Abstract
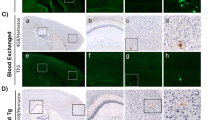
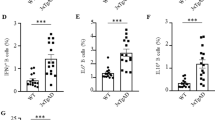
One hallmark of Alzheimer disease is the accumulation of amyloid β-peptide in the brain and its deposition as plaques. Mice transgenic for an amyloid β precursor protein (APP) mini-gene driven by a platelet-derived (PD) growth factor promoter (PDAPP mice), which overexpress one of the disease-linked mutant forms of the human amyloid precursor protein, show many of the pathological features of Alzheimer disease, including extensive deposition of extracellular amyloid plaques, astrocytosis and neuritic dystrophy1,2. Active immunization of PDAPP mice with human amyloid β-peptide reduces plaque burden and its associated pathologies3. Several hypotheses have been proposed regarding the mechanism of this response4,5. Here we report that peripheral administration of antibodies against amyloid β-peptide, was sufficient to reduce amyloid burden. Despite their relatively modest serum levels, the passively administered antibodies were able to enter the central nervous system, decorate plaques and induce clearance of preexisting amyloid. When examined in an ex vivo assay with sections of PDAPP or Alzheimer disease brain tissue, antibodies against amyloid β-peptide triggered microglial cells to clear plaques through Fc receptor-mediated phagocytosis and subsequent peptide degradation. These results indicate that antibodies can cross the blood–brain barrier to act directly in the central nervous system and should be considered as a therapeutic approach for the treatment of Alzheimer disease and other neurological disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Games, D. et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373, 523–527 (1995).
Masliah, E. et al. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer's disease. J. Neurosci. 16, 5795–57811 (1996).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999).
St. George-Hyslop, P.H. & Westaway, D.A. Alzheimer's disease: antibody clears senile plaques. Nature 400, 116–117 (1999).
Duff, K. Curing amyloidosis: will it work in humans? Trends Neurosci. 22, 485–486 (1999).
Paresce, D.M., Chung, H. & Maxfield, F.R. Slow degradation of aggregates of the Alzheimer's disease amyloid β-protein by microglial cells. J. Biol. Chem. 272, 29390–29397 (1997).
Chung, H., Brazil, M.I., Soe, T.T. & Maxfield, F.R. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer's amyloid β-peptide by microglial cells. J. Biol. Chem. 274, 32301–32308 (1999).
Thompson, E.J. & Keir, G. Laboratory investigation of cerebrospinal fluid proteins. Ann. Clin. Biochem. 27, 425–435 (1990).
Johnson-Wood, K. et al. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 94, 1550–1555 (1997).
Hyman, B.T. et al. Kunitz protease inhibitor-containing amyloid beta protein precursor immunoreactivity in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 51, 76–83 (1992).
Acknowledgements
We thank K. Hoenow for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bard, F., Cannon, C., Barbour, R. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6, 916–919 (2000). https://doi.org/10.1038/78682
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/78682
This article is cited by
-
The advent of Alzheimer treatments will change the trajectory of human aging
Nature Aging (2024)
-
Involvement of the glymphatic/meningeal lymphatic system in Alzheimer’s disease: insights into proteostasis and future directions
Cellular and Molecular Life Sciences (2024)
-
Stability in distribution for a stochastic Alzheimer’s disease model with reaction diffusion
Nonlinear Dynamics (2022)
-
Alzheimer disease neuropathology in a patient previously treated with aducanumab
Acta Neuropathologica (2022)
-
Structural biology of cell surface receptors implicated in Alzheimer’s disease
Biophysical Reviews (2022)