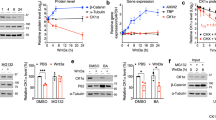
Abstract
In multicellular organisms, a tight control of cell death is required to ensure normal development and tissue homeostasis. Improper function of apoptotic or survival pathways can not only affect developmental programs but also favor cancer progression. Here we describe a novel apoptotic signaling pathway involving the transmembrane receptor Kremen1 and its ligand, the Wnt-antagonist Dickkopf1. Using a whole embryo culture system, we first show that Dickkopf1 treatment promotes cell survival in a mouse model exhibiting increased apoptosis in the developing neural plate. Remarkably, this effect was not recapitulated by chemical Wnt inhibition. We then show that Dickkopf1 receptor Kremen1 is a bona fide dependence receptor, triggering cell death unless bound to its ligand. We performed Wnt-activity assays to demonstrate that the pro-apoptotic and anti-Wnt functions mediated by Kremen1 are strictly independent. Furthermore, we combined phylogenetic and mutagenesis approaches to identify a specific motif in the cytoplasmic tail of Kremen1, which is (i) specifically conserved in the lineage of placental mammals and (ii) strictly required for apoptosis induction. Finally, we show that somatic mutations of kremen1 found in human cancers can affect its pro-apoptotic activity, supporting a tumor suppressor function. Our findings thus reveal a new Wnt-independent function for Kremen1 and Dickkopf1 in the regulation of cell survival with potential implications in cancer therapies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CNS:
-
central nervous system
- COSMIC:
-
catalogue of somatic mutations in cancer
- DCC:
-
deleted in colorectal cancer
- Dkk:
-
Dickkopf
- ECD:
-
extracellular domain
- GFP:
-
green fluorescent protein
- HA:
-
hemagglutinin
- HEK:
-
human embryonic kidney
- ICD:
-
intracellular domain
- Krm:
-
Kringle-coding gene marking the eye and the nose
- LRP:
-
low-density lipoprotein receptor-related protein
- TCGA:
-
the cancer genome atlas
- TUNEL:
-
terminal deoxynucleotidyl transferase dUTP nick end labeling
- Wnt:
-
wingless-related integration site
References
Goldschneider D, Mehlen P . Dependence receptors: a new paradigm in cell signaling and cancer therapy. Oncogene 2010; 29: 1865–1882.
Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE . The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 1998; 395: 801–804.
Llambi F, Causeret F, Bloch-Gallego E, Mehlen P . Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J 2001; 20: 2715–2722.
Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P . Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science 2003; 301: 843–846.
Tauszig-Delamasure S, Yu LY, Cabrera JR, Bouzas-Rodriguez J, Mermet-Bouvier C, Guix C et al. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc Natl Acad Sci USA 2007; 104: 13361–13366.
Nikoletopoulou V, Lickert H, Frade JM, Rencurel C, Giallonardo P, Zhang L et al. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature 2010; 467: 59–63.
Luchino J, Hocine M, Amoureux MC, Gibert B, Bernet A, Royet A et al. Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell 2013; 24: 673–685.
Anastas JN, Moon RT . WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013; 13: 11–26.
van Amerongen R, Nusse R . Towards an integrated view of Wnt signaling in development. Development 2009; 136: 3205–3214.
Nusse R, Varmus HE . Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982; 31: 99–109.
McMahon AP, Bradley A . The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 1990; 62: 1073–1085.
Angers S, Moon RT . Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 2009; 10: 468–477.
Cruciat CM, Niehrs C . Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol 2013; 5: a015081.
Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA . Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol 2001; 3: 683–686.
Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 2001; 411: 321–325.
Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He X . Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 2001; 11: 951–961.
Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 2002; 417: 664–667.
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C . Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998; 391: 357–362.
Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 2001; 1: 423–434.
Niehrs C . Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006; 25: 7469–7481.
Rubin JS, Barshishat-Kupper M, Feroze-Merzoug F, Xi ZF . Secreted WNT antagonists as tumor suppressors: pro and con. Front Biosci 2006; 11: 2093–2105.
Menezes ME, Devine DJ, Shevde LA, Samant RS . Dickkopf1: a tumor suppressor or metastasis promoter? Int J Cancer 2012; 130: 1477–1483.
Causeret F, Ensini M, Teissier A, Kessaris N, Richardson WD, Lucas de Couville T et al. Dbx1-expressing cells are necessary for the survival of the mammalian anterior neural and craniofacial structures. PLoS One 2011; 6: e19367.
Davidson G, Mao B, del Barco Barrantes I, Niehrs C . Kremen proteins interact with Dickkopf1 to regulate anteroposterior CNS patterning. Development 2002; 129: 5587–5596.
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 2009; 5: 100–107.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012; 19: 107–120.
Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA 2007; 104: 14700–14705.
Nakamura RE, Hackam AS . Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors 2010; 28: 232–242.
De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D et al. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol 2005; 277: 316–331.
Kunick C, Lauenroth K, Leost M, Meijer L, Lemcke T . 1-Azakenpaullone is a selective inhibitor of glycogen synthase kinase-3 beta. Bioorg Med Chem Lett 2004; 14: 413–416.
Goldman M, Craft B, Swatloski T, Ellrott K, Cline M, Diekhans M et al. The UCSC Cancer Genomics Browser: update 2013. Nucl Acids Res 2012; 41: D949–D954.
Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res 2011; 39: D945–D950.
Ellwanger K, Saito H, Clément-Lacroix P, Maltry N, Niedermeyer J, Lee WK et al. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol 2008; 28: 4875–4882.
Nakamura T, Aoki S, Kitajima K, Takahashi T, Matsumoto K, Nakamura T . Molecular cloning and characterization of Kremen, a novel kringle-containing transmembrane protein. Biochim Biophys Acta 2001; 1518: 63–72.
Nakamura T, Nakamura T, Matsumoto K . The functions and possible significance of Kremen as the gatekeeper of Wnt signalling in development and pathology. J Cell Mol Med 2008; 12: 391–408.
Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C et al. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell 2013; 12: 204–214.
Castets M, Broutier L, Molin Y, Brevet M, Chazot G, Gadot N et al. DCC constrains tumour progression via its dependence receptor activity. Nature 2011; 482: 534–537.
Fitamant J, Guenebeaud C, Coissieux MM, Guix C, Treilleux I, Scoazec JY et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci USA 2008; 105: 4850–4855.
Delloye-Bourgeois C, Fitamant J, Paradisi A, Cappellen D, Douc-Rasy S, Raquin MA et al. Netrin-1 acts as a survival factor for aggressive neuroblastoma. J Exp Med 2009; 206: 833–847.
Paradisi A, Maisse C, Coissieux MM, Gadot N, Lépinasse F, Delloye-Bourgeois C et al. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proc Natl Acad Sci USA 2009; 106: 17146–17151.
Paradisi A, Creveaux M, Gibert B, Devailly G, Redoulez E, Neves D et al. Combining chemotherapeutic agents and netrin-1 interference potentiates cancer cell death. EMBO Mol Med 2013; 5: 1821–1834.
Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 2009; 114: 371–379.
Glantschnig H, Hampton RA, Lu P, Zhao JZ, Vitelli S, Huang L et al. Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem 2010; 285: 40135–40147.
Pozzi S, Fulciniti M, Yan H, Vallet S, Eda H, Patel K et al. Scadden, H.M. Kronenberg, and N. Raje. In vivo and in vitro effects of a novel anti-Dkk1 neutralizing antibody in multiple myeloma. Bone 2013; 53: 487–496.
Qian J, Zheng Y, Zheng C, Wang L, Qin H, Hong S et al. Active vaccination with Dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood 2012; 119: 161–169.
Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res 2010; 70: 5326–5336.
Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr . Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood 2007; 109: 2106–2111.
Acknowledgements
We are grateful to Thierry Galli, Anne Camus, Stéphane Nedelec, Eva Coppola and Sonia Garel for helpful discussions and critical reading of the manuscript. We wish to thank Anne Camus and Yoko Arai for their help in implementing whole embryo cultures, Christine Vesque and Sylvie Schneider-Maunoury for providing zebrafish cDNA, De-Li Shi for providing xenopus cDNA, Thierry Galli for providing HEK cells, Eve Gazave for her help regarding pharmacological agents, Anne-Laure Todeschini for her help with luciferase assays, Fabien Fauchereau for TCGA data mining, Lisa Vigier and Annie Dutriaux for technical assistance, Betty Freret-Hodara for helpful comments, Elenat Marot, Natalia Maties and Sébastien Gravat for maintaining mouse colonies, as well as the ImagoSeine imaging facility (member of the France BioImaging infrastructure, supported by the French National Research Agency ANR-10-INSB-04 ‘Investments for the future’). FC is an Inserm researcher. AP is a CNRS investigator and member Team of the Ecole des Neurosciences de Paris (ENP). This work was supported by grants from the Agence Nationale de la Recherche (ANR-2011-BSV4-023-01), the Fondation pour la Recherche Médicale (FRM) (INE20060306503 and Equipe FRM DEQ20130326521), Ville de Paris (2006 ASES 102) and the Association pour la Recherche sur le Cancer (ARC) (SFI 2011 1203674) to AP and Comité de Paris de la Ligue contre le cancer (RS14/75-7 and RS15/75-46) to FC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by H Ichijo
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Causeret, F., Sumia, I. & Pierani, A. Kremen1 and Dickkopf1 control cell survival in a Wnt-independent manner. Cell Death Differ 23, 323–332 (2016). https://doi.org/10.1038/cdd.2015.100
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2015.100
This article is cited by
-
Dickkopf1 induces enteric neurogenesis and gliogenesis in vitro if apoptosis is evaded
Communications Biology (2023)
-
The Effect of hsa-miR-451b Knockdown on Biological Functions of Gastric Cancer Stem-Like Cells
Biochemical Genetics (2021)
-
Kremen1-induced cell death is regulated by homo- and heterodimerization
Cell Death Discovery (2019)
-
YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury
Cell Death & Disease (2018)