of antiapoptotic Bcl-2 proteins, has been reported to trigger cell death via autophagy. However, the underlying molecular mechanisms have remained elusive. Here, we identify GX15-070-stimulated assembly of the necrosome on autophagosomal membranes as a key event that connects GX15-070-stimulated autophagy to necroptosis. GX15-070 predominately induces a non-apoptotic form of cell death in rhabdomyosarcoma cells, as evident by lack of typical apoptotic features such as DNA fragmentation or caspase activation and by insensitivity to the broad-range caspase inhibitor zVAD.fmk. Instead, GX15-070 triggers massive accumulation of autophagosomes, which are required for GX15-070-induced cell death, as blockade of autophagosome formation by silencing of Atg5 or Atg7 abolishes GX15-070-mediated cell death. Co-immunoprecipitation studies reveal that GX15-070 stimulates the interaction of Atg5, a constituent of autophagosomal membranes, with components of the necrosome such as FADD, RIP1 and RIP3. This GX15-070-induced assembly of the necrosome on autophagosomes occurs in a Atg5-dependent manner, as knockdown of Atg5 abrogates formation of this complex. RIP1 is necessary for GX15-070-induced cell death, as both genetic and pharmacological inhibition of RIP1 by shRNA-mediated knockdown or by the RIP1 inhibitor necrostatin-1 blocks GX15-070-induced cell death. Similarly, RIP3 knockdown rescues GX15-070-mediated cell death and suppression of clonogenic survival. Interestingly, RIP1 or RIP3 silencing has no effect on GX15-070-stimulated autophagosome formation, underlining that RIP1 and RIP3 mediate cell death downstream of autophagy induction. Of note, GX15-070 significantly suppresses tumor growth in a RIP1-dependent manner in the chorioallantoic membrane model in vivo. In conclusion, GX15-070 triggers necroptosis by promoting the assembly of the necrosome on autophagosomes. These findings provide novel insights into the molecular mechanisms of GX15-070-induced non-apoptotic cell death.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ERMS:
-
embryonal rhabdomyosarcoma
- ARMS:
-
alveolar rhabdomyosarcoma
- BafA1:
-
Bafilomycin A1
- Nec-1:
-
necrostatin-1
- CAM:
-
chorioallantoic membrane
- TNFα:
-
tumor necrosis factor α
- RIP:
-
receptor-interacting protein
- RMS:
-
rhabdomyosarcoma
- zVAD.fmk:
-
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone
References
Lockshin RA, Zakeri Z . Cell death in health and disease. J Cell Mol Med 2007; 11: 1214–1224.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012; 19: 107–120.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Adams JM, Cory S . The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007; 26: 1324–1337.
Fulda S, Galluzzi L, Kroemer G . Targeting mitochondria for cancer therapy. Nat Rev Drug Discov 2010; 9: 447–464.
Schimmer AD, O'Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res 2008; 14: 8295–8301.
Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA 2007; 104: 19512–19517.
Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res 2008; 68: 3413–3420.
Heidari N, Hicks MA, Harada H . GX15-070 (obatoclax) overcomes glucocorticoid resistance in acute lymphoblastic leukemia through induction of apoptosis and autophagy. Cell Death Dis 2010; 1: e76.
Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest 2010; 120: 1310–1323.
Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P . Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther 2009; 8: 2084–2096.
Martin AP, Park MA, Mitchell C, Walker T, Rahmani M, Thorburn A et al. BCL-2 family inhibitors enhance histone deacetylase inhibitor and sorafenib lethality via autophagy and overcome blockade of the extrinsic pathway to facilitate killing. Mol Pharmacol 2009; 76: 327–341.
Wei Y, Kadia T, Tong W, Zhang M, Jia Y, Yang H et al. The combination of a histone deacetylase inhibitor with the Bcl-2 homology domain-3 mimetic GX15-070 has synergistic antileukemia activity by activating both apoptosis and autophagy. Clin Cancer Res 2010; 16: 3923–3932.
Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y . Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 2009; 10: 458–467.
Mizushima N, Yoshimori T, Ohsumi Y . The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 2011; 27: 107–132.
Fulda S . Autophagy and cell death. Autophagy 2012; 8: 1250–1251.
McCoy F, Hurwitz J, McTavish N, Paul I, Barnes C, O'Hagan B et al. Obatoclax induces Atg7-dependent autophagy independent of beclin-1 and BAX/BAK. Cell Death Dis 2010; 1: e108.
Pan J, Cheng C, Verstovsek S, Chen Q, Jin Y, Cao Q . The BH3-mimetic GX15-070 induces autophagy, potentiates the cytotoxicity of carboplatin and 5-fluorouracil in esophageal carcinoma cells. Cancer Lett 2010; 293: 167–174.
Wei CC, Ball S, Lin L, Liu A, Fuchs JR, Li PK et al. Two small molecule compounds, LLL12 and FLLL32, exhibit potent inhibitory activity on STAT3 in human rhabdomyosarcoma cells. Int J Oncol 2011; 38: 279–285.
Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T . The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal 2010; 3: re4.
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 2008; 135: 1311–1323.
He S, Wang L, Miao L, Wang T, Du F, Zhao L et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009; 137: 1100–1111.
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G . Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010; 11: 700–714.
Dagher R, Helman L . Rhabdomyosarcoma: an overview. Oncologist 1999; 4: 34–44.
Merlino G, Helman LJ . Rhabdomyosarcoma—working out the pathways. Oncogene 1999; 18: 5340–5348.
Margue CM, Bernasconi M, Barr FG, Schafer BW . Transcriptional modulation of the anti-apoptotic protein BCL-XL by the paired box transcription factors PAX3 and PAX3/FKHR. Oncogene 2000; 19: 2921–2929.
Pazzaglia L, Chiechi A, Conti A, Gamberi G, Magagnoli G, Novello C et al. Genetic and molecular alterations in rhabdomyosarcoma: mRNA overexpression of MCL1 and MAP2K4 genes. Histol Histopathol 2009; 24: 61–67.
Fulda S . Cell death pathways as therapeutic targets in rhabdomyosarcoma. Sarcoma 2012; 2012: 326210.
Hayes-Jordan A, Andrassy R . Rhabdomyosarcoma in children. Curr Opin Pediatr 2009; 21: 373–378.
Klionsky DJ . The autophagosome is overrated!. Autophagy 2011; 7: 353–354.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005; 1: 112–119.
Stupack DG, Teitz T, Potter MD, Mikolon D, Houghton PJ, Kidd VJ et al. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature 2006; 439: 95–99.
Hacker S, Dittrich A, Mohr A, Schweitzer T, Rutkowski S, Krauss J et al. Histone deacetylase inhibitors cooperate with IFN-gamma to restore caspase-8 expression and overcome TRAIL resistance in cancers with silencing of caspase-8. Oncogene 2009; 28: 3097–3110.
Gump JM, Thorburn A . Autophagy and apoptosis: what is the connection? Trends Cell Biol 2011; 21: 387–392.
Shen HM, Codogno P . Autophagy is a survival force via suppression of necrotic cell death. Exp Cell Res 2012; 318: 1304–1308.
Wu YT, Tan HL, Huang Q, Ong CN, Shen HM . Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy 2009; 5: 824–834.
Wu YT, Tan HL, Huang Q, Kim YS, Pan N, Ong WY et al. Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy 2008; 4: 457–466.
Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 2004; 304: 1500–1502.
Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem 2005; 280: 20722–20729.
Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, Luhrs KA et al. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci USA 2008; 105: 16677–16682.
Young MM, Takahashi Y, Khan O, Park S, Hori T, Yun J et al. Autophagosomal membrane serves as platform for intracellular death-inducing signaling complex (iDISC)-mediated caspase-8 activation and apoptosis. J Biol Chem 2012; 287: 12455–12468.
Pan JA, Ullman E, Dou Z, Zong WX . Inhibition of protein degradation induces apoptosis through a microtubule-associated protein 1 light chain 3-mediated activation of caspase-8 at intracellular membranes. Mol Cell Biol 2011; 31: 3158–3170.
Behrends C, Fulda S . Receptor proteins in selective autophagy. Int J Cell Biol 2012; 2012: 673290.
Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 2009; 137: 721–735.
Loeder S, Schirmer M, Schoeneberger H, Cristofanon S, Leibacher J, Vanlangenakker N et al. RIP1 is required for IAP inhibitor-mediated sensitization of childhood acute leukemia cells to chemotherapy-induced apoptosis. Leukemia 2012; 26: 1742.
Fulda S, Sieverts H, Friesen C, Herr I, Debatin KM . The CD95 (APO-1/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res 1997; 57: 3823–3829.
Acknowledgements
We thank D. Brücher for expert technical assistance, C. Hugenberg for expert secretarial assistance and Dr. J. Cinatl for help with microscopy. This work has been partially supported by grants from the Bundesministerium für Forschung und Technologie (01GM1104C) and the Deutsche Krebshilfe (to SF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Kroemer
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Basit, F., Cristofanon, S. & Fulda, S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ 20, 1161–1173 (2013). https://doi.org/10.1038/cdd.2013.45
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2013.45
Keywords
This article is cited by
-
BH3-mimetics: recent developments in cancer therapy
Journal of Experimental & Clinical Cancer Research (2021)
-
Essential role for autophagy protein VMP1 in maintaining neuronal homeostasis and preventing axonal degeneration
Cell Death & Disease (2021)
-
The role of necroptosis in cancer biology and therapy
Molecular Cancer (2019)
-
Accurate elimination of superfluous attachment cells is critical for the construction of functional multicellular proprioceptors in Drosophila
Cell Death & Differentiation (2019)
-
Current translational potential and underlying molecular mechanisms of necroptosis
Cell Death & Disease (2019)