Abstract
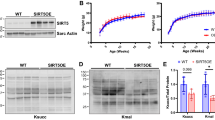
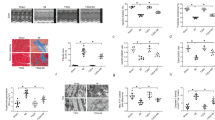
Over the past decade, endoplasmic reticulum (ER) stress has emerged as an important mechanism involved in the pathogenesis of cardiovascular diseases including heart failure. Cardiac therapy based on ER stress modulation is viewed as a promising avenue toward effective therapies for the diseased heart. Here, we tested whether sirtuin-1 (SIRT1), a NAD+-dependent deacetylase, participates in modulating ER stress response in the heart. Using cardiomyocytes and adult-inducible SIRT1 knockout mice, we demonstrate that SIRT1 inhibition or deficiency increases ER stress-induced cardiac injury, whereas activation of SIRT1 by the SIRT1-activating compound STAC-3 is protective. Analysis of the expression of markers of the three main branches of the unfolded protein response (i.e., PERK/eIF2α, ATF6 and IRE1) showed that SIRT1 protects cardiomyocytes from ER stress-induced apoptosis by attenuating PERK/eIF2α pathway activation. We also present evidence that SIRT1 physically interacts with and deacetylates eIF2α. Mass spectrometry analysis identified lysines K141 and K143 as the acetylation sites on eIF2α targeted by SIRT1. Furthermore, mutation of K143 to arginine to mimic eIF2α deacetylation confers protection against ER stress-induced apoptosis. Collectively, our findings indicate that eIF2α deacetylation on lysine K143 by SIRT1 is a novel regulatory mechanism for protecting cardiac cells from ER stress and suggest that activation of SIRT1 has potential as a therapeutic approach to protect the heart against ER stress-induced injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ron D, Walter P . Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8: 519–529.
Hetz C . The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 2012; 13: 89–102.
Csordas G, Thomas AP, Hajnoczky G . Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 1999; 18: 96–108.
Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 2007; 129: 1337–1349.
Tabas I, Ron D . Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 2011; 13: 184–190.
Minamino T, Kitakaze M . ER stress in cardiovascular disease. J Mol Cell Cardiol 2010; 48: 1105–1110.
Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA et al. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res 2006; 98: 1186–1193.
Severino A, Campioni M, Straino S, Salloum FN, Schmidt N, Herbrand U et al. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J Am Coll Cardiol 2007; 50: 1029–1037.
Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC . Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res 2006; 99: 275–282.
Shintani-Ishida K, Nakajima M, Uemura K, Yoshida K . Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun 2006; 345: 1600–1605.
Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M et al. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation 2010; 122: 361–369.
Ayala P, Montenegro J, Vivar R, Letelier A, Urroz PA, Copaja M et al. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp Mol Pathol 2012; 92: 97–104.
Ceylan-Isik AF, Sreejayan N, Ren J . Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol 2011; 50: 107–116.
Haigis MC, Sinclair DA . Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 2010; 5: 253–295.
Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 2007; 100: 1512–1521.
Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2010; 122: 2170–2182.
Planavila A, Iglesias R, Giralt M, Villarroya F . Sirt1 acts in association with PPARalpha to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res 2011; 90: 276–284.
Zhang Y, Xia Z, La Cour KH, Ren J . Activation of Akt rescues endoplasmic reticulum stress-impaired murine cardiac contractile function via glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeation pore opening. Antioxid Redox Signal 2011; 15: 2407–2424.
Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013; 339: 1216–1219.
Brooks WW, Conrad CH . Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: structural and functional correlates. Comp Med 2009; 59: 339–343.
Dalal S, Foster CR, Das BC, Singh M, Singh K . Beta-adrenergic receptor stimulation induces endoplasmic reticulum stress in adult cardiac myocytes: role in apoptosis. Mol Cell Biochem 2012; 364: 59–70.
Nichtova Z, Novotova M, Kralova E, Stankovicova T . Morphological and functional characteristics of models of experimental myocardial injury induced by isoproterenol. Gen Physiol Biophys 2012; 31: 141–151.
Wallner M, Duran JM, Mohsin S, Troupes CD, Vanhoutte D, Borghetti G et al. Acute catecholamine exposure causes reversible myocyte injury without cardiac regeneration. Circ Res 2016; 119: 865–879.
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 2007; 13: 365–376.
Castillero E, Akashi H, Pendrak K, Yerebakan H, Najjar M, Wang C et al. Attenuation of the unfolded protein response and endoplasmic reticulum stress after mechanical unloading in dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2015; 309: H459–H470.
D'Onofrio N, Vitiello M, Casale R, Servillo L, Giovane A, Balestrieri ML . Sirtuins in vascular diseases: emerging roles and therapeutic potential. Biochim Biophys Acta 2015; 1852: 1311–1322.
Winnik S, Auwerx J, Sinclair DA, Matter CM . Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J 2015; 36: 3404–3412.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P . Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005; 434: 113–118.
Gerhart-Hines Z, Dominy JE Jr, Blattler SM, Jedrychowski MP, Banks AS, Lim JH et al. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Mol Cell 2011; 44: 851–863.
Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R et al. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One 2009; 4: e8414.
Zhu H, Xia L, Zhang Y, Wang H, Xu W, Hu H et al. Activating transcription factor 4 confers a multidrug resistance phenotype to gastric cancer cells through transactivation of SIRT1 expression. PloS one 2012; 7: e31431.
Wang FM, Chen YJ, Ouyang HJ . Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem J 2011; 433: 245–252.
Guo R, Liu W, Liu B, Zhang B, Li W, Xu Y . SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: an insight into endoplasmic reticulum stress response mechanism. Int J Cardiol 2015; 191: 36–45.
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 2000; 6: 1099–1108.
Dey M, Velyvis A, Li JJ, Chiu E, Chiovitti D, Kay LE et al. Requirement for kinase-induced conformational change in eukaryotic initiation factor 2alpha (eIF2alpha) restricts phosphorylation of Ser51. Proc Natl Acad Sci USA 2011; 108: 4316–4321.
Donnelly N, Gorman AM, Gupta S, Samali A . The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci 2013; 70: 3493–3511.
Ghosh HS, Reizis B, Robbins PD . SIRT1 associates with eIF2-alpha and regulates the cellular stress response. Sci Rep 2011; 1: 150.
Sun T, Li X, Zhang P, Chen WD, Zhang HL, Li DD et al. Acetylation of beclin 1 inhibits autophagosome maturation and promotes tumour growth. Nat Commun 2015; 6: 7215.
Hetz C, Chevet E, Harding HP . Targeting the unfolded protein response in disease. Nat Rev Drug Discov 2013; 12: 703–719.
Cominacini L, Mozzini C, Garbin U, Pasini A, Stranieri C, Solani E et al. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic Biol Med 2015; 88 (Pt B): 233–242.
Giblin W, Skinner ME, Lombard DB . Sirtuins: guardians of mammalian healthspan. Trends Genet 2014; 30: 271–286.
Oakes SA, Papa FR . The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 2015; 10: 173–194.
Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C et al. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013; 154: 430–441.
Verde I, Vandecasteele G, Lezoualc'h F, Fischmeister R . Characterization of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br J Pharmacol 1999; 127: 65–74.
Shevchenko A, Wilm M, Vorm O, Mann M . Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 1996; 68: 850–858.
Pachl F, Ruprecht B, Lemeer S, Kuster B . Characterization of a high field Orbitrap mass spectrometer for proteome analysis. Proteomics 2013; 13: 2552–2562.
Acknowledgements
We thank N Viet Hung for Mass Spectrometry analysis (SFR A Lwoff Platform). We also thank F Lefebvre for helpful technical assistance and Valérie Domergue-Dupont and the animal core facility of IPSIT for efficient handling and preparation of the animals. We gratefully acknowledge C Longin and S Chat for electron microscopy work (TEM platform of INRA). We thank Dr R Fischmeister for continuous support and careful reading of the manuscript. WT and mutant (S52A) eIF2α encoding plasmids were kindly provided by D Ron (University of Cambridge). This work was supported by grants from LabEx LERMIT, FRM (Fondation pour la Recherche Médicale) to A Garnier (#DPM20121125546) and Région Ile de France CODDIM to R Ventura-Clapier (#cod110153). A Prola received a fellowship from Groupe de Réflexion sur la Recherche Cardiovasculaire (GRRC). A Guilbert received a fellowship from Région Ile de France CORDDIM. D Sinclair is supported by the Glenn Foundation for Medical Research, a gift from the Schulak Family, the Juvenile Diabetes Foundation, and a MERIT award from the NIH/NIA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DAS consults for GlaxoSmithKline, Metrobiotech, Ovascience and BigDataBio. The remaining authors declare no conflict of interest.
Additional information
Edited by A Villunger
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Prola, A., Pires Da Silva, J., Guilbert, A. et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ 24, 343–356 (2017). https://doi.org/10.1038/cdd.2016.138
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.138
This article is cited by
-
Sirtuin1 mitigates hypoxia-induced cardiomyocyte apoptosis in myocardial infarction via PHD3/HIF-1α
Molecular Medicine (2025)
-
Chronic environmental temperature affects protein expression in the eye of adult zebrafish (Danio rerio)
Scientific Reports (2025)
-
Cardiac intermediary metabolism in heart failure: substrate use, signalling roles and therapeutic targets
Nature Reviews Cardiology (2025)
-
Metabo-epigenetic circuits of heart failure: chromatin-modifying enzymes as determinants of metabolic plasticity
EMBO Molecular Medicine (2025)
-
Luteolin Inhibits Ferroptosis of HUVEC by Regulating the Sirt1/Nrf2 Pathway
Cell Biochemistry and Biophysics (2025)