Abstract
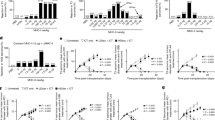
There is currently a need for vaccines that stimulate cell-mediated immunity—particularly that mediated by CD8+ cytotoxic T lymphocytes (CTLs)—against viral and tumor antigens. The optimal induction of cell-mediated immunity requires the presentation of antigens by specialized cells of the immune system called dendritic cells1 (DCs). DCs are unique in their ability to process exogenous antigens via the major histocompatibility complex (MHC) class I pathway2 as well as in their ability to activate naive, antigen-specific CD8+ and CD4+ T cells1,3. Vaccine strategies that target or activate DCs in order to elicit potent CTL-mediated immunity are the subject of intense research. We report here that whole recombinant Saccharomyces cerevisiae yeast expressing tumor or HIV-1 antigens potently induced antigen-specific, CTL responses, including those mediating tumor protection, in vaccinated animals. Interactions between yeast and DCs led to DC maturation, IL-12 production and the efficient priming of MHC class I- and class II-restricted, antigen-specific T-cell responses. Yeast exerted a strong adjuvant effect, augmenting DC presentation of exogenous whole-protein antigen to MHC class I- and class II-restricted T cells. Recombinant yeast represent a novel vaccine strategy for the induction of broad-based cellular immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–52 (1998).
Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nature Cell Biol. 1, 362–368 (1999).
Ridge, J.P., Di Rosa, F. & Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393, 474–8 (1998).
Yewdell, J.W., Norbury, C.C. & Bennink, J.R. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol. 73, 1–77 (1999).
Falo, L.D., Jr., Kovacsovics-Bankowski, M., Thompson, K. & Rock, K.L. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nature Med. 1, 649–53 (1995).
O'Hagan, D.T. Recent advances in vaccine adjuvants for systemic and mucosal administration. J. Pharm. Pharmacol. 50, 1–10 (1998).
Williams, D.L. et al. Development of a water-soluble, sulfated (1,3)—-D-glucan biological response modifier derived from Saccharomyces cerevisiae. Carbohydr. Res. 235, 247–57 (1992).
Rios-Hernandez, M., Dos-Santos, N.J., Silvia, C., Bello-Garciga, J.L. & Pedroso, M. Immunopharmacological studies of β-1,3-glucan. Arch. Med. Res. 25, 179–80 (1994).
McCabe, B.J. et al. Minimal determinant expressed by a recombinant vaccinia virus elicits therapeutic antitumor cytolytic T lymphocyte responses. Cancer Res. 55, 1741–7 (1995).
Brossart, P., Goldrath, A.W., Butz, E.A., Martin, S. & Bevan, M.J. Virus–mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J. Immunol. 158, 3270–6 (1997).
Cho, H.J. et al. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nature Biotechnol. 18, 509–14 (2000).
Hogquist, K.A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Murphy, K.M., Heimberger, A.B. & Loh, D.Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250, 1720–3 (1990).
Cella, M. et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184, 747–52 (1996).
Koch, F. et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184, 741–6 (1996).
De Smedt, T. et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184, 1413–24 (1996).
Roake, J.A. et al. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 181, 2237–47 (1995).
Franzusoff, A., Volpe, A.M., Josse, D., Pichuantes, S. & Wolf, J.R. Biochemical and genetic definition of the cellular protease required for HIV-1 gp160 processing. J. Biol. Chem. 270, 3154–9 (1995).
Schreuder, M.P., Deen, C., Boersma, W.J., Pouwels, P.H. & Klis, F.M. Yeast expressing hepatitis B virus surface antigen determinants on its surface: implications for a possible oral vaccine. Vaccine 14, 383–8 (1996).
Sallusto, F., Cella, M., Danieli, C. & Lanzavecchia, A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400 (1995).
Toda, S. et al. HIV-1-specific cell-mediated immune responses induced by DNA vaccination were enhanced by mannan-coated liposomes and inhibited by anti-interferon-gamma antibody. Immunology 92, 111–7 (1997).
Shibata, Y., Metzger, W.J. & Myrvik, Q.N. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J. Immunol. 159, 2462–7 (1997).
Inaba, K. et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188, 2163–73 (1998).
Mayordomo, J.I. et al. Therapy of murine tumors with p53 wild-type and mutant sequence peptide-based vaccines. J. Exp. Med. 183, 1357–65 (1996).
Acknowledgements
We thank M. Schleicher, K. Hance, J. Moore and L. Sylvan for technical assistance; G. Devendra for construction of the rVV-SF2-gp160 virus; T. Potter for OT-1 TCR transgenic mice; J. DeGregory for DO11.10 TCR transgenic mouse; P. Marrack, R. Kedl, B. Kotzin and S. Rozzo for discussion and useful suggestions. B. Moss for the vaccinia virus vSC8 (rVV-lac). This work has been supported by grants from the Cancer League of Colorado (A.C.S., A.F. and R.C.D.), the Colorado Advanced Technology Institute (D.B.), and by USPHS-NIH grants AI-01459 and AI-42704 (C.W.), AI-42688 (R.C.D.), AI-43143 (R.C.D and A.F.), AI-34747 (A.F.), AI-33299 (D.K.). This study made use of the Immunology and Tissue Culture Core Facilities of the University of Colorado Cancer Center supported by USPHS-NIH grant CA-46934.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Stubbs, A., Martin, K., Coeshott, C. et al. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med 7, 625–629 (2001). https://doi.org/10.1038/87974
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/87974
This article is cited by
-
Yeast-derived nanoparticles remodel the immunosuppressive microenvironment in tumor and tumor-draining lymph nodes to suppress tumor growth
Nature Communications (2022)
-
Lyophilized yeast powder for adjuvant free thermostable vaccine delivery
Applied Microbiology and Biotechnology (2021)
-
Yeast display platform technology to prepare oral vaccine against lethal H7N9 virus challenge in mice
Microbial Cell Factories (2020)
-
Formaldehyde effects on kanamycin resistance gene of inactivated recombinant Escherichia coli vaccines
Biotechnology Letters (2020)
-
Genetically engineered probiotic Saccharomyces cerevisiae strains mature human dendritic cells and stimulate Gag-specific memory CD8+ T cells ex vivo
Applied Microbiology and Biotechnology (2019)