Abstract
Background
Biliary atresia (BA) is an etiologically perplexing disease, manifested by neonatal cholestasis, repeated cholangitis, and progressive biliary fibrosis. MiR-155 has been implicated to modulate the immune response, which contributes to biliary injury. However, its potential role in the pathogenesis of BA has not been addressed so far.
Methods
The microRNA changes from BA patients and controls were identified via microarray. The immunomodulatory function of miR-155 was investigated via cell transfection and reporter assay. The lentiviral vector pL-miR-155 inhibitor was transfected into a mouse model to investigate its role in BA.
Results
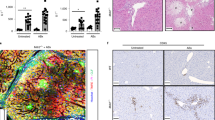
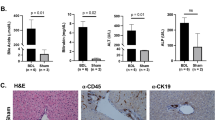
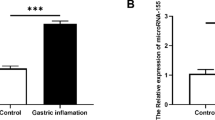
The expression of miR-155 in livers of BA patients was significantly increased, and an inverse correlation between miR-155 and suppressor of cytokine signaling 1 (SOCS1) was detected. MiR-155 overexpression promoted expressions of major histocompatibility complex (MHC) I, MHC II, Chemokine (C-X-C motif) ligand (CXCL) 9, CXCL10, monocyte chemotactic protein 1, and CXCL1 after IFN-γ stimulation, which could be suppressed by SOCS1 overexpression. Moreover, miR-155 overexpression activated JAK2/STAT3, thus enhancing the pro-inflammatory effect. Downregulating miR-155 reduced the incidence of BA in a rhesus monkey rotavirus-induced BA model.
Conclusion
Our results reveal a vital contribution of miR-155 upregulation and consequent SOCS1 downregulation to an immune response triggered via IFN-γ in BA.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mack CL, Feldman AG, Sokol RJ . Clues to the etiology of bile duct injury in biliary atresia. Semin Liver Dis 2012;32:307–316.
Davenport M . Biliary atresia: clinical aspects. Semin Pediatr Surg 2012;21:175–184.
Mack CL, Sokol RJ . Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res 2005;57:87R–94R.
Bessho K, Bezerra JA . Biliary atresia: will blocking inflammation tame the disease? Annu Rev Med 2011;62:171–185.
Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD . MicroRNAs: new regulators of immune cell development and function. Nat Immunol 2008;9:839–845.
Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC . miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 2012;135:73–88.
Guedes J, Cardoso AL, Pedroso de Lima MC . Involvement of microRNA in microglia-mediated immune response. Clin Dev Immunol 2013;2013:186872.
Thai TH, Calado DP, Casola S et al. Regulation of the germinal center response by microRNA-155. Science 2007;316:604–608.
Wang G, Tam LS, Li EK et al. Serum and urinary cell-free MiR-146a and MiR-155 in patients with systemic lupus erythematosus. J Rheumatol 2010;37:2516–2522.
Stanczyk J, Pedrioli DM, Brentano F et al. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 2008;58:1001–1009.
Junker A, Krumbholz M, Eisele S et al. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 2009;132:3342–3352.
Krebs DL, Hilton DJ . SOCS proteins: negative regulators of cytokine signaling. Stem Cells 2001;19:378–387.
Walhout AJ, Temple GF, Brasch MA et al. GATEWAY recombinational cloning: application to the cloning of large numbers of open reading frames or ORFeomes. Methods Enzymol 2000;328:575–592.
Guo Z, Hardin H, Montemayor-Garcia C et al. In situ hybridization analysis of miR-146b-5p and miR-21 in thyroid nodules: diagnostic implications. Endocr Pathol 2015;26:157–163.
Syal G, Fausther M, Dranoff JA . Advances in cholangiocyte immunobiology. Am J Physiol Gastrointest Liver Physiol 2012;303:G1077–G1086.
Davey GM, Heath WR, Starr R . SOCS1: a potent and multifaceted regulator of cytokines and cell-mediated inflammation. Tissue Antigens 2006;67:1–9.
Jiang S, Zhang HW, Lu MH et al. MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res 2010;70:3119–3127.
Zhang J, Cheng Y, Cui W, Li M, Li B, Guo L . MicroRNA-155 modulates Th1 and Th17 cell differentiation and is associated with multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol 2014;266:56–63.
Shukla GC, Singh J, Barik S . MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol 2011;3:83–92.
Bartel DP . MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–233.
Shivakumar P, Campbell KM, Sabla GE et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest 2004;114:322–329.
Ayres RC, Neuberger JM, Shaw J, Joplin R, Adams DH . Intercellular adhesion molecule-1 and MHC antigens on human intrahepatic bile duct cells: effect of pro-inflammatory cytokines. Gut 1993;34:1245–1249.
Chen XM, O'Hara SP, LaRusso NF . The immunobiology of cholangiocytes. Immunol Cell Biol 2008;86:497–505.
Al-Masri AN, Flemming P, Rodeck B, Melter M, Leonhardt J, Petersen C . Expression of the interferon-induced Mx proteins in biliary atresia. J Pediatr Surg 2006;41:1139–1143.
Jafri M, Donnelly B, Bondoc A, Allen S, Tiao G . Cholangiocyte secretion of chemokines in experimental biliary atresia. J Pediatr Surg 2009;44:500–507.
Shinkai M, Shinkai T, Puri P, Stringer MD . Increased CXCR3 expression associated with CD3-positive lymphocytes in the liver and biliary remnant in biliary atresia. J Pediatr Surg 2006;41:950–954.
Leon MP, Bassendine MF, Gibbs P, Thick M, Kirby JA . Immunogenicity of biliary epithelium: study of the adhesive interaction with lymphocytes. Gastroenterology 1997;112:968–977.
Marra F, DeFranco R, Grappone C et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol 1998;152:423–430.
Imai T, Hieshima K, Haskell C et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997;91:521–530.
Acknowledgements
Statement of financial support
This study received financial support from the National Key Clinical Specialty Construction Programs of China (2014–2016), the Shanghai ‘Non Key-in-Key Discipline’ Clinical medical centers (2014–2016), the Shanghai Hospital Development Center (SHDC12014106), the Natural Science Foundation of China (Nos. 81200257, 81300517, and 81370472), the Shanghai Hospital Development Center (SHDC12014106), the Shanghai Rising-Star Program (A type) (No. 15QA1400800), and The Science Foundation of Shanghai (No. 16411952200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary material is linked to the online version of the paper at
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, R., Dong, R., Yang, Y. et al. MicroRNA-155 modulates bile duct inflammation by targeting the suppressor of cytokine signaling 1 in biliary atresia. Pediatr Res 82, 1007–1016 (2017). https://doi.org/10.1038/pr.2017.87
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.87
This article is cited by
-
Epigenetic regulation of pediatric and neonatal immune responses
Pediatric Research (2022)
-
Epigenetic Mechanisms of Pancreatobiliary Fibrosis
Current Treatment Options in Gastroenterology (2019)
-
MicroRNA-29b/142-5p contribute to the pathogenesis of biliary atresia by regulating the IFN-γ gene
Cell Death & Disease (2018)