Abstract
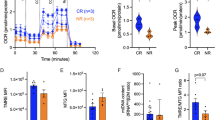
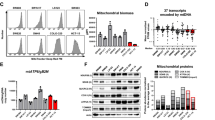
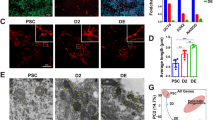
The relationship between mitochondrial metabolism and cell viability and differentiation in stem cells (SCs) remains poorly understood. In the present study, we compared mitochondrial physiology and metabolism between P19SCs before/after differentiation and present a unique fingerprint of the association between mitochondrial activity, cell differentiation and stemness. In comparison with their differentiated counterparts, pluripotency of P19SCs was correlated with a strong glycolytic profile and decreased mitochondrial biogenesis and complexity: round, low-polarized and inactive mitochondria with a closed permeability transition pore. This decreased mitochondrial capacity increased their resistance against dichloroacetate. Thus, stimulation of mitochondrial function by growing P19SCs in glutamine/pyruvate-containing medium reduced their glycolytic phenotype, induced loss of pluripotent potential, compromised differentiation and became P19SCs sensitive to dichloroacetate. Because of the central role of this type of SCs in teratocarcinoma development, our findings highlight the importance of mitochondrial metabolism in stemness, proliferation, differentiation and chemoresistance. In addition, the present work suggests the regulation of mitochondrial metabolism as a tool for inducing cell differentiation in stem line therapies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ANT:
-
adenine nucleotide translocase
- ADP:
-
adenosine diphosphate
- ATP5A:
-
ATP synthase subunit α
- COX IV:
-
cytochrome c oxidase subunit IV
- CSCs:
-
cancer stem cells
- CyP-D:
-
cyclophilin D
- ESCs:
-
embryonic stem cells
- ETC:
-
electron transport chain
- FCCP:
-
carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- HBSS:
-
Hank’s Balanced Salt Solution
- mPTP:
-
mitochondrial permeability transition pore
- MTCO1:
-
cytochrome c oxidase subunit I
- mTFA:
-
mitochondrial transcription factor A
- MTG:
-
MitoTracker Green
- MTR:
-
MitoTracker Red
- NANOG:
-
nanog homeobox protein
- NDUFB8:
-
NADH dehydrogenase (ubiquinone) 1 β subcomplex 8
- NMR:
-
nuclear magnetic resonance
- OCT4:
-
octamer-binding transcription factor
- OXPHOS:
-
oxidative phosphorylation
- P19dC:
-
differentiated P19 cell
- P19SC:
-
P19 stem cell
- PDH:
-
pyruvate dehydrogenase
- PDK:
-
pyruvate dehydrogenase kinase
- PGC-1:
-
peroxisomal proliferator-activated receptor coactivator 1
- PI:
-
propidium iodide
- RA:
-
retinoic acid
- ROS:
-
reactive oxygen species
- SCs:
-
stem cells
- SDHB:
-
succinate dehydrogenase (ubiquinone) iron-sulfur subunit
- SOX2:
-
sex determining region Y-box 2
- TMRM:
-
tetramethylrhodamine methyl ester
- TOM20:
-
translocase of outer mitochondrial membrane 20
- TROMA-1:
-
cytokeratin 8 Endo-A
- UQCRC2:
-
ubiquinol-cytochrome c reductase core protein II
- Δψm:
-
mitochondrial transmembrane electric potential
References
McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ . Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature 1982; 299: 165–167.
McBurney MW . P19 embryonal carcinoma cells. Int J Dev Biol 1993; 37: 135–140.
Mummery CL, Feijen A, Moolenaar WH, van den Brink CE, de Laat SW . Establishment of a differentiated mesodermal line from P19 EC cells expressing functional PDGF and EGF receptors. Exp Cell Res 1986; 165: 229–242.
Tang C, Ang BT, Pervaiz S . Cancer stem cell: target for anti-cancer therapy. FASEB J 2007; 21: 3777–3785.
Doss CG, Debajyoti C, Debottam S . Disruption of mitochondrial complexes in cancer stem cells through nano-based drug delivery: a promising mitochondrial medicine. Cell Biochem Biophys 2013; 67: 1075–1079.
Loureiro R, Mesquita KA, Oliveira PJ, Vega-Naredo I . Mitochondria in cancer stem cells: a target for therapy. Recent Pat Endocr Metab Immune Drug Discov 2013; 7: 102–114.
Watkins J, Basu S, Bogenhagen DF . A quantitative proteomic analysis of mitochondrial participation in p19 cell neuronal differentiation. J Proteome Res 2008; 7: 328–338.
Barbosa IA, Machado NG, Skildum AJ, Scott PM, Oliveira PJ . Mitochondrial remodeling in cancer metabolism and survival: potential for new therapies. Biochim Biophys Acta 2012; 1826: 238–254.
Poteet E, Choudhury GR, Winters A, Li W, Ryou MG, Liu R et al. Reversing the Warburg effect as a treatment for glioblastoma. J Biol Chem 2013; 288: 9153–9164.
Folmes CD, Dzeja PP, Nelson TJ, Terzic A . Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 2012; 11: 596–606.
Lonergan T, Bavister B, Brenner C . Mitochondria in stem cells. Mitochondrion 2007; 7: 289–296.
Gordon JW, Rungi AA, Inagaki H, Hood DA . Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol 2001; 90: 389–396.
Lindqvist A, Mei J, Sundler F, Erlanson-Albertsson C . Decreased UCP2 mRNA expression in rat stomach following vagotomy: novel role for UCP2 as free radical scavenger in the stomach? Nutr Neurosci 2004; 7: 217–222.
Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA 2013; 110: 5887–5892.
Machida K, Ohta Y, Osada H . Suppression of apoptosis by cyclophilin D via stabilization of hexokinase II mitochondrial binding in cancer cells. J Biol Chem 2006; 281: 14314–14320.
Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, Capaldi RA . Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res 2004; 64: 985–993.
Jho EH, Malbon CC . Galpha12 and Galpha13 mediate differentiation of P19 mouse embryonal carcinoma cells in response to retinoic acid. J Biol Chem 1997; 272: 24461–24467.
Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA . Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 2012; 11: 589–595.
St John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS et al. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells 2005; 7: 141–153.
Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A . Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update 2009; 15: 553–572.
Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab 2011; 14: 623–634.
Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ, Bian XW et al. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int J Cancer 2011; 129: 820–831.
Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P . TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med 2008; 205: 2397–2408.
Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A . Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 2010; 7: 150–161.
Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J 2011; 30: 4860–4873.
Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 2011; 14: 264–271.
Ye XQ, Wang GH, Huang GJ, Bian XW, Qian GS, Yu SC . Heterogeneity of mitochondrial membrane potential: a novel tool to isolate and identify cancer stem cells from a tumor mass? Stem Cell Rev 2011; 7: 153–160.
Kroemer G, Pouyssegur J . Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell 2008; 13: 472–482.
Schieke SM, Ma M, Cao L, McCoy JP Jr, Liu C, Hensel NF et al. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem 2008; 283: 28506–28512.
Ramos-Mejia V, Bueno C, Roldan M, Sanchez L, Ligero G, Menendez P et al. The adaptation of human embryonic stem cells to different feeder-free culture conditions is accompanied by a mitochondrial response. Stem Cells Dev 2012; 21: 1145–1155.
Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R et al. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 2010; 28: 661–673.
Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 2010; 329: 1492–1499.
Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U . Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells 2011; 29: 486–495.
Hom JR, Quintanilla RA, Hoffman DL, de Mesy Bentley KL, Molkentin JD, Sheu SS et al. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev Cell 2011; 21: 469–478.
Shakya A, Cooksey R, Cox JE, Wang V, McClain DA, Tantin D . Oct1 loss of function induces a coordinate metabolic shift that opposes tumorigenicity. Nat Cell Biol 2009; 11: 320–327.
Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 2011; 14: 537–544.
Prigione A, Adjaye J . Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int J Dev Biol 2010; 54: 1729–1741.
Denton RM . Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 2009; 1787: 1309–1316.
Villalobo A, Lehninger AL . Inhibition of oxidative phosphorylation in ascites tumor mitochondria and cells by intramitochondrial Ca2+. J Biol Chem 1980; 255: 2457–2464.
Porter GA Jr, Makuck RF, Rivkees SA . Reduction in intracellular calcium levels inhibits myoblast differentiation. J Biol Chem 2002; 277: 28942–28947.
Hausenloy D, Wynne A, Duchen M, Yellon D . Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 2004; 109: 1714–1717.
Hou T, Zhang X, Xu J, Jian C, Huang Z, Ye T et al. Synergistic triggering of superoxide flashes by mitochondrial Ca2+ uniport and basal reactive oxygen species elevation. J Biol Chem 2013; 288: 4602–4612.
Gao J, Sana R, Calder V, Calonge M, Lee W, Wheeler LA et al. Mitochondrial permeability transition pore in inflammatory apoptosis of human conjunctival epithelial cells and T cells: effect of cyclosporin A. Invest Ophthalmol Vis Sci 2013; 54: 4717–4733.
Mathupala SP, Ko YH, Pedersen PL . Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 2006; 25: 4777–4786.
Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CAt, Ramalho-Santos J et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 2011; 6: e20914.
Michelakis ED, Webster L, Mackey JR . Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer 2008; 99: 989–994.
Morfouace M, Lalier L, Bahut M, Bonnamain V, Naveilhan P, Guette C et al. Comparison of spheroids formed by rat glioma stem cells and neural stem cells reveals differences in glucose metabolism and promising therapeutic applications. J Biol Chem 2012; 287: 33664–33674.
Dagda RK, Cherra SJ 3rd, Kulich SM, Tandon A, Park D, Chu CT . Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem 2009; 284: 13843–13855.
Pendergrass W, Wolf N, Poot M . Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A 2004; 61: 162–169.
Elmore SP, Nishimura Y, Qian T, Herman B, Lemasters JJ . Discrimination of depolarized from polarized mitochondria by confocal fluorescence resonance energy transfer. Arch Biochem Biophys 2004; 422: 145–152.
Pereira GC, Branco AF, Matos JA, Pereira SL, Parke D, Perkins EL et al. Mitochondrially targeted effects of berberine [Natural Yellow 18, 5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo(5,6-a) quinolizinium] on K1735-M2 mouse melanoma cells: comparison with direct effects on isolated mitochondrial fractions. J Pharmacol Exp Ther 2007; 323: 636–649.
Thundathil J, Filion F, Smith LC . Molecular control of mitochondrial function in preimplantation mouse embryos. Mol Reprod Dev 2005; 71: 405–413.
Rasmussen HN, Rasmussen UF . Oxygen solubilities of media used in electrochemical respiration measurements. Anal Biochem 2003; 319: 105–113.
Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P et al. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 1999; 76: 725–734.
Bernardi P . The mitochondrial permeability transition pore: a mystery solved? Front Physiol 2013; 4: 95.
Verma G, Bhatia H, Datta M . JNK1/2 regulates ER-mitochondrial Ca2+ cross-talk during IL-1beta-mediated cell death in RINm5F and human primary beta-cells. Mol Biol Cell 2013; 24: 2058–2071.
Hoth M, Fanger CM, Lewis RS . Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol 1997; 137: 633–648.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990; 82: 1107–1112.
Acknowledgements
We thank Isabel Nunes Correia and Luisa Cortes for technical assistance. This work was performed with funding from the Portuguese Foundation for Science and Technology (FCT) co-funded by COMPETE/FEDER/National Budget (PTDC/QUI-BIQ/101052/2008 and PEst-C/SAU/LA0001/2013-2014). Vega-Naredo I acknowledges a Marie Curie Intra-European Fellowship from the EU Seventh Framework Programme (PIEF-GA-2009-251850) and the FCT (SFRH/BPD/86534/2012). The FCT also supported Mesquita KA (SFRH/BD/66138/2009). The present work was also supported by QREN project # 4832, reference ‘CENTRO-07-ST24-FEDER-002008’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by L Scorrano
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Vega-Naredo, I., Loureiro, R., Mesquita, K. et al. Mitochondrial metabolism directs stemness and differentiation in P19 embryonal carcinoma stem cells. Cell Death Differ 21, 1560–1574 (2014). https://doi.org/10.1038/cdd.2014.66
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2014.66
This article is cited by
-
Variation in mitochondrial DNA affects locomotor activity and sleep in Drosophila melanogaster
Heredity (2022)
-
Overexpression of TNFα induces senescence, autophagy and mitochondrial dysfunctions in melanoma cells
BMC Cancer (2021)
-
Exogenous NADPH ameliorates myocardial ischemia–reperfusion injury in rats through activating AMPK/mTOR pathway
Acta Pharmacologica Sinica (2020)
-
Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells
Cell Death & Disease (2019)
-
Regenerative abilities of mesenchymal stem cells through mitochondrial transfer
Journal of Biomedical Science (2018)