Abstract
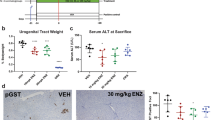
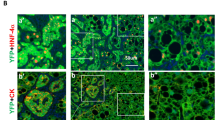
Aberrant cell death/survival has a critical role in the development of hepatocellular carcinoma (HCC). Caspase-2, a cell death protease, limits oxidative stress and chromosomal instability. To study its role in reactive oxygen species (ROS) and DNA damage-induced liver cancer, we assessed diethylnitrosamine (DEN)-mediated tumour development in caspase-2-deficient (Casp2−/−) mice. Following DEN injection in young animals, tumour development was monitored for 10 months. We found that DEN-treated Casp2−/− mice have dramatically elevated tumour burden and accelerated tumour progression with increased incidence of HCC, accompanied by higher oxidative damage and inflammation. Furthermore, following acute DEN injection, liver injury, DNA damage, inflammatory cytokine release and hepatocyte proliferation were enhanced in mice lacking caspase-2. Our study demonstrates for the first time that caspase-2 limits the progression of tumourigenesis induced by an ROS producing and DNA damaging reagent. Our findings suggest that after initial DEN-induced DNA damage, caspase-2 may remove aberrant cells to limit liver damage and disease progression. We propose that Casp2−/− mice, which are more susceptible to genomic instability, are limited in their ability to respond to DNA damage and thus carry more damaged cells resulting in accelerated tumourigenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- HCC:
-
hepatocellular carcinoma
- DEN:
-
diethylnitrosamine
- ROS:
-
reactive oxygen species
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate transaminase
- LDH:
-
lactate dehydrogenase
- ALP:
-
alkaline phosphatase
- TUNEL:
-
terminal deoxynucleotidyl transferase dUTP nick end labelling
- PCNA:
-
proliferating cell nuclear antigen
- HMGB1:
-
high-mobility group box 1
References
Puccini J, Dorstyn L, Kumar S . Caspase-2 as a tumour suppressor. Cell Death Differ 2013; 20: 1133–1139.
Puccini J, Shalini S, Voss AK, Gatei M, Wilson CH, Hiwase DK et al. Loss of caspase-2 augments lymphomagenesis and enhances genomic instability in Atm-deficient mice. Proc Natl Acad Sci USA 2013; 110: 19920–19925.
Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S . A tumor suppressor function for caspase-2. Proc Natl Acad Sci USA 2009; 106: 5336–5341.
Parsons MJ, McCormick L, Janke L, Howard A, Bouchier-Hayes L, Green DR . Genetic deletion of caspase-2 accelerates MMTV/c-neu-driven mammary carcinogenesis in mice. Cell Death Differ 2013; 20: 1174–1182.
Shalini S, Puccini J, Wilson CH, Finnie J, Dorstyn L, Kumar S . Caspase-2 protects against oxidative stress in vivo. Oncogene 2015; 34: 4995–5002.
Dorstyn L, Puccini J, Wilson CH, Shalini S, Nicola M, Moore S et al. Caspase-2 deficiency promotes aberrant DNA-damage response and genetic instability. Cell Death Differ 2012; 19: 1288–1298.
Kim JU, Shariff MIF, Crossey MME, Gomez-Romero M, Taylor-Robinson SD . Hepatocellular carcinoma: Review of disease and tumor biomarkers. World J Hepatol 2016; 8: 471–484.
Tang ZY . Hepatocellular carcinoma—cause, treatment and metastasis. World J Gastroenterol 2001; 7: 445–454.
Vesselinovitch SD, Koka M, Mihailovich N, Rao KV . Carcinogenicity of diethylnitrosamine in newborn, infant, and adult mice. J Cancer Res Clin Oncol 1984; 108: 60–65.
Verna L, Whysner J, Williams GM . N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 1996; 71: 57–81.
Maeda S, Kamata H, Luo JL, Leffert H, Karin M . IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005; 121: 977–990.
Wei JC, Meng FD, Qu K, Wang ZX, Wu QF, Zhang LQ et al. Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1. Acta Pharmacol Sin 2015; 36: 241–251.
Buitrago-Molina LE, Marhenke S, Longerich T, Sharma AD, Boukouris AE, Geffers R et al. The degree of liver injury determines the role of p21 in liver regeneration and hepatocarcinogenesis in mice. Hepatology 2013; 58: 1143–1152.
Wree A, Johnson CD, Font-Burgada J, Eguchi A, Povero D, Karin M et al. Hepatocyte-specific Bid depletion reduces tumor development by suppressing inflammation-related compensatory proliferation. Cell Death Differ 2015; 22: 1985–1994.
Qiu W, Wang X, Leibowtiz B, Yang W, Zhang L, Yu J . PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology 2011; 54: 1249–1258.
Teoh N, Pyakurel P, Dan YY, Swisshelm K, Hou J, Mitchell C et al. Induction of p53 renders ATM-deficient mice refractory to hepatocarcinogenesis. Gastroenterology 2010; 138: 1155–1165, e1151–e1152.
Liedtke C, Zschemisch NH, Cohrs A, Roskams T, Borlak J, Manns MP et al. Silencing of caspase-8 in murine hepatocellular carcinomas is mediated via methylation of an essential promoter element. Gastroenterology 2005; 129: 1602–1615.
Bai L, Ni HM, Chen X, DiFrancesca D, Yin XM . Deletion of Bid impedes cell proliferation and hepatic carcinogenesis. Am J Pathol 2005; 166: 1523–1532.
Weber A, Boger R, Vick B, Urbanik T, Haybaeck J, Zoller S et al. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology 2010; 51: 1226–1236.
Pierce RH, Vail ME, Ralph L, Campbell JS, Fausto N . Bcl-2 expression inhibits liver carcinogenesis and delays the development of proliferating foci. Am J Pathol 2002; 160: 1555–1560.
Wilson CH, Shalini S, Filipovska A, Richman TR, Davies S, Martin SD et al. Age-related proteostasis and metabolic alterations in Caspase-2-deficient mice. Cell Death Dis 2015; 6: e1597.
Shalini S, Dorstyn L, Dawar S, Kumar S . Old, new and emerging functions of caspases. Cell Death Differ 2015; 22: 526–539.
Shalini S, Dorstyn L, Wilson C, Puccini J, Ho L, Kumar S . Impaired antioxidant defence and accumulation of oxidative stress in caspase-2-deficient mice. Cell Death Differ 2012; 19: 1370–1380.
Yang S, Chang C, Wei R, Shiue Y, Wang S, Yeh Y . Involvement of DNA damage response pathways in hepatocellular carcinoma. Bio Med Res Int 2014; ;2014: 153867.
Hacker HJ, Mtiro H, Bannasch P, Vesselinovitch SD . Histochemical profile of mouse hepatocellular adenomas and carcinomas induced by a single dose of diethylnitrosamine. Cancer Res 1991; 51: 1952–1958.
Schug TT, Li X . Sirtuin 1 in lipid metabolism and obesity. Ann Med 2011; 43: 198–211.
Garcia-Rodriguez JL, Barbier-Torres L, Fernandez-Alvarez S, Gutierrez-de Juan V, Monte MJ, Halilbasic E et al. SIRT1 controls liver regeneration by regulating bile acid metabolism through farnesoid X receptor and mammalian target of rapamycin signaling. Hepatology 2014; 59: 1972–1983.
Zhang YY, Zhou LM . Sirt3 inhibits hepatocellular carcinoma cell growth through reducing Mdm2-mediated p53 degradation. Biochem Biophys Res Commun 2012; 423: 26–31.
Farber E . Liver cell cancer: insights into the pathogenesis of hepatocellular carcinoma in humans from experimental hepatocarcinogenesis in the rat. Monogr Pathol 1987; 28: 199–222.
Kolaja KL, Klaunig JE . Vitamin E modulation of hepatic focal lesion growth in mice. Toxicol Appl Pharmacol 1997; 143: 380–387.
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M . Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006; 160: 1–40.
Kawanishi S, Hiraku Y, Murata M, Oikawa S . The role of metals in site-specific DNA damage with reference to carcinogenesis. Free Radic Biol Med 2002; 32: 822–832.
Heindryckx F, Colle I, Van Vlierberghe H . Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol 2009; 90: 367–386.
Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 2010; 40: 893–904.
Dhanasekaran DN, Reddy EP . JNK signaling in apoptosis. Oncogene 2008; 27: 6245–6251.
Das M, Garlick DS, Greiner DL, Davis RJ . The role of JNK in the development of hepatocellular carcinoma. Genes Dev 2011; 25: 634–645.
Meek DW . Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer 2009; 9: 714–723.
Wu X, Bayle JH, Olson D, Levine AJ . The p53-mdm-2 autoregulatory feedback loop. Genes Dev 1993; 7: 1126–1132.
de Visser KE, Coussens LM . The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol 2006; 13: 118–137.
Mantovani A, Allavena P, Sica A, Balkwill F . Cancer-related inflammation. Nature 2008; 454: 436–444.
Grivennikov SI, Greten FR, Karin M . Immunity, inflammation, and cancer. Cell 2010; 140: 883–899.
Grivennikov SI, Karin M . Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 2010; 21: 11–19.
Johnson C, Han Y, Hughart N, McCarra J, Alpini G, Meng F . Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer 2012; 1: 58–70.
Liu Y, Fuchs J, Li C, Lin J . IL-6, a risk factor for hepatocellular carcinoma: FLLL32 inhibits IL-6-induced STAT3 phosphorylation in human hepatocellular cancer cells. Cell Cycle 2010; 9: 3423–3427.
Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007; 317: 121–124.
Manzl C, Peintner L, Krumschnabel G, Bock F, Labi V, Drach M et al. PIDDosome-independent tumor suppression by Caspase-2. Cell Death Diff 2012; 19: 1722–1732.
O'Reilly LA, Ekert P, Harvey N, Marsden V, Cullen L, Vaux DL et al. Caspase-2 is not required for thymocyte or neuronal apoptosis even though cleavage of caspase-2 is dependent on both Apaf-1 and caspase-9. Cell Death Differ 2002; 9: 832–841.
Acknowledgements
We thank Alyshea Collaco for injecting mice and the staff at the SA Pathology animal resource facility for maintaining the mouse strains. This work was supported by the National Health and Medical Research Council (NHMRC) of Australia project grant (1021456), a Cancer Council Collaborative Research Fellowship to LD, an NHMRC Early Career Research Fellowship to CW (1073771) and NHMRC Senior Principal Research Fellowships (1002863 and 1103006) to SK.
Author contributions
SS, LD and SK conceptualised and designed the study; SS, AN, JP, CW and NS performed experiments; SS, AN, LD, CW and SK analysed the data; JF helped with tumour histopathology analyses; SS, CW and SK wrote and revised the paper. All authors commented on the manuscript drafts and edited the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Shalini, S., Nikolic, A., Wilson, C. et al. Caspase-2 deficiency accelerates chemically induced liver cancer in mice. Cell Death Differ 23, 1727–1736 (2016). https://doi.org/10.1038/cdd.2016.81
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2016.81
This article is cited by
-
The p53-caspase-2 axis in the cell cycle and DNA damage response
Experimental & Molecular Medicine (2021)
-
Transcriptome profiling of caspase-2 deficient EμMyc and Th-MYCN mouse tumors identifies distinct putative roles for caspase-2 in neuronal differentiation and immune signaling
Cell Death & Disease (2019)
-
p53 accumulation following cytokinesis failure in the absence of caspase-2
Cell Death & Differentiation (2018)
-
Caspase-2 deficiency enhances whole-body carbohydrate utilisation and prevents high-fat diet-induced obesity
Cell Death & Disease (2017)
-
Caspase-2-mediated cell death is required for deleting aneuploid cells
Oncogene (2017)