Abstract
Background:
The genetic architecture of obesity is multifactorial. We have previously identified a quantitative trait locus (QTL) on rat chromosome 10 in a F2 cross of Wistar Ottawa Karlsburg (WOKW) and Dark Agouti (DA) rats responsible for obesity-related traits. The QTL was confirmed in congenic DA.WOKW10 rats. To pinpoint the region carrying causal genes, we established two new subcongenic lines, L1 and L2, with smaller refined segments of chromosome 10 to identify novel candidate genes.
Methods:
All lines were extensively characterized under different diet conditions. We employed transcriptome analysis in visceral adipose tissue (VAT) by RNA-Seq technology to identify potential underlying genes in the segregating regions. Three candidate genes were measured in human paired samples of VAT and subcutaneous (SC) AT (SAT) (N=304) individuals with a wide range of body weight and glucose homeostasis parameters.
Results:
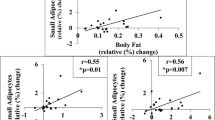
DA.WOKW and L1 subcongenic lines were protected against body fat gain under high-fat diet (HFD), whereas L2 and DA had significantly more body fat after high-fat feeding. Interestingly, adipocyte size distribution in SAT and epigonadal AT of L1 subcongenic rats did not undergo typical ballooning under HFD and the number of preadipocytes in AT was significantly elevated in L2 compared with L1 and parental rats. Transcriptome analysis identified three candidate genes in VAT on rat chromosome 10. In humans, these candidate genes were differentially expressed between SAT and VAT. Moreover, HID1 mRNA significantly correlates with parameters of obesity and glucose metabolism.
Conclusions:
Our data suggest novel candidate genes for obesity that map on rat chromosome 10 in an interval 102.2–104.7 Mb and are strongly associated with body fat mass regulation, preadipocyte number and adipocyte size in rats. Among those genes, AT head involution defective (HID1) mRNA expression may be relevant for human fat distribution and glucose homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dominiczak AF, Clark JS, Jeffs B, Anderson NH, Negrin CD, Lee WK et al. Genetics of experimental hypertension. J Hypertens 1998; 16: 1859–1869.
Galli J, Fakhrai-Rad H, Kamel A, Marcus C, Norgren S, Luthman H . Pathophysiological and genetic characterization of the major diabetes locus in GK rats. Diabetes 1999; 48: 2463–2470.
Klimes I, Weston K, Kovacs P, Gasperikova D, Jezova D, Kvetnansky R et al. Mapping of genetic loci predisposing to hypertriglyceridaemia in the hereditary hypertriglyceridaemic rat: analysis of genetic association with related traits of the insulin resistance syndrome. Diabetologia 2003; 46: 352–358.
Kovács P, Klöting I . Quantitative trait loci on chromosomes 1 and 4 affect lipid phenotypes in the rat. Arch Biochem Biophys 1998; 354: 139–143.
Kovács P, van den Brandt J, Klöting I . Genetic dissection of the syndrome X in the rat. Biochem Biophys Res Commun 2000; 269: 660–665.
Klöting I, Kovács P, van den Brandt J . Quantitative trait loci for body weight, blood pressure, blood glucose, and serum lipids: linkage analysis with wild rats (Rattus norvegicus. Biochem Biophys Res Commun 2001; 284: 1126–1133.
Kovács P, Klöting I . Mapping of quantitative trait loci for body weight on chromosomes 1 and 4 in the rat. Biochem Mol Biol Int 1998; 44: 399–405.
Stewart TP, Kim HY, Saxton AM, Kim JH . Genetic and genomic analysis of hyperlipidemia, obesity and diabetes using (C57BL/6 J × TALLYHO/JngJ) F2 mice. BMC Genomics 2010; 11: 713.
Davis RC, van Nas A, Castellani LW, Zhao Y, Zhou Z, Wen P et al. Systems genetics of susceptibility to obesity-induced diabetes in mice. Physiol Genomics 2012; 44: 1–13.
Inomata H, Watanabe T, Iizuka Y, Liang Y, Mashimo T, Nabika T et al. Identification of quantitative trait loci for cardiac hypertrophy in two different strains of the spontaneously hypertensive rat. Hypertens Res 2005; 28: 273–281.
Baguhl R, Wilke B, Klöting N, Klöting I . Genes on rat chromosomes 3, 5, 10, and 16 are linked with facets of metabolic syndrome. Obesity (Silver Spring) 2009; 17: 1215–1219.
van den Brandt J, Kovács P, Klöting I . Features of the metabolic syndrome in the spontaneously hypertriglyceridemic Wistar Ottawa Karlsburg W (RT1u Haplotype) rat. Metabolism 2000; 49: 1140–1144.
Su Z, Korstanje R, Tsaih S, Paigen B . Candidate genes for obesity revealed from a C57BL/6 J x 129S1/SvImJ intercross. Int J Obes (Lond) 2008; 32: 1180–1189.
Kern M, Kosacka J, Hesselbarth N, Brückner J, Heiker JT, Flehmig G et al. Liver-restricted Repin1 deficiency improves whole-body insulin sensitivity, alters lipid metabolism, and causes secondary changes in adipose tissue in mice. Diabetes 2014; 63: 3295–3309.
Klöting N, Blüher M, Klöting I . The polygenetically inherited metabolic syndrome of WOKW rats is associated with insulin resistance and altered gene expression in adipose tissue. Diabetes Metab Res Rev 2006; 22: 146–154.
Martin M . Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011; 17: 10–12.
Quinlan AR, Hall IM . BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 2010; 26: 841–842.
Stokowy T, Eszlinger M, Świerniak M, Fujarewicz K, Jarząb B, Paschke R et al. Analysis options for high-throughput sequencing in miRNA expression profiling. BMC Res Notes 2014; 7: 144.
Ruschke K, Illes M, Kern M, Klöting I, Fasshauer M, Schön MR et al. Repin1 maybe involved in the regulation of cell size and glucose transport in adipocytes. Biochem Biophys Res Commun 2010; 400: 246–251.
Klöting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab 2007; 6: 79–87.
Blüher M, Unger R, Rassoul F, Richter V, Paschke R . Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or Type II diabetes. Diabetologia 2002; 45: 210–216.
Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010; 299: E506–E515.
Bilak A, Su TT . Regulation of Drosophila melanogaster pro-apoptotic gene hid. Apoptosis 2009; 14: 943–949.
Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K . Mitochondrial disruption in Drosophila apoptosis. Dev Cell 2007; 12: 793–806.
Tanaka-Matakatsu M, Xu J, Cheng L, Du W . Regulation of apoptosis of rbf mutant cells during Drosophila development. Dev Biol 2009; 326: 347–356.
Mesa R, Luo S, Hoover CM, Miller K, Minniti A, Inestrosa N et al. HID-1, a new component of the peptidergic signaling pathway. Genetics 2011; 187: 467–483.
Havrylov S, Rzhepetskyy Y, Malinowska A, Drobot L, Redowicz MJ . Proteins recruited by SH3 domains of Ruk/CIN85 adaptor identified by LC-MS/MS. Proteome Sci 2009; 7: 21.
Loïodice I, Alves A, Rabut G, van Overbeek M, Ellenberg J, Sibarita J et al. The entire Nup107-160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Mol Biol Cell 2004; 15: 3333–3344.
Esaki K, Yoshinaga S, Tsuji T, Toda E, Terashima Y, Saitoh T et al. Structural basis for the binding of the membrane-proximal C-terminal region of chemokine receptor CCR2 with the cytosolic regulator FROUNT. FEBS J 2014; 281: 5552–5566.
Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006; 116: 115–124.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Ito A, Suganami T, Yamauchi A, Degawa-Yamauchi M, Tanaka M, Kouyama R et al. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem 2008; 283: 35715–35723.
Knight MJ, Leettola C, Gingery M, Li H, Bowie JU . A human sterile alpha motif domain polymerizome. Protein Sci 2011; 20: 1697–1706.
Klöting N, Blüher M . Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord 2014; 15: 277–287.
Acknowledgements
We thank Viola Döbel, Eva Böge, Manuela Thiemicke and Susan Berthold of University of Leipzig for technical assistance. This work was supported by the research group ‘SFB1052’, B1 (to MB) and B4 (to NK) funded by Deutsche Forschungsgemeinschaft, and by the Federal Ministry of Education and Research (BMBF), Germany, IFB AdiposityDiseases’ FKZ: 01EO1501 (to NK, AW and PK) and DZD (82DZD00601).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
NK conceived the research ideas, supervised the project and wrote manuscript. AW researched data and wrote manuscript. IK and MB reviewed/edited the manuscript. MK performed human adipose tissue expression analysis. PK reviewed manuscript and contributed to discussion. MS conceived the research ideas and reviewed/edited the manuscript. NK is the guarantor of this work, had full access to all data in the study and takes responsibility for the integrity of the data and data analysis.
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Weingarten, A., Turchetti, L., Krohn, K. et al. Novel genes on rat chromosome 10 are linked to body fat mass, preadipocyte number and adipocyte size. Int J Obes 40, 1832–1840 (2016). https://doi.org/10.1038/ijo.2016.127
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ijo.2016.127
This article is cited by
-
Characterizing the metabolic divide: distinctive metabolites differentiating CAD-T2DM from CAD patients
Cardiovascular Diabetology (2024)