Abstract
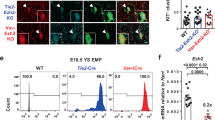
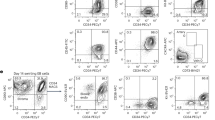
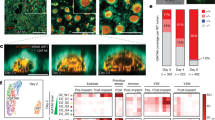
Loss-of-function studies have demonstrated the essential role of Notch in definitive embryonic mouse hematopoiesis. We report here the consequences of Notch gain-of-function in mouse embryo hematopoiesis, achieved by constitutive expression of Notch1 intracellular domain (N1ICD) in angiopoietin receptor tyrosine kinase receptor-2 (Tie2)-derived enhanced green fluorescence protein (EGFP+) hematovascular progenitors. At E9.5, N1ICD expression led to the absence of the dorsal aorta hematopoietic clusters and of definitive hematopoiesis. The EGFP+ transient multipotent progenitors, purified from E9.5 to 10.5 Tie2-Cre;N1ICD yolk sac (YS) cells, had strongly reduced hematopoietic potential, whereas they had increased numbers of hemogenic endothelial cells. Late erythroid cell differentiation stages and mature myeloid cells (Gr1+, MPO+) were also strongly decreased. In contrast, EGFP+ erythro-myeloid progenitors, immature and intermediate differentiation stages of YS erythroid and myeloid cell lineages, were expanded. Tie2-Cre;N1ICD YS had reduced numbers of CD41++ megakaryocytes, and these produced reduced below-normal numbers of immature colonies in vitro and their terminal differentiation was blocked. Cells from Tie2-Cre;N1ICD YS had a higher proliferation rate and lower apoptosis than wild-type (WT) YS cells. Quantitative gene expression analysis of FACS-purified EGFP+ YS progenitors revealed upregulation of Notch1-related genes and alterations in genes involved in hematopoietic differentiation. These results represent the first in vivo evidence of a role for Notch signaling in YS transient definitive hematopoiesis. Our results show that constitutive Notch1 activation in Tie2+ cells hampers YS hematopoiesis of E9.5 embryos and demonstrate that Notch signaling regulates this process by balancing the proliferation and differentiation dynamics of lineage-restricted intermediate progenitors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- 7-AAD:
-
7-aminoactinomycin D
- AGM:
-
aorta-gonads-mesonephros
- BM:
-
bone marrow
- DAPI:
-
4′,6-diamidino-2-phenylindole
- EdU:
-
5-ethinyl-2′-deoxyuridine
- EGFP:
-
enhanced green fluorescence protein
- FSC:
-
forward scatter
- HSC:
-
hematopoietic stem cells
- MFI:
-
mean fluorescence intensity
- MK:
-
megakaryocyte
- mAbs:
-
monoclonal antibodies
- N1ICD:
-
Notch1 intracellular domain
- P-Sp:
-
para-aortic splanchnopleura region
- PI:
-
propidium iodide
- qRT-PCR:
-
quantitative real-time PCR
- SSC:
-
side scatter
- Tie2:
-
tyrosine kinase receptor-2
- vWF:
-
von Willebrand factor
- WT:
-
wild type
- YS:
-
yolk sac
References
Artavanis-Tsakonas S, Rand MD, Lake RJ . Notch signaling: cell fate control and signal integration in development. Science 1999; 284: 770–776.
Radtke F, Fasnacht N, Macdonald HR . Notch signalling in the immune system. Immunity 2010; 32: 14–27.
Lieber T, Kidd S, Alcamo E, Corbin V, Young MW . Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev 1993; 7: 1949–1965.
Rebay I, Fehon RG, Artavanis-Tsakonas S . Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell 1993; 74: 319–329.
Struhl G, Fitzgerald K, Greenwald I . Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell 1993; 74: 331–345.
Milner LA, Kopan R, Martin DI, Bernstein ID . A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood 1994; 83: 2057–2062.
Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991; 66: 649–661.
Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 2004; 306: 269–271.
Jonsson JI, Xiang Z, Pettersson M, Lardelli M, Nilsson G . Distinct and regulated expression of Notch receptors in hematopoietic lineages and during myeloid differentiation. Eur J Immunol 2001; 31: 3240–3247.
Singh N, Phillips RA, Iscove NN, Egan SE . Expression of notch receptors, notch ligands, and fringe genes in hematopoiesis. Exp Hematol 2000; 28: 527–534.
Walker L, Carlson A, Tan-Pertel HT, Weinmaster G, Gasson J . The notch receptor and its ligands are selectively expressed during hematopoietic development in the mouse. Stem Cells 2001; 19: 543–552.
Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lievre F, Marcos MA . Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature 1993; 364: 67–70.
Marcos MA, Godin I, Cumano A, Morales S, García-Porrero JA, Dieterlen-Lievre F et al. Developmental events from hemopoietic stem cells to B-cell populations and Ig repertories. Immunol Rev 1994; 137: 155–171.
Silver L, Palis J . Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood 1997; 89: 1154–1164.
Palis J, Robertson S, Kennedy M, Wall C, Keller G . Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 1999; 126: 5073–5084.
Jaffredo T, Nottingham W, Liddiard K, Bollerot K, Pouget C . de Bruijn M. From hemangioblast to hematopoietic stem cell: an endothelial connection? Exp Hematol 2005; 33: 1029–1040.
Cumano A, Dieterlen-Lievre F, Godin I . Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 1996; 86: 907–916.
Cumano A, Godin I . Pluripotent hematopoietic stem cell development during embryogenesis. Curr Opin Immunol 2001; 13: 166–171.
Medvinsky A, Dzierzak E . Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 1996; 86: 897–906.
Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E . Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1994; 1: 291–301.
Orkin SH, Zon L . Hematopoiesis: an evolving paradigm for stem cell biology. Cell 2008; 132: 631–644.
Carlesso N, Aster JC, Sklar J, Scadden DT . Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood 1999; 93: 838–848.
Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med 2000; 192: 1365–1372.
Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT . Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 2002; 99: 2369–2378.
Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med 2000; 6: 1278–1281.
Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 2003; 18: 699–711.
Robert-Moreno A, Espinosa L, Sanchez MJ, de la Pompa JL, Bigas A . The notch pathway positively regulates programmed cell death during erythroid differentiation. Leukemia 2007; 21: 1496–1503.
Hamaguchi I, Huang XL, Takakura N, Tada J, Yamaguchi Y, Kodama H et al. In vitro hematopoietic and endothelial cell development from cells expressing TEK receptor in murine aorta-gonad-mesonephros region. Blood 1999; 93: 1549–1556.
Ema M, Yokomizo T, Wakamatsu A, Terunuma T, Yamamoto M, Takahashi S . Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin, and CD34 in the mouse embryo. Blood 2006; 108: 4018–4024.
Del Monte G, Grego-Bessa J, Gonzalez-Rajal A, Bolos V, De La Pompa JL . Monitoring Notch1 activity in development: Evidence for a feedback regulatory loop. Dev Dyn 2007; 236: 2594–2614.
Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M . Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 2001; 230: 230–242.
Murtaugh LC, Stanger BZ, Kwan KM, Melton DA . Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA 2003; 100: 14920–14925.
Luna-Zurita L, Prados B, Grego-Bessa J, Luxan G, del Monte G, Benguria A et al. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J Clin Invest 2010; 120: 3493–3507.
Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T . Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 2004; 18: 2469–2473.
Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A . RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development 2005; 132: 1117–1126.
Robert-Moreno A, Guiu J, Ruiz-Herguido C, Lopez ME, Ingles-Esteve J, Riera L et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J 2008; 27: 1886–1895.
Yoon MJ, Koo BK, Song R, Jeong HW, Shin J, Kim YW et al. Mind bomb-1 is essential for intraembryonic hematopoiesis in the aortic endothelium and the subaortic patches. Mol Cell Biol 2008; 28: 4794–4804.
Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G . The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 2009; 457: 892–895.
Yamasaki S, Nobuhisa I, Ramadan A, Taga T . Identification of a yolk sac cell population with hematopoietic activity in view of CD45/c-Kit expression. Dev Growth Differ 2011; 53: 870–877.
Asari S, Sakamoto A, Okada S, Ohkubo Y, Arima M, Hatano M et al. Abnormal erythroid differentiation in neonatal bcl-6-deficient mice. Exp Hematol 2005; 33: 26–34.
McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J et al. A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood 2011; 117: 4600–4608.
Xu M, Matsuoka S, Yang F, Ebihara Y, Manabe A, Tanaka R et al. Evidence for the presence of murine primitive megakarycytopoiesis in the early yolk sac. Blood 2001; 97: 2016–2022.
Bertrand JT, Jalil A, Klaine M, Jung S, Cumano A, Godin I . Three pathways to mature macrophages in the early mouse yolk sac. Blood 2005; 106: 3004–3011.
Pimanda JE, Ottersbach K, Knezevic K, Kinston S, Chan WY, Wilson NK et al. Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci USA 2007; 104: 17692–17697.
Friedman AD . Transcriptional control of granulocyte and monocyte development. Oncogene 2007; 26: 6816–6828.
Alhashem YN, Vinjamur DS, Basu M, Klingmuller U, Gaensler KM, Lloyd JA . Transcription factors KLF1 and KLF2 positively regulate embryonic and fetal beta-globin genes through direct promoter binding. J Biol Chem 2011; 286: 24819–24827.
Medvinsky A, Rybtsov S, Taoudi S . Embryonic origin of the adult hematopoietic system: advances and questions. Development 2011; 138: 1017–1031.
Tang Y, Harrington A, Yang X, Friesel RE, Liaw L . The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis 2010; 48: 563–567.
Takakura N, Huang XL, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD et al. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 1998; 9: 677–686.
Li Z, Chen MJ, Stacy T, Speck NA . Runx1 function in hematopoiesis is required in cells that express Tek. Blood 2006; 107: 106–110.
Oh P, Lobry C, Gao J, Tikhonova A, Loizou E, Manet J et al. In vivo mapping of notch pathway activity in normal and stress hematopoiesis. Cell Stem Cell 2013; 13: 190–204.
Zeuner A, Francescangeli F, Signore M, Venneri MA, Pedini F, Felli N et al. The Notch2-Jagged1 interaction mediates stem cell factor signaling in erythropoiesis. Cell Death Differ 2010; 18: 371–380.
Venkatesh DA, Park KS, Harrington A, Miceli-Libby L, Yoon JK, Liaw L . Cardiovascular and hematopoietic defects associated with Notch1 activation in embryonic Tie2-expressing populations. Circ Res 2008; 103: 423–431.
Poirault-Chassac S, Six E, Catelain C, Lavergne M, Villeval J-L, Vainchenker W et al. Notch/Delta4 signalling inhibits human megakaryocytic terminal differentiation. Blood 2010; 116: 5670–5678.
Liu H, Chi AW, Arnett KL, Chiang MY, Xu L, Shestova O et al. Notch dimerization is required for leukemogenesis and T-cell development. Genes Dev 2010; 24: 2395–2407.
Orford KW, Scadden DT . Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 2008; 9: 115–128.
Serrano N, Cortegano I, Ruiz C, Alia M, de Andres B, Rejas MT et al. Megakaryocytes promote hepatoepithelial liver cell development in E11.5 mouse embryos by cell-to-cell contact and by vascular endothelial growth factor a signaling. Hepatology 2012; 56: 1934–1945.
de la Pompa JL, Wakeham A, Correira KM, Samper E, Brown S, Aguilera RJ et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 1997; 124: 1139–1148.
Gozalbo-Lopez B, Andrade P, Terrados G, de Andres B, Serrano N, Cortegano I et al. A role for DNA polymerase mu in the emerging DJH rearrangements of the postgastrulation mouse embryo. Mol Cell Biol 2009; 29: 1266–1275.
Acknowledgements
We are grateful to B Palacios, P Martínez and C Ruiz for husbandry, Dr. M Alía and C Prado for their technical cytometry assistance and Dr. N Serrano and Dr. B de Andrés for helpful discussions. We thank S Bartlett (CNIC) for English editing. IC is supported by a fellowship from the Ministerio de Economía y Competitividad (MINECO). PMR has been supported by a PhD fellowship (FPU program, MINECO) and by the Fundación Leticia Castillejo. This work was funded by grants SAF2007-65265, SAF2009-12596, SAF2012-33916, and ISCIII08/0685 (MINECO) and SAL-0304-2006 (Madrid Regional Government) to MLG; SAF2007-62445 and SAF2010-17555, RD12/0019/0003 and RD12/0042/0005 (MINECO) to JLdlP. The CNIC is supported by the MINECO and the Pro-CNIC Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by R De Maria
Author contributions
MARM, MLG and JLP designed research; IC, PMR, LL and MSA performed research; IC and MLG contributed analytical tools; IC, PMR, MARM, MLG and JLP analyzed and interpreted data; IC and MLG performed statistical analysis; JLP, MLG, IC and PMR wrote the manuscript.
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Cortegano, I., Melgar-Rojas, P., Luna-Zurita, L. et al. Notch1 regulates progenitor cell proliferation and differentiation during mouse yolk sac hematopoiesis. Cell Death Differ 21, 1081–1094 (2014). https://doi.org/10.1038/cdd.2014.27
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2014.27