Abstract
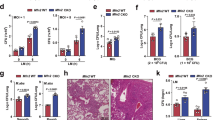
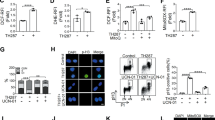
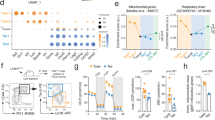
Cell reprogramming technology has allowed the in vitro control of cell fate transition, thus allowing for the generation of highly desired cell types to recapitulate in vivo developmental processes and architectures. However, the precise molecular mechanisms underlying the reprogramming process remain to be defined. Here, we show that depleting p53 and p21, which are barriers to reprogramming, yields a high reprogramming efficiency. Deletion of these factors results in a distinct mitochondrial background with low expression of oxidative phosphorylation subunits and mitochondrial fusion proteins, including mitofusin 1 and 2 (Mfn1/2). Importantly, Mfn1/2 depletion reciprocally inhibits the p53-p21 pathway and promotes both the conversion of somatic cells to a pluripotent state and the maintenance of pluripotency. Mfn1/2 depletion facilitates the glycolytic metabolic transition through the activation of the Ras-Raf and hypoxia-inducible factor 1α (HIF1α) signaling at an early stage of reprogramming. HIF1α is required for increased glycolysis and reprogramming by Mfn1/2 depletion. Taken together, these results demonstrate that Mfn1/2 constitutes a new barrier to reprogramming, and that Mfn1/2 ablation facilitates the induction of pluripotency through the restructuring of mitochondrial dynamics and bioenergetics.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- 2-DG:
-
2-deoxy-D-glucose
- AP:
-
alkaline phosphatase
- CM:
-
conditioned medium
- Drp1:
-
dynamin-related protein 1
- ESCs:
-
embryonic stem cells
- hESCs:
-
human ESCs
- HIF1α:
-
hypoxia-inducible factor 1α
- iPSC:
-
induced pluripotent stem cell
- KO:
-
knockout
- LDHA:
-
lactate dehydrogenase isoform A
- MACS:
-
magnetic-activated cell sorting
- MEFs:
-
mouse embryonic fibroblasts
- Mfn1/2:
-
mitofusin 1 and 2
- NEAA:
-
non-essential amino acids
- OSKM:
-
Oct4, Sox2, Klf4, and c-Myc
- OXPHOS:
-
oxidative phosphorylation
- PSCs:
-
pluripotent stem cells
- ROS:
-
reactive oxygen species
- RT:
-
room temperature
- UM:
-
unconditioned medium
- WT:
-
wild-type
References
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676.
Theunissen TW, Jaenisch R . Molecular Control of Induced Pluripotency. Cell Stem Cell 2014; 14: 720–734.
Buganim Y, Faddah DA, Cheng AW, Itskovich E, Markoulaki S, Ganz K et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 2012; 150: 1209–1222.
Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 2009; 460: 1140–1144.
Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 2009; 460: 1145–1148.
Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 2009; 460: 1132–1135.
Guo S, Zi X, Schulz VP, Cheng J, Zhong M, Koochaki SH et al. Nonstochastic reprogramming from a privileged somatic cell state. Cell 2014; 156: 649–662.
Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 2005; 7: 165–171.
Brosh R, Assia-Alroy Y, Molchadsky A, Bornstein C, Dekel E, Madar S et al. p53 counteracts reprogramming by inhibiting mesenchymal-to-epithelial transition. Cell Death Differ 2013; 20: 312–320.
Bieging KT, Mello SS, Attardi LD . Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer 2014; 14: 359–370.
Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 2011; 14: 264–271.
Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J . The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 2010; 28: 721–733.
Westermann B . Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 2010; 11: 872–884.
Son MY, Choi H, Han YM, Cho YS . Unveiling the critical role of REX1 in the regulation of human stem cell pluripotency. Stem Cells 2013; 31: 2374–2387.
Vazquez-Martin A, Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Vellon L, Menendez JA . Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging 2012; 4: 393–401.
Wang W, Cheng X, Lu J, Wei J, Fu G, Zhu F et al. Mitofusin-2 is a novel direct target of p53. Biochem Biophys Res Commun 2010; 400: 587–592.
Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D et al. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol 2004; 6: 872–883.
Chen KH, Dasgupta A, Ding J, Indig FE, Ghosh P, Longo DL . Role of mitofusin 2 (Mfn2) in controlling cellular proliferation. FASEB J 2014; 28: 382–394.
Prigione A, Rohwer N, Hoffmann S, Mlody B, Drews K, Bukowiecki R et al. HIF1alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells 2014; 32: 364–376.
Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z et al. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 2014; 14: 592–605.
Wenger RH, Stiehl DP, Camenisch G . Integration of oxygen signaling at the consensus HRE. Sci STKE 2005; 2005: re12.
Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 2000; 275: 25130–25138.
Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S . Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 2009; 5: 237–241.
Son MJ, Jeong BR, Kwon Y, Cho YS . Interference with the mitochondrial bioenergetics fuels reprogramming to pluripotency via facilitation of the glycolytic transition. Int J Biochem Cell Biol 2013; 45: 2512–2518.
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC . Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 2003; 160: 189–200.
Naon D, Scorrano L . At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim Biophys Acta 2014; 1843: 2184–2194.
West AP, Shadel GS, Ghosh S . Mitochondria in innate immune responses. Nat Rev Immunol 2011; 11: 389–402.
Detmer SA, Chan DC . Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 2007; 8: 870–879.
Farrand L, Byun S, Kim JY, Im-Aram A, Lee J, Lim S et al. Piceatannol enhances cisplatin sensitivity in ovarian cancer via modulation of p53, X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial fission. J Biol Chem 2013; 288: 23740–23750.
Son MJ, Son MY, Seol B, Kim MJ, Yoo CH, Han MK et al. Nicotinamide overcomes pluripotency deficits and reprogramming barriers. Stem Cells 2013; 31: 1121–1135.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF; 2010-020272(3), and 2012M3A9C7050224) and the KRIBB/NST research initiative program (NAP-09-3) grants funded by the Korea government (MSIP).
Author Contributions
Myung Jin Son: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript. Youjeong Kwon: collection and/or assembly of data, data analysis and interpretation. Mi-Young Son, Hoon-Sung Choi, and Binna Seol: collection and/or assembly of data. Seung-Wook Ryu: conception and design, data interpretation, final approval of manuscript. Chulhee Choi: conception and design, final approval of manuscript. Yee Sook Cho: financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by DR Green
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Son, M., Kwon, Y., Son, MY. et al. Mitofusins deficiency elicits mitochondrial metabolic reprogramming to pluripotency. Cell Death Differ 22, 1957–1969 (2015). https://doi.org/10.1038/cdd.2015.43
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2015.43
This article is cited by
-
Emerging roles of mitochondrial functions and epigenetic changes in the modulation of stem cell fate
Cellular and Molecular Life Sciences (2024)
-
MFN2 knockdown promotes osteogenic differentiation of iPSC-MSCs through aerobic glycolysis mediated by the Wnt/β-catenin signaling pathway
Stem Cell Research & Therapy (2022)
-
Metabostemness in cancer: Linking metaboloepigenetics and mitophagy in remodeling cancer stem cells
Stem Cell Reviews and Reports (2022)
-
Mitofusin-2 modulates the epithelial to mesenchymal transition in thyroid cancer progression
Scientific Reports (2021)
-
Critical requirement of SOS1 RAS-GEF function for mitochondrial dynamics, metabolism, and redox homeostasis
Oncogene (2021)