Abstract
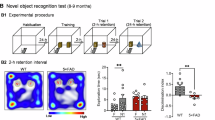
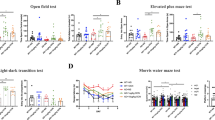
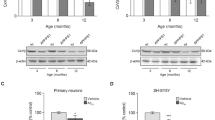
Epidemiological studies indicate that isolated persons have increased risk of developing Alzheimer's disease (AD). This study investigated the cellular mechanisms of how social isolation influenced amyloid β peptide (Aβ) accumulation and affected the severity of AD-associated cognitive decline in a mouse model of AD. Amyloid precursor protein (APP) and presenilin 1 (PS1) double-transgenic (APP/PS1) mice were placed either in isolation or in group from postnatal day 28 and tested for cognitive performance at the age of 3 months with fear-conditioning paradigms. We found that social isolation accelerated impairment of contextual fear memory in the APP/PS1 mice. The magnitude of long-term potentiation in the hippocampal CA1 neurons was significantly lower in the isolated APP/PS1 mice compared with group APP/PS1 and wild-type mice. Hippocampal level of Aβ was significantly elevated in the isolated APP/PS1 mice, which was accompanied by an increased calpain activity and p25/p35 ratio. In addition, surface expression of GluR1 subunit of AMPA receptor was decreased by social isolation. The association of p35, and α-CaMKII was significantly less in the isolated APP/PS1 mice indicating that their interaction was impaired. These results suggest that social isolation exacerbates memory deficit by increasing Aβ level, leading to the increased calpain activity, conversion of p35 to p25 and decrease in association of p35, α-CaMKII, and GluR1, resulting in the endocytosis of AMPA receptors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barria A, Muller D, Derkach V, Griffith LC, Soderling TR (1997). Regulatory phosphorylation of AMPA-type glutamate receptors by CaMKII during long-term potentiation. Science 276: 2042–2045.
Billings LM, Oddo S, Green KN, McGaugh JL, Laferla FM (2005). Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron 45: 675–688.
Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N et al (2009). The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry 14: 95–105.
Chan A, Tchantchou F, Rogers EJ, Shea TB (2009). Dietary deficiency increases presenilin expression, gamma-secretase activity, and Abeta levels: potentiation by ApoE genotype and alleviation by S-adenosyl methionine. J Neurochemistry 110: 831–836.
Cruz JC, Tseng H, Goldman JA, Shih H, Tsai LH (2003). Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40: 471–483.
Davis M (2006). Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol 61: 741–756.
Dewachter I, Filipkowski RK, Priller C, Ris L, Neyton J, Croes S et al (2009). Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol Aging 30: 241–256.
Dhavan R, Greer PL, Morabito MA, Orlando LR, Tsai LH (2002). The cyclin-dependent kinase 5 activators p35 and p39 interact with the α-subunit of Ca2/calmodulin-dependent protein kinase II and a-actinin-1 in a calcium-dependent manner. J Neurosci 22: 7879–7891.
Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG (2004). Modulation for hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg 2567) mutant mice by isolation stress. Neuroscience 127: 601–609.
Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP et al (2003). Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA 100: 4162–4167.
Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH (2005). Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron 48: 825–838.
Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM (2006). Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J Neurosci 26: 9047–9056.
Gu Z, Liu W, Yan Z (2009). {beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem 284: 10639–10649.
Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL et al (1992). Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359: 322–325.
Hsiao YH, Chen PS, Lin CH, Gean PW (2008). N-acetylcysteine prevents β-amyloid toxicity by retarding proteolytic degradation of a cyclin-dependent kinase 5 activator in cultured cortical neurons. J Neurosci Res 86: 2685–2695.
Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S et al (2006). AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron 52: 831–843.
Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP et al (2001). Metabolic regulation of brain Abeta by neprilysin. Science 292: 1550–1552.
Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA et al (2004). Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13: 159–170.
Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS et al (2006). Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer's disease model. FASEB J 20: 729–731.
Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R et al (1997). Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci USA 94: 1550–1555.
Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH et al (1987). The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736.
Kim JJ, Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science 256: 675–677.
Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM (2005). Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci 25: 8843–8853.
Knobloch M, Farinelli M, Konietzko U, Nitsch RM, Mansuy IM (2007). Abeta oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J Neurosci 27: 7648–7653.
Knobloch M, Mansuy IM (2008). Dendritic spine loss and synaptic alterations in Alzheimer's disease. Mol Neurobiol 37: 73–82.
Koo EH, Squazzo SL (1994). Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem 269: 17386–17389.
Kusakawa G, Saito T, Onuki R, Ishiguto K, Kishimoto T, Hisanaga S (2000). Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem 275: 17166–17172.
Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000). Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405: 360–364.
Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X et al (2003). Enhanced proteolysis of betaamyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40: 1087–1093.
Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D (2009). Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62: 788–801.
Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J et al (2003). Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol 183: 600–609.
Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L et al (1999). Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol 155: 853–862.
Mammen AL, Kameyama K, Roche WK, Huganir RL (1997). Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole 4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem 272: 32528–32533.
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K et al (1999). Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol 46: 860–866.
Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH (1996). The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev 10: 816–825.
Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J et al (2003). Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron 38: 555–565.
Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai LH (1998). p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J Biol Chem 273: 24057–24064.
Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH (1999). Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402: 615–622.
Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ et al (1999). Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem 274: 18851–18856.
Phillips RG, LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285.
Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M et al (2009). Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry 66: 384–392.
Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM et al (1992). Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258: 126–129.
Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY et al (2005). Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 8: 1051–1058.
Srivareerat M, Tran TT, Alzoubi KH, Alkadhi KA (2009). Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in β-Amyloid rat model of Alzheimer's disease. Biol Psychiatry 65: 918–926.
Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O (2004). Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol 55: 801–814.
Tsai LH, Delalle I, Caviness Jr VS, Chae T, Harlow E (1994). p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371: 419–423.
Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL et al (2007). Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry 64: 234–240.
Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB et al (2010). Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model. Proc Natl Acad Sci USA 107: 19014–19019.
Zhao CT, Li K, Li JT, Zheng W, Liang XJ, Geng AQ et al (2009). PKCdelta regulates cortical radial migration by stabilizing the Cdk5 activator p35. Proc Natl Acad Sci USA 106: 21353–21358.
Zhao WQ, Santini F, Breese R, Ross D, Zhang XD, Stone DJ et al (2010). Inhibition of calcineurin-mediated endocytosis and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid β oligomer-induced synaptic disruption. J Biol Chem 285: 7619–7632.
Acknowledgements
This study was supported by Grants NSC98-2321-B-006-009 from the National Science Council, NHRI-EX97-9716NI from the National Health Research Institute and Landmark Project (R026) of the National Cheng-Kung University of Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Hsiao, YH., Chen, P., Chen, SH. et al. The Involvement of Cdk5 Activator p35 in Social Isolation-Triggered Onset of Early Alzheimer’s Disease-Related Cognitive Deficit in the Transgenic Mice. Neuropsychopharmacol 36, 1848–1858 (2011). https://doi.org/10.1038/npp.2011.69
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2011.69
Keywords
This article is cited by
-
RPS23RG1 modulates tau phosphorylation and axon outgrowth through regulating p35 proteasomal degradation
Cell Death & Differentiation (2021)
-
Impact of social relationships on Alzheimer’s memory impairment: mechanistic studies
Journal of Biomedical Science (2018)
-
Citalopram Ameliorates Synaptic Plasticity Deficits in Different Cognition-Associated Brain Regions Induced by Social Isolation in Middle-Aged Rats
Molecular Neurobiology (2017)
-
Dynamic Regulation of AMPAR Phosphorylation In Vivo Following Acute Behavioral Stress
Cellular and Molecular Neurobiology (2016)
-
One is the deadliest number: the detrimental effects of social isolation on cerebrovascular diseases and cognition
Acta Neuropathologica (2015)