Abstract
Ancient architectural color paintings are highly susceptible to outdoor environmental conditions. This study explores the feasibility of fluorinated ethylene vinyl ether (FEVE) composites incorporating TiO₂ and SiO2 nanoparticles (F-T/S) as a protective coating for preservation. Characterization results revealed uniformly dispersed nanoparticles without agglomeration or phase changes. The F-T/S films demonstrated enhanced hydrophobicity, UV resistance, and mechanical strength. Nano-SiO2 and Nano-TiO2 significantly improved water resistance ability and UV durability respectively, and mechanical tests also confirmed good tensile strength and hardness. The composites also exhibited strong antifungal activity against Aspergillus niger, Aspergillus flavus, and Penicillium citrinum species. Applied to surface of model film samples and pigment layer, the composites maintained excellent durability and visual integrity after 480-hour degradation cycles. These findings highlight F-T/S as a multifunctional conservation material, offering simultaneous mechanical reinforcement, environmental protection, and antifungal properties for conservation of heritage paintings.
Similar content being viewed by others
Introduction
The Pavilion of Rain Flower (Fig. 1a, b), originally built in the 14th year of the Qianlong period (1749), is located in the northwest corner of the Forbidden City. It was rebuilt in the 44th year of the Qianlong period and serves as a Tibetan Buddhist tantric hall. As the largest Buddhist hall within the palace, it remains the most well-preserved Tibetan Buddhist tantric temple in China, holding great value for the study of Tibetan Buddhism1. The decorative paintings on the eaves and beams of buildings, known as ancient architectural color paintings2,3,4, and are an essential part of China’s cultural heritage (Fig. 1c). These paintings typically consist of three layers, including the wooden layer, the ground layer, and the pigment layer. The wooden layer refers to the basic structure of the building, and the ground layer is made from a mixture of tung oil5, pig’s blood6, lime, brick dust, flour7, lime water, and ramie, used to cover the imperfections of the wood. The pigment layer is composed of a mixture of pigments and glues, such as animal glue8,9, gelatin, and fish glue10. Many ancient architectural paintings in China are over a hundred years old, particularly those on exterior eaves, and have endured over a century of exposure to environmental factors (e.g., temperature, humidity, light, wind, sand, rainwater, UV radiation, etc.), biological factors (e.g., microorganisms, bird droppings, etc.). These factors, along with the composition of the materials, have led to deterioration issues such as ground layer detachment (Fig. 1e), pigment layer peeling (Fig. 1f), and mold growth (Fig. 1g). Water and ultraviolet radiation not only weaken material adhesion but also accelerate weathering and mold growth11. To mitigate these issues, researchers have explored various reinforcement materials for restoration12.
a The location of the Palace Museum in Beijing; (b) the Pavilion of Rain Flower in the Palace Museum; (c) cross-section of color paintings on the wooden beams in the Pavilion of Rain Flower; (d) cross-section structure of the color paintings; (e) mold growth and dust contamination (the red circles in the image indicate mold growth); (f) detachments of mortar; (g) exfoliation of pigment layer.
Early conservation efforts primarily relied on inorganic materials for restoration, including calcium-barium systems (e.g., calcium hydroxide and calcium carbonate13) and sodium silicate systems (e.g., high-modulus potassium silicate solutions14). These materials effectively reinforced stone, bone, soil, and paper artifacts. Inorganic materials are known for their compatibility and resistance to aging, long service life, and low cost. However, they also present limitations, including weak adhesion, poor permeability, and limited strengthening capacity14,15.
As conservation techniques have advanced, synthetic organic materials have become widely adopted due to their high strength, corrosion resistance, and fast effectiveness16. These materials include both traditional and synthetic types. Traditional materials refer to those made from the original materials of the relics, such as tung oil, lacquer, and flour used for painting ground layer restoration, and animal glues, fish glue, gelatin, egg white, and rice paste used as adhesive for pigment layers17. However, gelatins may cause the pigment layer to peel or harden and also promote mold growth18. Tung oil used in the ground layer may seep out and stain the painting’s surface19. Synthetic materials are primarily classified into acrylic esters (e.g., B7220 and AC3321,22), silicone materials (including siloxane, siloxane monomers, and organic silicon copolymers), fluororesins, e.g., trifuorovinyl chloride and vinyl ether copolymer, fluorinated ethylene vinyl ether (FEVE) resin23, vinyl materials (such as polyvinyl acetate (PAVC)24, polyvinyl butyral (PVB), and polystyrene (PS)), and epoxy resins (such as those based on epichlorohydrin and bisphenol A or polyols12). Over time, these reinforcement materials have shown various strengths and weaknesses. For example, epoxy resins have good durability, viscosity, and mechanical properties25 but suffer from poor UV resistance, degradation26, low impact toughness, and brittleness27. Acrylic materials offer high strength but are highly sensitive to light intensity and fluctuation in temperature-humidity28. Silicone materials retain hydrophobicity and breathability but have poor film-forming properties and are prone to cracking29. Fluororesins are excellent in weather resistance, stain resistance, and low VOC emissions, but have moderate long-term antimicrobial properties30. Vinyl materials provide good transparency after film formation but suffer from poor aging resistance and yellowing31.
With the advancement of nanotechnology, nanomaterials and their unique structures have been widely applied to restore and protect cultural heritage32,33,34. Many common inorganic nanoparticles, such as SiO₂, TiO₂, Ca(OH)₂, MgO, and their composite materials, have been considered for conservation purposes. The design and preparation of superhydrophobic and bacteriostatic composite coatings using these particles has become a promising approach for stone protection35,36. For instance, Yutong Shao et al. introduced nano-SiO2 for antibacterial protection in silk artifacts37, and Jing Li et al. used TiO2 nanocomposites for multifunctional restoration of paper-based objects, including reinforcement, deacidification, UV protection, and antibacterial preservation38. Despite their beneficial reinforcement effects, these materials have shown some issues over time, such as detachment and mold growth. Earlier studies have evaluated four existing reinforcement materials for ancient architectural color paintings19. FEVE fluorocarbon resin has received considerable attention in the coating field due to its structural characteristics. The fluoropolymer units protect unstable vinyl ether units from oxidation, while the alkyl ether (or ester) side chains make the resin soluble and transparent, while carboxyl groups ensure the wettability and adhesion of pigments, and hydroxyl groups provide crosslinking sites39,40. FEVE offers excellent weather resistance, stain resistance, and low VOC emissions. However, it still has drawbacks, such as low density, poor antimicrobial properties, and weathering resistance. By adding small amounts of Nano-SiO2 and Nano-TiO2, FEVE’s light-shielding ability, surface energy41,42, and antimicrobial performance43,44 can be significantly improved, compensating for its limitations and enhancing its application in cultural heritage preservation.
This study introduces a composite of Nano-SiO₂, Nano-TiO₂, and FEVE. The hydroxyl groups on the surface of nano-SiO2 are replaced by amino groups by the silane coupling agent KH550. The epoxy group of fluoropolymer reacts with amino groups or hydroxyl groups on KH550-SiO2, then adsorbs onto the surface of TiO₂ nanoparticles via fluorosilane through silane hydrolysis condensation reaction, forming FEVE-TiO2/SiO2 (F-T/S). Characterizations were conducted to explore the dispersion, hydrophobicity, UV protection, and antifungal properties of this composite, with the aim of assessing its potential to slow the degradation effects after treatment. The properties of this composite for reinforcing ancient architectural color paintings are evaluated. This material has also been tested on a small scale for the color paintings on the western annex of The Pavilion of Rain Flower in the Palace Museum, aiming to provide insights for the restoration and preservation of color-painted cultural heritage.
Methods
Preparation of the reinforcement reagents
The fluorinated ethylene vinyl ether (FEVE) was purchased from the Dalian Zhenbang Co., Ltd. (Dalian, China), and was prepared for use. Nano-SiO₂ (15 nm), Nano-TiO₂ (20 nm) and KH550 were sourced from Aladdin Co., Ltd. (Shanghai, China). was also supplied by Aladdin Co., Ltd. The interface effects, size effects, and scattering coefficients of 15 nm SiO₂ are comparable to those of air or transparent matrices. Since the particle size of spherical scatterers is much smaller than the wavelength of visible light, Mie scattering is significantly reduced, making it advantageous for the preparation of transparent layers45,46.
Preparation of KH550-SiO2
Nano-SiO₂ has hydroxyl groups on its surface, but these are not reactive enough to bond with fluoropolymers, so modification of the nano-SiO₂ is required. The hydroxyl groups on the surface of nano-SiO₂ are replaced with amino groups from the silane coupling agent KH550. The amino groups can react with hydroxyl groups on the substrate surface and epoxy groups in fluoropolymers, making KH550 a suitable coupling agent.
Modification with nano-SiO2
1 g of dried nano-SiO₂ is added to 50 mL of 75% ethanol and stirred at room temperature for 30 min. Triethylamine is then added dropwise. The reaction flask with the solution is connected to a condenser and placed in a water bath at 85 °C, where 2 g of KH550 is added, and the mixture is magnetically stirred and refluxed for 5 h. Afterward, the solution is centrifuged for 5 min, purified with 95% ethanol, repeated three times, and dried at 120 °C for 3 h.
The reaction of KH550-SiO2 with aqueous fluoropolymer
KH550-SiO₂ is added to a solution containing the fluoropolymer and stirred magnetically. the epoxy groups of the fluoropolymer react with some of the amino groups on KH550-SiO₂, and the other amino groups on SiO₂ undergo ionic adsorption with the hydroxyl groups on the substrate surface, thus connecting KH550-SiO₂ and the fluoropolymer to the substrate surface.
Incorporation with TiO2
The FEVE-SiO₂ mixture is combined with 15 nm TiO₂ nanoparticles. The fluorosilane is adsorbed onto the surface of TiO₂ nanoparticles via the hydrolysis-condensation reaction of silane. This modification of the TiO₂ nanoparticles is achieved by electrostatic adsorption. The modified TiO₂ particles are then attached to the surface of a specific substrate by spraying or immersion. 3 mL FEVE was dissolved in 97 mL deionized water to prepare the FEVE solution, and then different concentrations of TiO₂/SiO₂ (TiO₂:SiO₂ = 1:1) at 0.05%, 0.07%, 0.1%, and 0.2% in w/v are dissolved in the FEVE solution, labeled as F-T/S-1, F-T/S-2, F-T/S-3, and F-T/S-4, respectively with 3% FEVE serving as the control group (Fig. 2).
Preparation of films
The FEVE and F-T/S solutions were cast onto 76 × 26 mm microscope slides and air-dried under room conditions for 12 h. These films were used for all characterization except the mechanical tests. Since not all films could be easily removed from the slides, the reinforcing agents were also evenly coated onto Xuan paper—a homogeneous, high-quality traditional Chinese paper—to evaluate the tensile strength of the reinforcement films.
Preparation model samples with pigment layer
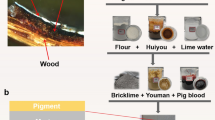
The mortar layer was prepared by mixing tung oil, flour, lime, and brick ash (provided by the Palace Museum) following the traditional recipe ratios (Fig. 3). This mixture was uniformly applied to 5 × 14.5 cm wooden boards (Fig. 3a) and air-dried at room conditions (T = 25 ± 1 °C, RH = 50% ± 1%) for 30 days (Fig. 3b) under ambient light to stabilize the properties of the paint layers. Three types of commonly used pigment powders (ultramarine, cinnabar, and Paris green) with uniform particle sizes, sourced from Beijing Tianya Pigment Co., Ltd., were dissolved in a 3% gelatin solution. These solutions were applied evenly to the mortar layers using brushes (Fig. 3c), followed by air drying for one week under room conditions.
Degradation of model samples
Given the exposure of the pigment layers to continuous outdoor sunlight, fluctuating temperatures and relative humidity, leading to pigment flaking47, and considering the difficulty of controlling the temperature and humidity outdoors, the stability of the reinforcing materials was evaluated under UV radiation. Artificial UV degradation was carried out using a UVB lamp (180 W, 340 nm, OSRAM Co., Ltd., China) for 480 h at a distance of 10 cm, applied to both the films on slides and model painting samples.
SEM and EDX analysis
The samples were mounted on a conductive stage using conductive adhesive and then coated with a thin layer of gold to enhance conductivity. The microstructure was examined, and elemental analysis was performed using a scanning electron microscope coupled with energy-dispersive X-ray spectroscopy (SEM–EDX, SU8020, Hitachi, Japan). The acceleration voltage was set to 10–15 kV, with a beam spot intensity of 30 and a working distance of 8.8 mm.
Fourier-Transform Infrared Spectroscopy (FTIR)
KBr was dried in an oven at 180 °C and dried for 24 h; then, 150 mg of dried KBr was ground as the blank sample. Subsequently, 2 mg of ground pigment samples were mixed with KBr powder, pressed into pellets, and analyzed using an FTIR spectroscope (Thermo Scientific Nicolet iS10, Waltham, USA). The spectra were recorded in the wavelength range of 4000–450 cm−1 with a spectral resolution of 1 cm−1.
X-Ray diffraction
A Rigaku Smart Lab 9 high-resolution X-ray diffractometer (XRD, Rigaku Corporation, Tokyo, Japan) was used for analysis under the following conditions: Cu Kα radiation (λ = 1.54056 Å), 2θ range of 20–80°, an acceleration voltage of 45 kV, a tube current of 200 mA, and a scanning speed of 5°/min.
Contact angle test
The video optical contact angle tester (Dataphysics, OCA20, Stuttgart, Germany) was used to capture images of water droplets, with each droplet containing 2 μL of water. The droplet velocity was set to 1.635 mm/s, and the CCD inclination was 30°. Contact angle measurements were taken at five different spots on the films (F-T/S-1, F-T/S-2, F-T/S-3, and FEVE), and the average values were calculated for analysis.
Ultraviolet near-infrared spectrometer
A UV-vis near-infrared spectrophotometer (PerkinElmer, Lambda 950, USA) was used to collect transmittance (%) of the films across wavelengths ranging from 250–800 nm, as well as UV absorption spectrum of the films across wavelengths ranging from 300–500 nm.
Pencil hardness test
The pencil hardness test is used to determine the hardness of a film by moving pencil tips of known hardness over the coating at a 45° angle to the horizontal with a force of 7.5 N. The results are graded on a 17-point scale, ranging from 9B (softest) to 9H (hardest), based on the standard ISO 15184:2012(E).
Mechanical characterization
The tensile strength was measured 10 times for each type of reinforced paper sample (15 × 1.5 cm) using a universal testing machine (QT-1136PC, Guangzhou, China) with the tensile speed at 10 mm/min, and the average data were used for analysis. This strain rate was chosen due to that it was difficult to test the brittle samples at higher strain rates.
Water vapor transmission property
The water vapor transmission properties were explored for the reinforcement agents according to the standard ISO 12572:2016 with some modifications. Each agent was uniformly applied to model painting samples and left to air dry for 12 h. The coated surfaces of these model painting samples were then positioned over the opening of a collecting bottle, containing 100 mL of deionized water. The interface between the model painting samples and the bottle was sealed using Vaseline to prevent external airflow. Subsequently, this entire setup was placed in an oven and dried at 35 °C for 21 days. To evaluate the water vapor permeability of the reinforcement agents, the weight of the assembly was measured before and after the drying process. This procedure was repeated four times, and the average was calculated.
Anti-fungal experiment
A 500 μL solution of Aspergillus flavus, Aspergillus niger, and Penicillium citrinum (2 × 105 CFU/mL) was evenly smeared on the surface of both reinforced and unreinforced mock samples. The samples were then dried and placed on PDA medium at 28 °C for 3–5 days to observe mold growth, which was recorded. After culturing, the samples were fixed with 2.5% glutaraldehyde, dehydrated using an ethanol gradient (30%–100%), and dried. The samples were then gold-coated and analyzed for microstructure using SEM.
Colorimetric measurements
The CIE LAB system was used to evaluate the color changes of the same spot on the model painting samples before and after UV degradation following the equation48,49
where ∆E is the color difference, ∆L, ∆a, and ∆b denotes lightness change, the degrees of difference in red and green and the degrees of difference in yellow and blue respectively. This measurement was conducted three times, and the average data were used for analysis. Model sample color measurements were carried out according to the standard ISO 7724-1 (2003).
Confocal laser scanning microscopy (CLSM)
The surface topography of the samples was measured using a VK-X250 laser confocal scanning microscope (Keyence, Japan). Each measurement was performed over an area of 1024 × 768 μm². Three measurements were taken for each sample at a magnification of 10× using the Multi-File Analyzer software. Surface roughness parameters were determined in accordance with ISO 25178-2.
Results
Structural characterization of F-T/S
As shown in Fig. 4, the SEM and EDX images of FEVE and F-T/S materials reveal that the FEVE film has a smooth surface, with only C, O, and F elements detected, which are evenly distributed. After modification, the F-T/S film retains a similar surface morphology to the unmodified FEVE film, maintaining its smoothness and clear texture. EDX data indicate that Si and Ti are uniformly distributed across the film surface without forming agglomerates. This suggests that TiO2 and SiO2 modifications to FEVE can be evenly dispersed on the substrate surface without clumping.
a SEM image (2000 ×) of the FEVE film, (b–f) distribution of C, O and F elements, and XRF analysis diagram; (g) SEM image (2000 ×), of the F-T/S-1 film; (h–o) distribution of C, O, F, Si, and Ti elements, and XRF analysis diagram, elements are uniformly distributed on the surface of the F-T/S films.
The FTIR spectra (Fig. 5a) indicate that after modification, the F-T/S material shows a peak around 3500–3000 cm−1, corresponding to the -NH2 group (from the silane coupling agent KH550), and a peak at 3310–3350 cm−1 is attributed to the -NH- group, suggesting that -NH2 bonds to the substrate during after synthesis. A peak near 1090 cm−1 corresponds to the Si-O-Si antisymmetric stretching vibration50,51, and at 800 cm−1, a symmetric stretching vibration of the Si-O bond is observed. The peak around 1374 cm−1 corresponds to Ti-O-Ti absorption, indicating that the modification process does not alter the nanomaterials. Additionally, the C-O stretching vibration in the 1300–1030 cm−1 region shows higher absorption compared to unmodified FEVE, further supporting the successful grafting of nano-TiO₂ and nano-SiO₂ onto FEVE. As shown in Fig. 5b, the synthesis process did not induce any phase changes in nano-TiO2 and nano-SiO2. The F-T/S diffraction pattern displays a wide broad peak at 2θ = 15°, indicating the presence of amorphous organic and polymer phases, and the yellow dashed cycle indicates the presence of TiO2.
Properties of the films
Reinforcement materials for color painting layers should exhibit high transparency to minimize their impact on visual aesthetics52. As shown in Fig. 6a, the transparency of films (FEVE, F-T/S-1, F-T/S-2, F-T/S-3, F-T/S-4) was evaluated in the wavelength range of 300–800 nm. Among them, FEVE exhibited the highest transparency, approaching 100%19, indicating less impact on the visual appearance of the underlying layer. F-T/S-4 had the lowest transparency, suggesting a potentially greater visual impact. Other modified materials (F-T/S-1, F-T/S-2, F-T/S-3) displayed intermediate transparency levels, showing a negative correlation between concentration and transparency.
a Light transmittance of the FEVE and F-T/S films; (b) ultraviolet absorption of t FEVE and F-T/S films; (c) Contact angles of the films before and after 480 hUV degradation (d) pencil hardness and shear strength of films before and after 480-hUV degradation; (e) tensile strength of samples made by coating reinforcement agents on the Xuan paper before and after 480-hUV degradation.
As shown in Fig. 1g, since the color paintings are exposed to intense UV radiation and rainwater outdoors, the surface pigment layers have severely detached. Preventing UV-induced degradation is therefore crucial for their preservation. To assess the UV-resistance performance of the reinforcement films, their UV absorption capacity was measured using a UV-vis spectrophotometer (Fig. 6b). FEVE alone exhibited the lowest UV absorption, while the incorporation of TiO2 significantly enhanced the UV blocking capacity of the F-T/S materials. Among them, F-T/S-4 exhibited the strongest UV absorption, indicating a positive correlation between UV absorption and concentration.
Considering the exposure of the paintings to rainwater or potential leakage in ancient buildings, nano-SiO2 was incorporated into FEVE to improve its hydrophobic performance. Nano-SiO2 has a surface rich in hydroxyl groups53, which, after modification with KH550, forms hydrophobic side chains, thereby enhancing hydrophobicity. Figure 6c shows the contact angles of the coatings, where a larger angle indicates better water resistance. Before degradation, all films exhibited high contact angles. After degradation, the contact angles decreased with a decline in hydrophobicity. Among the samples, FEVE showed the most significant reduction in contact angle (22.6%). F-T/S-1 and F-T/S-2 also experienced decreases of 24.6% and 20.7%, respectively, but F-T/S-2 maintained a relatively high contact angle after degradation (73.45°). F-T/S-3 and F-T/S-4 showed smaller decreases of 10.2% and 7.8%, respectively, with final contact angles of 86.25o and 89.62°, demonstrating excellent hydrophobic performance. Overall, the incorporation of SiO2 in F-T/S materials significantly improved their initial hydrophobicity and effectively mitigated degradation over time. In particular, F-T/S-3 and F-T/S-4, exhibited better durability and hydrophobic stability, making them ideal for applications requiring long-term hydrophobic performance. In contrast, FEVE and F-T/S-1 showed greater hydrophobicity loss after degradation, making them more suitable for environments with relatively lower durability requirements.
As shown in Fig. 6d, the pencil hardness test results before and after UV degradation indicate the mechanical properties of the films. Before degradation, both FEVE and F-T/S-1 exhibited a hardness of 2H, showing good initial surface hardness, while F-T/S-2, F-T/S-3, and F-T/S-4 exhibited a hardness of 3H, indicating enhanced mechanical properties. After UV degradation, the hardness of FEVE and F-T/S-1 decreased to H, while F-T/S-2, F-T/S-3, and F-T/S-4 decreased from 3H to 2H, showing only one-level reduction. This suggests that F-T/S films possess better resistance to mechanical degradation compared to FEVE, demonstrating superior durability and UV degradation resistance. Notably, F-T/S-2, F-T/S-3, and F-T/S-4 retained higher hardness levels after degradation, making them more suitable for applications requiring long-term stability and mechanical strength.
The tensile lap-shear strength tests were conducted to explore the changes in the mechanical properties of different film materials before and after UV degradation (Fig. 6e). Before degradation, all samples had a tensile lap-shear strength of around 6 MPa. After UV degradation, all samples experienced a reduction in strength to varying degrees. FEVE showed the most significant decline, decreasing to 4.91 MPa with a 21.2% drop, indicating relatively poor UV degradation resistance. F-T/S materials demonstrated superior degradation resistance. F-T/S-1 exhibited the best stability, with only a 3.5% reduction (from 6.21 MPa to 5.99 MPa). F-T/S-2 and F-T/S-3 experienced moderate reductions of 5.5% and 4.4%, respectively, while F-T/S-4 had the smallest decrease of 3.3%, maintaining good mechanical stability despite its initially lower strength. These results suggest that F-T/S modifications effectively enhance the UV durability of FEVE, making them more suitable for long-term applications as protective coatings.
Antimycotic
In the study of microbial deterioration on color paintings, Aspergillus and Penicillium species were identified as major pathogens54. Therefore, this study focused on three specific fungi, including Aspergillus niger, Aspergillus flavus, and Penicillium citrinum. F-T/S-1 was evenly applied to the surfaces of three pigment layers (ultramarine, cinnabar, and Paris green), and fungal spore suspensions of A. niger, A. flavus, and P. citrinum were sprayed onto the F-T/S-1 treated pigment layers. After incubation, nearly no fungal growth was observed on the surfaces or edges of the reinforced pigment layers (Fig. 7a). SEM (Fig. 7b) examination further confirmed the absence of significant fungal growth on the reinforced samples. In contrast, untreated pigment layers exhibited substantial fungal colonization, with visible spore clusters observed under SEM. These findings demonstrate that the F-T/S reinforcement material presents good antifungal properties, providing effective protection for color paintings against mold contamination.
Application of coatings to the samples
As shown in Fig. 8, the permeability test data before and after UV degradation demonstrate the changes in the permeability of different modified materials. Before degradation, FEVE exhibited the highest permeability (3.93 g), while the F-T/S materials showed a gradual decrease in permeability from F-T/S-1 (3.91 g) to F-T/S-4 (3.39 g). This suggests that with increasing concentrations of SiO2 and TiO2, the permeability of the materials decreases, possibly due to the densification of the structure caused by the higher concentration of SiO2 and TiO2. After UV degradation, the permeability of all samples increased to varying extents. FEVE showed the largest increase (10.4%), indicating the formation of more microcracks, which substantially enhanced its permeability. In contrast, the F-T/S materials showed smaller changes in permeability, with F-T/S-1 and F-T/S-2 increasing by 5.9% and 3.0%, respectively. F-T/S-3 and F-T/S-4 showed the smallest increases (3.2% and 2.7%, respectively), indicating that higher-concentration formulations demonstrated greater structural stability during degradation. In summary, FEVE showed a significant increase in permeability during degradation, which may affect its long-term stability. The F-T/S materials, particularly F-T/S-3 and F-T/S-4, maintained better permeability resistance.
As shown in Fig. 9, the color difference (ΔE) of three pigment layers (ultramarine, Paris green, and cinnabar) treated with different concentrations of FEVE and F-T/S reinforcement agents was analyzed. After 480 h of UV degradation, samples treated with FEVE led to a significant color change in all three pigment layers. A ΔE value greater than 3 is considered a noticeable color change, and the results suggest that samples treated with F-T/S materials improved the UV absorption ability of the sample treated with FEVE. For the ultramarine pigment layer, the ΔE of the sample treated with FEVE after degradation was 7.39, while the pigment layer treated with F-T/S-3 had the lowest ΔE value (2.64), showing minimal impact on color and the best protective effect. In the Paris green pigment layer, the ΔE value of the sample treated with FEVE after degradation was 4.34, while F-T/S-2 had the lowest ΔE value (2.44). Similarly, for the cinnabar pigment layer, the ΔE value of the sample treated with FEVE was 5.32, and the sample treated with F-T/S-3 had the lowest ΔE value (2.99). The color comparison shown in Fig. 9b further confirms the data. Overall, F-T/S materials show significantly better protective performance than FEVE for the pigment layer, with F-T/S-3 performing best in ultramarine and cinnabar pigment layers, and F-T/S-2 being most effective in Paris green. Among all F-T/S materials, F-T/S-3 is as the most suitable reinforcement agent for maintaining the color integrity of all three pigment layers.
Based on surface roughness (SDR) test results, the effects of different reinforcement agents and their concentrations on the surface properties of three pigment layers (ultramarine, Paris green, and cinnabar) were evaluated. A lower SDR value indicates a smoother surface and better protective effect. For the ultramarine pigment layer (Fig. 10g), FEVE slightly improved surface smoothness, reducing the SDR value from 6.397 to 6.25. Among the ultramarine pigment layer treated with F-T/S materials, F-T/S-2 exhibited the best surface improvement with the lowest SDR value (4.76). In contrast, the pigment layer treated with F-T/S-4 had the highest SDR value (7.55), surpassing that of the untreated group, suggesting that its higher concentration may not be favorable for surface smoothness. In the Paris green pigment layer, FEVE significantly reduced the SDR value to 3.05, while F-T/S-3 achieved the lowest SDR (2.56), indicating optimal surface smoothness. Although F-T/S-4 had a slightly higher SDR value (3.83) compared to other F-T/S formulations, it still performed better than the untreated group. For the cinnabar pigment layer, FEVE treatment slightly increased the SDR value to 5.81, suggesting limited effectiveness in surface smoothness enhancement. F-T/S-3 demonstrated the best performance, reducing the SDR value to 3.23, relatively improving surface smoothness. F-T/S-4 also improved the surface quality, with an SDR value of 4.72, which was slightly higher than F-T/S-3 but still lower than the untreated and FEVE-treated samples. In summary, F-T/S materials significantly present better performance than FEVE in improving the surface smoothness of pigment layers. Among them, F-T/S-3 demonstrates the best overall performance across all three pigment layers, making it the optimal choice for surface optimization and long-term protection. F-T/S-2 also showed a good reinforcement effect, particularly for the ultramarine and Paris green pigment layers.
Discussion
This study evaluates the properties of F-T/S composites, incorporating Nano-SiO₂ and Nano-TiO₂ into FEVE resin, for the conservation of ancient architectural color paintings. The materials were systematically characterized to assess their structural homogeneity and stability. Structural analysis confirmed that the SiO2 and TiO2 nanoparticles were evenly dispersed within the FEVE matrix without forming agglomerates, ensuring a stable and functional composite. XRD and FTIR results showed that the nanoparticles retained their original phase composition, indicating that the modification process did not alter the structure of TiO2 and SiO2, which is crucial for maintaining the performance and longevity of the coatings.
The F-T/S composites demonstrated significant improvements in key properties compared to pure FEVE. The incorporation of Nano-SiO2 and Nano-TiO2 enhanced the hydrophobicity of the films, with higher contact angles observed in F-T/S composites, indicating better water resistance. Additionally, the UV absorption capacity of the modified films was significantly improved, with F-T/S-4 exhibiting the highest UV absorption, demonstrating a direct correlation between TiO2 concentration and UV protection. Mechanical testing revealed that the F-T/S composites displayed higher tensile strength and hardness than FEVE. The antifungal performance of the F-T/S composites indicated that the composites effectively inhibited fungal growth, F-T/S showed promising results in protecting against common fungal pathogens such as Aspergillus niger, Aspergillus flavus, and Penicillium citrinum. In practical applications, the F-T/S composites were tested on model color painting surfaces, where they demonstrated good durability, UV resistance, and protection against undesirable environmental factors. The results showed that F-T/S-3 provided the best overall protection, with minimal impact on the visual appearance of the paintings, making it an ideal candidate for long-term preservation.
In summary, the F-T/S composites, with their enhanced mechanical properties, UV protection, antifungal resistance, and hydrophobicity, offer a promising solution for the conservation of cultural heritage. F-T/S-3, in particular, proves to be the most effective formulation for preserving ancient color paintings. This research provides a valuable foundation for the development of advanced protective coatings in cultural heritage conservation.
Data availability
No datasets were generated or analysed during the current study.
References
Xie, J. Y. H. A Preliminary Study on the Standard of Disease Assessment of the Color Painting of Cultural Heritage Buildings: Taking the Color Painting of Yuhua Pavilion Building Eaves as an Example. 2023:258–260. https://doi.org/10.19875/j.cnki.jzywh.2023.08.085.
Ai, Guo, S. et al. Pigment identification of colored drawings from Wuying Hall of the Imperial Palace by micro-Raman spectroscopy and energy dispersive X-ray spectroscopy. 37, 230–234 (2006).
Chen, E., Zhang, B., Zhao, F., Wang, C. J. H. S. Pigments and binding media of polychrome relics from the central hall of Longju temple in Sichuan, China. 7, 1–8 (2019).
Lei, Z., Wu, W., Shang, G., Wu, Y. & Wang, J. Study on colored pattern pigments of a royal Taoist temple beside the Forbidden City (Beijing, China). Vibrational Spectrosc. 92, 234–244 (2017).
Wang, N., He, L., Zhao, X. & Simon, S. Comparative analysis of eastern and western drying-oil binding media used in polychromic artworks by pyrolysis–gas chromatography/mass spectrometry under the influence of pigments. Microchemical J. 123, 201–210 (2015).
Fu, P. et al Investigation of Ancient Architectural Painting from the Taidong Tomb in the Western Qing Tombs, Hebei, China. Coatings [Internet]. 10, (2020).
Peifan, Q. et al. Study and Restoration of the Yi Ma Wu Hui Layer of the Ancient Coating on the Putuo Zongcheng Temple. Int. J. Architectural Herit. 15, 1707–1721 (2021).
Liu, L. Z. B. & Yang, H. The Analysis of the Colored Paintings from the Yanxi Hall in theForbidden City. 38:2054–2063 (2018).
Ma, Z. et al. Chromatographic, Microscopic, and Spectroscopic Characterization of a Wooden Architectural Painting from the Summer Palace, Beijing, China. Anal. Lett. 52, 1670–1680 (2019).
Zou, W. & Yeo, S.-Y. Investigation on the Painting Materials and Profile Structures Used in Ancient Chinese Folk Architectural Paintings by Multiple Analytical Methods. Coatings [Internet]. 12, (2022).
Fierascu, R. C., Doni, M. & Fierascu, I. Selected Aspects Regarding the Restoration/Conservation of Traditional Wood and Masonry Building Materials: A Short Overview of the Last Decade Findings. 10,1164 (2020).
Wei, Y. & Dong, X. The protection and effect of ancient architecture painting in Shanshan Guild Hall, Luoyang :26-8+5+84 (2011).
Zhu, J. et al. Nano Ca(OH)2: A review on synthesis, properties and applications. J. Cultural Herit. 50, 25–42 (2021).
Chen, W., Dai, P., Yuan, P. & Zhang, J. Effect of inorganic silicate consolidation on the mechanical and durability performance of sandstone used in historical sites. Constr. Build. Mater. 121, 445–452 (2016).
Ossola, F. et al. New calcium alkoxides for consolidation of carbonate rocks. Influence of precursors’ characteristics on morphology, crystalline phase and consolidation effects. 36, 2618–2624 (2012).
Xie, A., Mao, S.-W., Chen, T.-J., Yang, H. & Zhang M. J. R. M. Microstructure and properties of cerium oxide/polyurethane elastomer composites. 40, 3685–3693 (2021).
Yang, Y. & Fei, X. Detection and protection of painted wood parts of Yanzong Temple in Yangzhou. 88–95 (2022).
Abdel-Haliem, M. E. F., Sakr, A. A., Ali, M. F., Ghaly, M. F. & Sohlenkamp, C. Characterization of Streptomyces isolates causing colour changes of mural paintings in ancient Egyptian tombs. Microbiological Res. 168, 428–437 (2013).
Han, K. et al. Evaluation of commonly used reinforcement materials for color paintings on ancient wooden architecture in China. 12,122 (2024).
Ntelia, E. & Karapanagiotis, I. Superhydrophobic Paraloid B72. Prog. Org. Coat. 139, 105224 (2020).
Zhang, Y., Li, X., Zhu, J., Wang, S. & Wei, B. Hybrids of CNTs and acrylic emulsion for the consolidation of wall paintings. Prog. Org. Coat. 124, 185–192 (2018).
Carretti, E. & Dei, L. Physicochemical characterization of acrylic polymeric resins coating porous materials of artistic interest. Prog. Org. Coat. 49, 282–289 (2004).
Fu, P. et al. Modified Graphene-FEVE Composite Coatings: Application in the Repair of Ancient Architectural Color Paintings. Coatings [Internet]. 10, (2020).
LIi, Y. Z. L. & Wang, L. Characterization, properties of polyvinyl acetate emulsion and its application in the conservation of painted cultural relics. 33, 22–32. https://doi.org/10.16334/j.cnki.cn31-1652/k.20201201987 (2021).
Liu, J. et al. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 113, 101353 (2021).
Rodriguez-Mella, Y., López-Morán, T., López-Quintela, M. A. & Lazzari, M. Durability of an industrial epoxy vinyl ester resin used for the fabrication of a contemporary art sculpture. Polym. Degrad. Stab. 107, 277–284 (2014).
Chen, Z. et al. Application of epoxy resin in cultural relics protection. Chin. Chem. Lett. 35, 109194 (2024).
Xu, J. et al. Nano-silica/fluorinated polyacrylate composites as surface protective coatings for simulated stone cultural relic protection. 139, e52953 (2022).
Zhang, X WX. & Dai, W. Organosilicon in situ polymerization formed organic-inorganic hybrid network structure to protect highly deteriorated ancient wood. 60–67 (2024).
Zhu, Z. Q. J., Duan, H. & Cao, Y. Study on the sealing material of modified fluororesin stone cultural relics. 39–43 https://doi.org/10.14030/j.cnki.scaa.2007.05.012 (2007).
Ma, C., Liang, Q., Kou, C., Zhang, H. & Wang, H. J. P. S., Series B. Preparation of Poly (methyl methacrylate-co-butyl acrylate) Modified by Vinyltriethoxysilane and Its Properties for Stone Relics Protection. 64, 725–732 (2022).
Baglioni, P. & Giorgi, R. Soft and hard nanomaterials for restoration and conservation of cultural heritage. Soft Matter 2, 293–303 (2006).
Colangiuli, D., Lettieri, M., Masieri, M. & Calia, AJSotTE. Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. 650, 2919–2930 (2019).
Yu, X. et al. SiO2 nanoparticle-based superhydrophobic spray and multi-functional surfaces by a facile and scalable method. Ceram. Int. 45, 15741–15744 (2019).
Munafò, P., Goffredo, G. B. & Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 84, 201–218 (2015).
Azadi, N., Parsimehr, H. & Ershad-Langroudi, A. J. P. Rubber, Composites. Cultural heritage protection via hybrid nanocomposite coating. 49,414–424 (2020).
Shao, Y. et al. Antimicrobial protection of two controlled release silver nanoparticles on simulated silk cultural relic. J. Colloid Interface Sci. 652, 901–911 (2023).
Li, J., Ma, R., Wu, P. & Quan, M. CMC-Ca(OH)2-TiO2 Nanocomposite for Paper Relics Multifunctional Restoration: Strengthening, Deacidification, UV Effect Resistance, and Antimicrobial Protection. Coatings [Internet]. 14 (2024).
Takayanagi, T. & Yamabe, MJPiOC. Progress of fluoropolymers on coating applications: Development of mineral spirit soluble polymer and aqueous dispersion. 40, 185–190 (2000).
Sumi, N., Kimura, I., Ataku, M., Maekawa, T. & Parker, RJJCT. New Advances in Waterborne Fluoropolymer Technology: A Water-based Dispersion for Industrial Coatings Formulations. 6, 24–28 (2009).
Tian, S., Gao, W., Liu, Y., Kang, W. & Yang, H. Effects of surface modification Nano-SiO2 and its combination with surfactant on interfacial tension and emulsion stability. Colloids Surf. A: Physicochemical Eng. Asp. 595, 124682 (2020).
Zhao, J. et al. Construction of a durable superhydrophobic surface based on the oxygen inhibition layer of organosilicon resins. Thin Solid Films. 717, 138467 (2021).
Zheng, Y. et al. Innovative natural antimicrobial natamycin incorporated titanium dioxide (nano-TiO2)/ poly (butylene adipate-co-terephthalate) (PBAT) /poly (lactic acid) (PLA) biodegradable active film (NTP@PLA) and application in grape preservation. Food Chem. 400, 134100 (2023).
Pan, Y. et al. Enhanced Bacterial and Biofilm Adhesion Resistance of ALD Nano-TiO2 Coatings Compared to AO Coatings on Titanium Abutments. Int. J. Nanomed. 19, 11143–11159 (2024).
Park, E. J., Sim, J. K., Jeong, M.-G., Seo, H. O. & Kim, Y. D. Transparent and superhydrophobic films prepared with polydimethylsiloxane-coated silica nanoparticles. RSC Adv. 3, 12571–12576 (2013).
Kim, S.-K., Wang, X., Ando, S. & Wang, X. Highly transparent triethoxysilane-terminated copolyimide and its SiO2 composite with enhanced thermal stability and reduced thermal expansion. Eur. Polym. J. 64, 206–214 (2015).
Mistretta, M. C. et al. Durability of Biodegradable Polymers for the Conservation of Cultural Heritage. Front. Mater. 6 https://doi.org/10.3389/fmats.2019.00151 (2019).
Bourguignon, E. et al. Calcium alkoxides as alternative consolidants for wall paintings: Evaluation of their performance in laboratory and on site, on model and original samples, in comparison to conventional products. J. Cultural Herit. 29, 54–66 (2018).
Ibrahim, M. M., Mohamed, S. O., Hefni, Y. K. & Ahmed, A. I. Nanomaterials for Consolidation and Protection of Egyptian Faience form Matteria, Egypt. J. Nano Res. 56, 39–48 (2019).
Ling, L., Bu, L., Jie, D. & Junping, Z. Roles of silanes and silicones in forming superhydrophobic and superoleophobic materials. https://doi.org/10.1039/C6TA05441B (2016).
Li, Y., Zhang, Z., Wang, M., Men, X. & Xue, Q JJmca. Environmentally-Safe, Substrate-Independent and Repairable Nanoporous Coatings: Large-Scale Preparation, High Transparency and Antifouling Property. https://doi.org/10.1039/C7TA05112C (2017).
Bagniuk, J., Białek-Kostecka, D., Forczek-Sajdak, A., Kaszowska, Z. & Skrzelowska J. J. C. R, Application. Color changes in wall paintings under the influence of consolidation with synthetic polymers. 44, 772–782 (2019).
Liu, T. et al. Superhydrophobicity and regeneration of PVDF/SiO2 composite films. Appl. Surf. Sci. 396, 1443–1449 (2017).
Teri, G. Study on characteristic analysis and control of mold disease in Wenhua Hall of the Imperial Palace [master] (2020).
Acknowledgements
This research was financially supported by the Fundamental Research Funds for the Central Universities (GK202304013), Shaanxi Key Research and Development Program of China (2024GX-YBXM-560), Youth Open Project from Nanjing Museum (NBKFKT0101) and Emperor Qinshihuang’s Mausoleum Site Museum (Qkfkt202407). Special thanks go to the Fundamental Innovation Project in the School of Materials Science and Engineering (SNNU), National Natural Science Foundation of China (No. 22102094).
Author information
Authors and Affiliations
Contributions
Conceptualization, K.H.; resources, Z.B.; software, investigation, formal analysis. D.H.; and X.L.; methodology, data curation, K.L.; and H.Y.; writing—original draft preparation, M.G.; and Y.R.; writing—review and editing, Y.L. (Yuhu Li); visualization, B.M.; supervision, project administration, and funding acquisition, Y. L. (Yujia Luo).; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, K., Ge, M., Li, K. et al. Conservation of color paintings on the Rain Flower Pavilion using a multi-functional composite coating. npj Herit. Sci. 13, 266 (2025). https://doi.org/10.1038/s40494-025-01812-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s40494-025-01812-w