Abstract
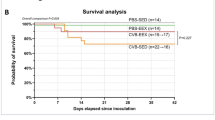
Cardiovascular disease is one of the leading causes of death worldwide, and has been associated with many environmental risk factors1. Recent evidence has indicated the involvement of pathogens such as viruses as causative agents, and specifically identified the coxsackievirus B serogroup as the leading culprit2,3. Not only has coxsackievirus B3 (CB3) been identified from patients with cardiovascular disease4, but also infection of mice with CB3 strains can reproduce human clinical heart disease in rodents5,6. Several mechanisms have been proposed in an attempt to distinguish between pathology mediated by direct viral destruction of cardiac muscle cells7,8 or by the virus-induced immune response directed at infected myocytes9,10,11 or at ‘mimicked’ epitopes shared between viral and cardiac antigens12,13,14. To distinguish between these mechanisms, we infected a unique mouse that diminishes the extent of infection and spread of the virus, but allows complete immunity to the virus. Transgenic mice expressing interferon-γ in their pancreatic β cells failed to develop CB-3-induced myocarditis. This work challenges the idea of the function of the immune response and ‘molecular mimicry’ in the CB-3-induced autoimmune myocarditis model, and instead favors the idea of virus-mediated damage. These results emphasize the benefit of reducing the level of viremia early during infection, thereby reducing the incidence of virus-mediated heart damage and autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Felker, G.M., Hu, W., Hare, J.M., Hruban, R.H., Baughman, K.L. & Kasper, E.K. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine 78, 270– 83 (1999).
Martino, T.A., Liu, P. & Sole, M.J. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ. Res. 74, 182–188 ( 1994).
Baboonian, C., Davies, M.J., Booth, J.C. & McKenna, W.J. Coxsackie B viruses and human heart disease. Curr. Top. Microbiol. Immunol. 223, 31–52 ( 1997).
Baboonian, C. & Treasure, T. Meta-analysis of the association of enteroviruses with human heart disease. Heart 78 , 539–543 (1997).
Rose, N.R. & Hill, S.L. The pathogenesis of postinfectious myocarditis. Clin. Immunol. Immunopathol. 80, S92–S99 (1996).
Godman, G.C., Bunting, H. & Melnick, J.L. The Histopathology of coxsackievirus infection in mice. I Morphologic observations with four different viral types. Am. J. Pathol 28, 223–258 (1952).
Chow, L.H., Beisel, K.W. & McManus, B.M. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab. Invest. 66, 24–31 (1992).
McManus, B.M. et al. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin. Immunol. Immunopatho. 68, 159–69 ( 1993).
Hashimoto, I., Tatsumi, M. & Nakagawa, M. The role of T lymphocytes in the pathogenesis of Coxsackie virus B3 heart disease. Br. J. Exp. Pathol. 64, 497–504 (1983).
Henke, A., Huber, S., Stelzner, A. & Whitton, J.L. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J. Virol. 69, 6720–6728 ( 1995).
Wolfgram, L.J., Beisel, K.W. & Rose, N.R. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J. Exp. Med. 161, 1112– 1121 (1985).
Badorff, C. et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nature Med. 5, 320–326 (1999).
Gauntt, C.J. et al. Anti-Coxsackievirus B3 neutralizing antibodies with pathological potential. Eur. Heart J. 12, 124– 129 (1991).
Gauntt, C.J. et al. Epitopes shared between coxsackievirus B3 (CVB3) and normal heart tissue contribute to CVB3-induced murine myocarditis. Clin. Immunol. Immunopathol. 68, 129–134 (1993).
Sarvetnick, N., Liggitt, D., Pitts, S., Hansen, S. & Stewart, T. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-γ. Cell 52, 773–782 ( 1988).
Gu, D.-L. & Sarvetnick, N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development 118, 33–46 (1993).
Horwitz, M.S., Krahl, T., Fine, C., Lee, J. & Sarvetnick, N. Protection from lethal coxsackievirus-induced pancreatitis by expression of gamma interferon. J. Virol. 73, 1756–1766 (1999).
Rose, N.R., Wolfgram, L.J., Herskowitz, A. & Beisel, K.W. Postinfectious autoimmunity: two distinct phases of coxsackievirus B3-induced myocarditis. Ann. NY Acad. Sci. 475, 146 –156 (1986).
Hohenadl, C., Klingel, K., Rieger, P., Hofschneider, P.H. & Kandolf, R. Investigation of the coxsackievirus B3 nonstructural proteins 2B, 2C, and 3AB: generation of specific polyclonal antisera and detection of replicating virus in infected tissue. J. Virol. Meth. 47, 279–295 (1994).
Schnurr, D.P., Cao, Y. & Schmidt, N.J. Coxsackievirus B3 persistence and myocarditis in N:NIH(S) II nu/nu and +/nu mice. J. Gen. Virol. 65, 1197–1201 (1984).
Lowenstein, C.J. et al. Nitric oxide inhibits viral replication in murine myocarditis. J. Clin. Invest. 97, 1837– 1843 (1996).
Saura, M. et al. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity 10, 21– 28 (1999).
Ramsingh, A.I., Lee, W.T., Collins, D.N. & Armstrong, L.E. T cells contribute to disease severity during coxsackievirus B4 infection. J. Virol. 73, 3080–3086 ( 1999).
Horwitz, M.S., Evans, C.F., McGavern, D.B., Rodriguez, M. & Oldstone, M.B.A. Primary demyelination in transgenic mice expressing interferon-γ. Nature Med. 3, 1037–1041 (1997).
Acknowledgements
We thank L. Tucker, B. Yeung and L. Mocnik for screening and maintaining our mouse colony and M. Wood for his time and effort on the confocal microscope. We also thank M. Krakowski, M. Flodstrom, C. King, H. Fox and M. Buchmeier for discussions. M.S.H. has received an American Diabetes Association Career Development Award. A.L.C. was supported by a Juvenile Diabetes Foundation International Postdoctoral Fellowship. N.S. was supported by a Diabetes Interdisciplinary Research Program grant from the Juvenile Diabetes Foundation International. This is manuscript number 12676-IMM from The Scripps Research Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horwitz, M., La Cava, A., Fine, C. et al. Pancreatic expression of interferon-γ protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat Med 6, 693–697 (2000). https://doi.org/10.1038/76277
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/76277
This article is cited by
-
Lower risk of primary Sjogren’s syndrome in patients with dengue virus infection: a nationwide cohort study in Taiwan
Clinical Rheumatology (2021)
-
Remodeling and dedifferentiation of adult cardiomyocytes during disease and regeneration
Cellular and Molecular Life Sciences (2014)
-
Could interferon-gamma be a therapeutic target for treating heart failure?
Heart Failure Reviews (2014)
-
Autoimmunological features in inflammatory cardiomyopathy
Clinical Research in Cardiology (2007)
-
Diagnosis and management of viral myocarditis
Current Treatment Options in Cardiovascular Medicine (2007)