Abstract
Background
The treatment of obesity is complex and requires long-term multidisciplinary care. While behavioral, pharmacological and psychological therapies are integral, bariatric surgery remains the most effective intervention. Therapeutic success is influenced by factors such as comorbidities and potentially by the experience of the treatment facilities. This rapid review evaluates the evidence of volume-outcome associations in bariatric surgery, focusing on the endpoint of mortality.
Methods
We performed a rapid review of the literature published after 2000, including adult patients (≥18 years) who underwent bariatric surgery for weight loss, or weight loss and diabetes management. Searches across EMBASE, MEDLINE, PubMed, and Cochrane Trials yielded 3540 records. The primary outcome was mortality; secondary outcomes included complications, morbidity and hospital stay. Mortality results were stratified by type of mortality and synthesized according to volume type (hospital or surgeon). Study quality was assessed using the ISPOR and ROBINS-E tools. An additional synthesis was conducted for studies above a quality score cutoff.
Results
Thirty-six studies met the inclusion criteria. Of these, 12 studies examined the association between hospital volume and mortality, and five studies focused on surgeon volume. Eight of the hospital volume studies and four of the surgeon volume studies reported a positive association with reduced mortality; some showed mixed results. Most studies also linked higher volumes to fewer complications and shorter hospital stays. However, the focus was predominantly on hospital volume and short-term mortality, with limited evaluations of long-term outcomes or weight loss success. Overall study quality varied, with noted limitations including arbitrary volume thresholds.
Conclusion
Higher hospital and surgeon volumes were associated with lower short-term mortality, fewer complications and shorter hospital stays. The association was more pronounced in higher-quality studies. Future research should aim to standardize volume definitions to improve comparability and support policy efforts to centralize care and enhance patient outcomes.
Similar content being viewed by others
Introduction
Obesity is a growing global health concern. As of 2022, one in eight people worldwide was living with obesity [1]. It is associated with a substantial loss of disability-free life years and increased premature mortality [2], estimated to reduce life expectancy by 2–4 years and to contribute to higher excess mortality than smoking [3]. The treatment management of obesity is complex and typically involves long-term multidisciplinary care across multiple medical disciplines and the integration of pharmacological and psychological therapies [4]. To date, bariatric surgery has emerged as the most effective therapeutic component for the treatment of obesity [5] and its associated diseases, such as diabetes type 2. While mortality rates are low [6], the effective treatment of obesity is complicated by its related comorbidities.
Postoperative complications occur in approximately 5–10% of patients and include bleeding, anastomotic leak, and infection [7]. Moreover, obesity itself is a risk factor for adverse short-term surgical events [8], and long-term postoperative mortality is mediated by accompanying comorbidities [9]. Several European countries—including Austria, Switzerland, Denmark, the United Kingdom and the Netherlands—have implemented minimum volume standards for bariatric surgery. Aiming to improve surgical outcomes and reduce complications and mortality, those standards range from 25 to 200 procedures a year and hospital, however with varying types of procedures involved [5, 10, 11]. These policy decisions were informed by studies reporting reduced mortality rates and complications when surgeries are performed by experienced surgeons and high-volume hospitals. For instance, a U.S. study of 14,716 patients reported lower odds ratios of hospital and short-term mortality in hospitals performing more than 100 procedures annually, compared to those performing fewer than 50 [12]. Similarly, another US study involving 14,714 patients from a retrospective registry identified a significant interaction between hospital and surgeon volume, further supporting the volume-outcome relationship, with high-volume hospitals defined as performing ≥300 procedures annually [13]. A large Scandinavian registry study of 49,977 bariatric surgeries reported improved composite hospital mortality and 90-day-reintervention rates for medium volumes (7–25 procedures/year), although no significant effect was observed for higher volumes [14].
Multiple studies with large sample sizes have also demonstrated significant associations between higher hospital volume and reduced perioperative morbidity, including lower rates of readmissions, reoperations, and specific complications [15,16,17,18]. Length of stay (LOS) was reported to be significantly shorter in high-volume hospitals and when surgeries were performed by high-volume surgeons [16, 19, 20].
While this volume-outcome relationship has been assessed in several studies, only a limited number of up-to-date systematic reviews are currently available. An umbrella review published in 2016 [21] summarizes three systematic reviews, including a health technology assessment from 2011 [22, 23] and 2012 [24], concluding that hospital and surgeon volume were inversely related to outcomes. However, these earlier works were partially focused on economic evaluation, did not capture the evidence published over the past decade and representing a gap in contemporary evidence, during which time both the volume and nature of bariatric procedures have evolved [25]. Additionally, in 2016 the International Federation for the Surgery of Obesity and Metabolic Disorders (ISFO) issued a joined statement recommending the consideration of weight loss surgery for the management of diabetes, thereby redefining the indications for bariatric procedures [26]. Since the introduction of laparoscopic techniques in 2000 [27], this review aims to systematically assess, update, and synthesize the evidence on the volume–outcome relationship in bariatric surgery generated over the past 23 years, thus addressing a gap in the current literature.
Methods
We conducted a rapid review aiming to inform clinicians and health policy decision makers. This type of review was chosen against the backdrop of hospital reform in Germany, where hospital planning will be based on defined services in the future. Bariatric surgery is one of these defined services [28, 29]. We applied Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 [30], slightly adapted for rapid reviews based on the updated guidance on methods used in Cochrane rapid reviews methods guidance for rapid reviews of effectiveness [31]. The review was prospectively registered at PROSPERO platform (CRD42023398566) [32].
Eligibility criteria
The following inclusion criteria were applied:
Population
Adult patients (≥18 years) undergoing invasive bariatric procedures for weight loss with or without diabetes. Stratification by indication was not performed, as available evidence suggests similar effects on mortality and life expectancy regardless of diabetes status [33]. Studies were excluded if more than 10% of the study population had malignant disease and the results were reported jointly for cancer and non-cancer patients. Studies with ≤10% of patients with malignancies were included even if data were not reported separately.
Intervention/exposure
The relative volume of invasive bariatric procedures was investigated at the hospital and surgeon level. This included the relative volume of high-volume surgeons compared to other surgeons or high-volume hospitals compared to hospitals with lower volumes of surgery procedures. Studies were excluded if more than 10% of the study population received other interventions and the results were only reported jointly. Studies with ≤10% of patients receiving other interventions were included even if data were not reported separately.
Context
Studies comparing at least two hospitals or surgeons in terms of bariatric surgery volume, explicitly distinguishing between high- and low-volume providers: (1) Low-volume hospitals vs. high-volume hospitals, and (2) Low surgeon caseload vs. high surgeon caseload.
Outcomes
Studies were included if they reported at least one of the following:
-
(1)
Mortality: all-cause mortality across any timeframe, including hospital mortality (perioperative, in-hospital mortality, in-hospital mortality among patients with complications (failure-to-rescue (FTR)), short-term (all cause 30-/90-day), and intermediate/long-term all-cause (1-/5-year) mortality.
-
(2)
Perioperative morbidity: adverse events of the intervention, re-intervention, re-admission and complications.
-
(3)
Disease-related morbidity: (long-term) weight/body mass index (BMI) reduction, comorbidity-related readmission rates (e.g., diabetes, arterial hypertension, sleep apnea).
-
(4)
Health-related quality of life (HRQoL): measured by validated instruments.
-
(5)
Length of stay (LOS) in hospital or intensive care units (ICU).
Study design
Eligible designs included randomized controlled trials (RCTs), controlled observational or interventional studies or trials (retrospective and prospective cohort studies). Systematic reviews were screened to identify relevant references.
Other
Only peer-reviewed publications from the year 2000 onward, in English or German were included. Full-text availability was required.
Information sources and search strategy
A systematic literature search was conducted in PubMed via Pubmed, the Cochrane Library, EMBASE via Ovid and MEDLINE via Ovid on June 25, 2023. The search strategy was first developed in PubMed and subsequently adapted for the other databases. It combined controlled vocabulary—using Medical Subject Headings (MeSH) terms in PubMed/MEDLINE and the Cochrane Library, and Emtree terms in Embase—with free-text synonyms to capture hospital/surgeon volume (e.g., “Hospitals, High-Volume,” “surgical volume”), bariatric surgery (e.g., “Bariatric Surgery,” “gastric bypass”), obesity (e.g., “Obesity,” “overweight”), and diabetes (e.g., “Diabetes Mellitus,” “type 2 diabetes”). Logical operators, proximity searches, wildcards, and truncation were applied, with no restrictions on language or publication date. Exclusions were limited to non-human studies and non-research publication types (e.g., case reports, editorials). Full search details are provided in Appendix 1. Reference lists of eligible systematic reviews were cross-checked to ensure that all relevant studies were identified.
Selection process
Following the methods of the Cochrane Rapid Reviews Methods Group, two reviewers (AC, CH) independently screened the titles and abstracts of a randomly selected 20% sample of search results using EndNote9.1. If agreement between reviewers was at least 90%, one reviewer screened the remaining records; otherwise, another 10% sample would have been screened until a 90% agreement was reached. One reviewer screened the remaining abstracts (AC). Full texts of all potentially relevant records were retrieved and screened by one reviewer (AC) of the research team against the review inclusion criteria. Any uncertainties in the review process were resolved through consultation with a second reviewer (CH). Studies excluded from full-text screening are found in Appendix 2.
Data extraction and synthesis preparation
An Excel-based data extraction form was developed and piloted. One reviewer performed data extraction (AC). A second reviewer (CH) verified data accuracy. In case of uncertainties or missing data, the study authors were contacted via correspondence email. Discrepancies were solved through discussion.
Data items extracted:
-
Basic study characteristics (e.g., country, study period, data source, sample size, surgery type, statistics and covariables).
-
Volume definitions as main exposure of interest: (e.g., volume categories, case volume cutoffs or averages), as well as number of patients and number of units per case volume category.
-
Outcomes and effect estimates (e.g., odds ratios (OR), risk ratios (RR), hazard ratios (HR), regression weights or percentages) and confidence intervals (CI) with the connected volume definition and reference categories, if reported. P-values were extracted for univariable results and in case CI were not reported.
-
Model type (e.g., univariate or multivariate), with a focus on full models when multiple analyses were reported.
-
Mortality outcomes were extracted and tabulated in three categories in accordance with PROSPERO protocol: (1) Hospital mortality; (2) short-term mortality; (3) intermediate/long-term mortality. Additional outcomes included (1) perioperative morbidity; (2) disease-related morbidity; (3) HRQoL; (4) LOS in hospital or ICU use.
Synthesis method
We conducted a structured synthesis to summarize the findings of included studies according to the Synthesis Without Meta-analysis (SWiM) guideline [34]. We did not conduct a meta-analysis due to clinically and methodologically insufficiently homogenous studies, similarly to other systematic reviews [24]. Findings were synthesized by outcome type and stratified by volume category (hospital or surgeon or both). Mortality was the primary endpoint, and results were prioritized accordingly. Quality scores (see below) were used to rank studies, with high-quality studies appearing at the top of the summary tables. Separate analyses by mortality type were conducted to compare results from higher-quality studies according to their certainty and confidence.
Risk of bias and reporting bias assessment
To evaluate the quality of included studies, the checklist of the International Society for Pharmacoeconomics and Outcome Research (ISPOR) was used [35]. The checklist contains 27 questions that focus on challenges specific to retrospective databases, disease registries and national survey data, considering the domains of the database, study methodology and study conclusions on the study level. Questions are scored as “Yes”, “Partially”, “No”, or “Not Applicable” (NA). As this instrument lacks a summary for the aggregated judgment measure, response counts for each study are listed next to their results. To evaluate risk of bias in the selection of the reported results, domain 7 of the ROBINS-E (Risk of Bias in Non-randomized Studies—of Exposure; Domain 7: Risk of bias in selection of the reported result) was applied for the exposure of being treated by surgeons or hospitals with their respective volumes [36]. This domain comprises five items on selective reporting with the response options “Yes”, “Probably Yes”, “Probably No”, “No”, or “No Information”. Item 7.1 (reporting in accordance with an available, pre-determined analysis plan) was uniformly assessed as “No Information” as none of the studies included such a plan. This is in accordance with the algorithms’ bias judgment, which assesses a study to “low risk”, “some concerns”, “high risk” or “very high” risk of reporting bias. The assessments were performed by one researcher (AC). Uncertainties were solved through discussion with a second researcher (CH).
To assess overall quality, the ISPOR checklist was appended with the ROBINS-E (domain 7) judgment, as the ISPOR checklist lacks a comprehensive appraisal of reporting quality. A composite Quality Score was calculated using an aggregate of ISPOR points and ROBINS-E Risk:
Outcomes were reported with both assessments and ranked within each volume-stratum (hospital or surgeon) based on the composite score to guide synthesis.
Results
Study selection
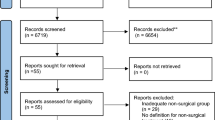
The literature extraction encompassed n = 3540 records for screening. After removal of duplicates and title/abstract screening, 75 titles were assessed with full-text screening, n = 36 publications met the inclusion criteria and were included in the review (Fig. 1). The most common reason for exclusion was wrong context.
Intervention sample sizes spanned between n = 933 and a maximum of n = 446,127. Among the included studies, 20 focused on hospital volume, eight on surgeon volume, and eight assessed both hospital and surgeon volume. Most studies came from the United States (69.4%), followed by Canada (8.3%). Other countries, including Denmark, Sweden, Finland, Italy, Australia, Taiwan and Brazil contributed single figures in terms of publications. Samples date back to the 1980s in two papers, while the most recent populations were sampled in 2018. All but two studies—both authored by the same first author—used retrospective databases or registries (the Longitudinal Assessment of Bariatric Surgery (LABS) clinical cohort from 2005–2007) [37, 38].
An overview of case volume categories, their case volume and corresponding units per case volume categories is provided in Appendix 3.
Table 1A–C shows the study characteristics by volume type, including the number of hospitals or surgeons involved (units), surgical indications and procedures, as well as statistics deployed for analysis and covariables.
Association between hospital/surgeon volume and mortality, incl. quality appraisal
In total, 17 studies reported mortality outcomes. Results were stratified by volume type and tabulated with their ISPOR and ROBINS-E score. Studies that analyzed both hospital and surgeon volume appear twice in the tables and are marked with an asterisk (*). Based on inference-based findings (i.e., reporting confidence intervals or p values), Tables 2 and 3 show a total of 33 effect estimates, 23 related to hospital volume, seven related to surgeon volume, and one study addressing the interaction between surgeon and hospital volume for two mortality outcomes appears in both tables [13]. Two studies presented non-inference-based results, which were therefore excluded from the quantitative synthesis [39, 40]. Among the 12 hospital volume studies with inferential analysis, eight found significant associations between higher volume and lower mortality, while two studies reported mixed results [41, 42]. Regarding surgeon volume, four out of five studies found a significant volume-mortality association. One study, which assessed combined mortality and morbidity, reported mixed findings [42]. One study was excluded from the synthesis because it employed an interaction term between hospital and surgeon volume that could not be clearly assigned to either category. However, that study reports significant associations between hospital and short-term mortality for the interactions of surgeon and hospital volumes [13].
For studies analyzing hospital volume, mixed effects were found in an earlier 2007 study [41]. Reported results were stratified by procedures, with significant hospital-volume effects observed for overall short-term mortality and vertical-banded gastroplasty (VBG) procedures, but not for other procedures. However, the inference was based on univariable tests of percentages and lacks adjustment for confounding variables. A similar limitation was noted in a 2005 study, which did not find a statistically significant effect [43]. A more recent large population-based registry study found a significant effect, but the exposure pattern was inconsistent: medium-volume hospitals were associated with significantly lower mortality compared to low-volume hospitals, while high-volume hospitals showed no significant difference [14]. Two studies reported composite outcomes including death, with the former contributing mixed effects by different, surgery-specific volume definitions [42, 44]. Although two other older studies did not report any inferential statistics, one reported a lower percentage of hospital mortality in higher-volume hospitals, while the other observed no meaningful variation in mortality percentages across volume levels [39, 40].
Studies analyzing surgeon volume partly overlapped with those on hospital volume. Two publications by the same author used composite endpoints including death, both demonstrating significant volume-outcome effects [37, 38]. These studies were based on the same sample but applied different volume definitions. In addition to these, several studies that showed significant hospital volume effects also reported significant surgeon volume associations [12, 13]. The only study rejecting an association found non-significant effects for 90-day major composite mortality and morbidity following sleeve gastrectomy (SG), for both surgeon and hospital volume [42]. An older 2009 study initially planned to assess both hospital and surgeon volume, but later excluded hospital volume from modeling process [45]; therefore, it is included only under surgeon volume results.
Mortality by mortality type
Figure 2 summarizes study results by type of mortality outcome—categorized as in-hospital, short-term, or intermediate/long-term mortality—and by overall study conclusion (significant, non-significant, mixed, or unknown when no inference was available). Studies were grouped by hospital volume and surgeon volume. This additional counting method was produced to decrease the effect of multiple reported results (here as “mixed”) and provide a more balanced overview on the evidence. In total, two studies reported no significant association between hospital volume and in-hospital mortality. In contrast, eight studies identified a positive relationship, while two studies lacked inferential statistics and were categorized as unknown. Only one study measured intermediate/long-term mortality in relation to hospital volume. Mixed results were found in three studies: two examining short-term mortality using hospital volume, and one using surgeon volume. Overall, the evidence tends to support a significant volume-outcome relationship. As a sensitivity analysis, studies with unknown effects may conservatively be treated as non-significant to assess the robustness of overall conclusions.
High-quality studies
After isolating high-quality studies based on predefined quality scoring criteria, a total of three surgeon volume and nine hospital volume entries remained. Notably, all three studies in the surgeon volume category also included analyses of hospital volume. Among the nine hospital volume studies, one showed mixed results [42], one did not demonstrate a clear exposure-outcome relationship [14], and one lacked inferential analysis and was limited to descriptive statistics [39]. Overall, restricting the synthesis to higher-quality studies as defined in this review supports a consistent volume-mortality relationship, indicating that higher provider volume is associated with better mortality outcomes. This is further corroborated by restricting the analysis to studies employing adjusted models, with those studies exceeding the quality score cutoff delineated by a bold line (Appendix 4).
Hospital and surgeon volume: outcome associations and quality appraisal
Perioperative morbidity was significantly related to hospital volume in ten studies. Six studies reported mixed effects, while two studies reported no association. Mixed effects were usually related to analysis of different complications (e.g., specific vs. overall), re-operations or some differing volume definitions [17, 20, 39, 46,47,48]. Two studies lacked a clear exposure pattern, one of which also failed to demonstrate such a pattern for mortality outcomes [14, 39]. A large U.S. study including 12 states (2014) found significant associations for overall complications, but none for reoperation [39]. A 2004 study focusing on Roux-En-Y gastric bypass (RYGB) procedures reported significant effects for overall complications, and single complications such as pulmonary, medical, wound complications, and readmission, but not for pneumonia, thrombosis or embolisms, and postprocedural hemorrhage [47]. The study with the largest sample size, also from the U.S., found significant associations between volume and serious complications for both laparoscopic adjustable gastric banding (LAGB) and LRYGB volume. However, significant volume–reoperation associations were only found for LAGB in the time-blocks of 2006–2007 and 2008–2009, but not in 2010–2011 [48]. All four studies using disease-related morbidity found a significant association with hospital volume [16, 43, 49, 50]. However, only one of them examined specific morbidity outcomes (total weight loss after 1 year) [16]. LOS was significantly associated with hospital volume in seven studies [12, 16, 19, 43, 47, 51, 52], while one study did not find a significant association [15], and another provided non-interpretable results [45].
For surgeon volume, three studies found a significant association with perioperative morbidity. The other three reported mixed results. A recent 2021 study using a large U.S. database of RYGB procedures found significant improvement in complications when using aggregate complications, but none of the single complications resulted in significance [53]. Only one study using a large sample from Canada analyzed overall disease-related morbidity in relation to surgeon volume, reporting a significant association [54]. All six studies assessing LOS in relation to surgeon volume found significant associations [12, 19, 20, 45, 52, 55].
No studies in either volume category used validated quality of life measures. A comprehensive overview of additional outcomes and quality appraisal details is provided in Appendix 5. Furthermore, Appendix 4 provides an overview of studies using adjusted models across all outcome domains.
Quality assessment of included studies
The median quality score across all studies, as described in Section “Risk of bias and reporting bias assessment”, was 11.5 points. A total of 17 studies exceeded this cutoff and were classified as “higher quality”, including nine studies addressing mortality-related outcomes. Assessment of reporting quality using ROBINS-E (domain 7) demonstrated variability. Three studies were identified with a very high risk of reporting bias. Twenty-two studies were assessed at high risk of reporting bias. In contrast, 11 studies were evaluated as having a low risk of bias. Study quality, including reporting quality, suffered from a lack of justification or validation of exposure definitions. Only few studies comprehensively reported the numeric volume ranges or cutoffs, contextualized these with specific nomenclature (e.g., “high”; “middle” or “low”), and provided justifying citations, sensitivity analyses or explanation for their choices such as data- or context-driven approaches to defining volume categories. Notably, several ISPOR quality assessment items emerged as critical constraints on study quality. These included the description of reliability, validity and quality checks, the rationale for study design selection, dealing with censoring and missing data, explanation of statistical modeling approaches, discussion of influential cases, testing of statistical assumptions, dealing with multiple tests and model prediction assessment.
Discussion
This rapid review provides updated evidence on the volume-outcome relationship in bariatric surgery for adults indicated for weight loss with or without diabetes. Our results suggest better outcomes—specifically lower mortality and complication rates—when surgery is performed at centers and/or by surgeons with higher volumes. This correlation aligns with the “practice makes perfect” hypothesis, which suggests that increased procedural volume enhances technical skills, fosters greater familiarity with complex conditions, and improves the management of complications [56]. Furthermore, high-volume hospitals are more likely to have access to superior resources and infrastructure, as they are more often located in urban areas and tend to attract more referrals and patient visits—factors that, in turn, further improve outcomes [57]. Remarkably, none of the studies included assessed quality of life as an outcome. Consequently, the body of evidence can map outcomes such as mortality and complications but remains partially blind to patient-reported outcomes that are relevant to the therapeutic goals of multidisciplinary obesity management, highlighting a gap in the evaluation of patient-centered measures. Future research may address this gap by employing validated instruments, such as the EQ-5D-5L for overall health related quality of life, SF-36 or obesity surgery-specific instruments like the Bariatric Analysis and Reporting Outcome System (BAROS), which assess weight loss, comorbidities, and quality of life [58].
The review also points to methodological concerns in the studies included. Some studies evaluated mortality outcomes using aggregate measures that included mortality among other endpoints [37, 38, 42, 44]. While this aggregation increases statistical power, it diminishes the ability to discern which specific outcomes are influenced by surgical volume. Nevertheless, this issue was limited to a subset of studies, and the overall conclusions remained robust when these were excluded. Moreover, most studies primarily focused on short-term or hospital-based mortality, with limited exploration of intermediate or long-term mortality. Similarly, disease-related morbidity was assessed less frequently compared to perioperative morbidity. The predominant focus on short-term outcomes likely reflects data availability and incomplete follow-up in registries but restricts insights into volume-outcome relationships to certain outcome domains. Among adjusted studies, those using morbidity as an outcome reported positive associations with hospital volume in both categorical and continuous analyses. Lower-quality studies more frequently yielded mixed results, typically due to some complications lacking statistical significance. These studies more often assessed surgeon volume, while the highest quality study analyzing morbidity reported significant associations with surgeon volume of LRYGB and sleeve gastrectomy (LSG) [20]. Only a few studies on disease-related morbidity met the high-quality threshold; one of such study used a composite of mortality and various complications, showing statistically significant associations for hospital volumes of SG procedures only [42]. Two lower-quality studies, both below the quality threshold, examining the association between disease-related morbidity and surgeon volume, reported one significant [54] and one non-significant association [50] for different but overlapping procedures. Across both lower and higher-quality studies, LOS was significantly associated with surgical and hospital volume, whether analyzed incrementally or categorically. It was also observed that mortality studies were generally older compared to those assessing complications or morbidity, indicating a shift in research focus over time. Despite some overlap with older systematic reviews [22,23,24], this rapid review includes more recent studies of higher quality, providing updated insights into the volume-outcome relationship.
One notable limitation of this review is the lack of standardized volume categorizations across studies. Many reports did not employ literature-based or data-driven categorizations of volumes, complicating the interpretation of volume-outcome correlations. This aspect contributed to a downgrade in quality during the appraisal. Certain studies defined volume according to policy-relevant criteria, such as the Bariatric Surgery Center of Excellence criteria recommendations [13, 19, 54, 59], applied changepoint analysis [20], or performed sensitivity analyses [60]. Analyses restricted to lower volumes may fail to detect effects that manifest only beyond a certain threshold, while high cutoffs can exceed the point of diminishing marginal returns in the volume-outcome association, as shown in one included study [20]. However, this rapid review observed evidence in favor of higher volumes across various volume definitions, including different forms of marginal analyses (Appendix 4). Studies with higher quality were more likely to use data-driven or policy-oriented approaches and transparently report volume thresholds, positively influencing their assessment under ROBINS-E criteria. Approaches not identified in the included literature encompass spline models, receiver-operator curves, and chi-square automatic interaction detection algorithms, as exemplified in a nationwide 2019 study of digestive cancer surgery [61]. While these data-driven methods can detect cutoffs, their generalizability may be limited when applied to the same dataset used for effect estimation.
The absence of causal frameworks, such as propensity score matching methods, in the included studies restricts their findings to associative results. Furthermore, this analysis is not equipped to recommend specific cutoff points for minimum volume standards, nor to synthesize the magnitude or clinical significance of effects. The heterogeneity in volume categorization, procedures, adjustment variables, and statistical modeling approaches across studies complicates estimation of these sources of variation. Future research should aim to quantify clinical benefit and identify policy-relevant volume thresholds using meta-analytic approaches.
Some studies employed limited covariables [40] and basic descriptive analyses [47, 55], while others used extensive adjustment for patient risk factors, facility characteristics or surgeon-specific variables, and morbidity-related variables, even accounting for time, cohort or cluster effects in their models [16, 39, 59]. However, even studies deploying a broader range of adjustments may be subject to residual confounding, as micro-level patient characteristics are often not captured in registries and hence remain unaccounted for. While the quality appraisal based on ISPOR checklist combined with ROBINS-E instrument contains assessments of data source reliability, validity, and reporting, the underreporting of adverse events, such as complications or morbidity, cannot be excluded. Items receiving negative assessments often concerned data quality checks, the discussion of influential cases, and the handling of censoring, in addition to other statistical considerations. Accordingly, the distribution of acceptable and lower study quality may restrict the quality of synthesized evidence and the conclusions of this review. In a synthesis of higher-quality studies, we found acceptable quality to sustain the conclusions. Yet, this review lacked an appraisal of publication bias, such that the skew towards positive results may stem from a lack of published null results. Additionally, the quality score was defined based on practical considerations, with the aim of enabling within-study comparisons. The score has not been validated, and the applied quality threshold is solely anchored in the quality points assigned within this review. Assessments for both instruments are reported for each study.
Geographically, the majority of the populations analyzed originated from the United States, with fewer studies representing non-Western, educated, industrial, rich and democratic (WEIRD) countries. This distribution reflects the higher prevalence of obesity in WEIRD countries but limits the generalizability of our results to these geographical regions. European health systems, for example, differ significantly in terms of access, cost structures, and the organization of bariatric surgery [62]. Broadening the scope of research to include more diverse healthcare contexts would improve the external validity of these findings.
We also found some overlaps in the data sources used, particularly with population-based registries. For instance, two reports using the Scandinavian Obesity Surgery Registry included overlapping observation periods, although the newer report extended the analysis by four additional years and differed in volume definitions [16, 51]. Similarly, a study from 2020 analyzed a Nordic obesity cohort [14], which was extended by up to 8 years in a 2023 publication that analyzed different outcomes [60]. Two 2016 and 2017 studies by the same author inquired the Bariatric Outcomes Longitudinal Database, but were included as they differed in analyzed interventions [18, 63]. The most meaningful overlap was observed in two studies by the same first author, using the Longitudinal Assessment of Bariatric Surgery (LABS) database, with the later 2013 study differing only in additional outcomes and volume definitions [37, 38].
Although studies using US-based registries and databases occasionally demonstrated overlap, they often differed in methodological designs, outcomes analyzed, type of surgery and regions of patient population. Such overlaps were carefully considered during data synthesis to prevent redundancy, as substantial overlap can lead to inflated study results without contributing new information. However, none of the studies exhibited complete overlaps without distinguishing features in methodology or procedures analyzed.
The findings of this rapid review are comparable with previous literature, including a systematic review from 2012 that analyzed volume-outcome relationships in bariatric surgery. That review, which included studies up to 2011, reported similar conclusions regarding improved outcomes with higher procedural volumes [24]. Although this systematic review did not explicitly exclude studies on surgical indications for malignancies, it found both strong evidence supporting volume-outcome associations and generally acceptable study quality, as assessed by the Newcastle-Ottawa Quality Assessment scale. In contrast, our review identified a trend towards higher-quality studies in more recent publications, suggesting improvements in methodological rigor over time.
Significant limitations of this study relate to methodological limitations inherent to rapid reviews. Only one reviewer screened all titles and extracted data. Although other reviewers verified data accuracy and were consulted in case of uncertainty, the risk of single-reviewer bias in screening and quality assessments may occur [64]. Furthermore, database coverage was limited, and synthesis depth was restricted by both heterogeneity and time constraints. The review process faced additional challenges related to the data extraction of results. Multiple studies lacked comprehensive reporting on volume definitions and intervention characteristics, which led to quality downgrades during assessment. Variability in reporting surgical indications and diagnostic codes further complicated study selection, necessitating inferred interpretation based on outcomes and study aims. To address reporting discrepancies, ROBINS-E domain 7 was included in the quality assessment. Moreover, some studies presented high-density results through multiple model variations. In these cases, priority was given to fully adjusted models that included volume as a key variable, ensuring that synthesized results reflected the most methodologically sound evidence. Given the multidimensional nature of obesity, the study underlines the importance of considering bundled care models that centralize expertise and resources in high-volume hospitals. Such a model could enhance consistency in outcomes and optimize the quality of care delivered. Notably, few studies in the review analyzed success metrics beyond mortality and complication rates, such as quality of life, BMI reduction, diabetes management, or other morbidity-related outcomes. This observation may stem from the widespread acceptance of bariatric surgery as an effective intervention, making further scrutiny of its impact on these parameters seem redundant, as previously evidenced in meta-analyses focusing on morbidity outcomes [48].
In conclusion, the studies included in this review indicate a relationship between hospital and surgeon volume and patient outcomes. For hospital volume, most reports identified an association with short-term mortality. As highlighted in the existing literature, there is a predominant focus on short-term outcomes, while robust evidence supporting a volume-outcome relationship for long-term mortality and morbidity remains unsubstantiated in this review. Given that long-term mortality rates following bariatric surgery are generally low, this tendency may adequately capture the relevant mortality risks in the context of bariatric surgery. Similarly, perioperative and postoperative complications were more frequently analyzed and demonstrated improvements with increasing procedural volumes.
The methodological quality of included studies varied, reflecting inconsistent reporting and a lack of standardized volume definitions. Future research should prioritize the substantiation of volume definitions, including the provision of relevant citations or the application of data-driven methodologies to delineate which volume thresholds qualify as “high” or “low.” A more rigorous and transparent validation of volume cutoffs would not only enhance the comparability of studies but also support the generalizability of findings. This, in turn, could inform evidence-based policy decisions on the centralization of bariatric care to optimize patient outcomes. Furthermore, to enhance comparability across studies, researchers investigating volume-outcome effects across different medical disciplines should converge on standardized reference categories for volume levels in modeling and inference. Such standardization would facilitate more consistent evidence synthesis and policy recommendations. Additionally, prospective cohort studies incorporating validated HRQoL instruments could address the current gap regarding possible volume-outcome associations with long-term therapeutic benefits and quality of life improvements. The strong heterogeneity noted in this and other reviews may be mitigated through studies based on international registries and harmonized procedural definitions, while future reviews could account for this variability by applying meta-analytic methods to estimate the clinical significance of reductions in mortality or complications across volume definitions. Similarly, primary studies using causal frameworks could provide more robust estimates of clinical benefit, thereby avoiding the limitations inherent to purely associative analyses.
Data availability
The data extracted in this work are available in the publication, its appendices, and from the corresponding author on reasonable request.
References
World Health Organization. Obesity and overweight. (World Health Organization Fact Sheets, 2024). https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. PLoS Med. 2020;17:e1003198. https://doi.org/10.1371/journal.pmed.1003198.
Ward ZJ, Willett WC, Hu FB, Pacheco LS, Long MW, Gortmaker SL. Excess mortality associated with elevated body weight in the USA by state and demographic subgroup: a modelling study. eClinicalMedicine. 2022;48:101429. https://linkinghub.elsevier.com/retrieve/pii/S2589537022001596.
Sharaiha RZ, Shikora S, White KP, Macedo G, Toouli J, Kow L. Summarizing consensus guidelines on obesity management: a joint, multidisciplinary venture of the International Federation for the Surgery of Obesity & Metabolic Disorders (IFSO) and World Gastroenterology Organisation (WGO). J Clin Gastroenterol. 2023;57:967–76. https://doi.org/10.1097/MCG.0000000000001916.
Kolen AM, Romeijn MM, Holthuijsen DDB, Janssen L, Greve JWM, Leclercq WKG, et al. Current preoperative strategies applied in the Dutch bariatric centers: a national survey. Clin Obes. 2021;11:e12461. https://doi.org/10.1111/cob.12461.
White GE, Courcoulas AP, King WC, Flum DR, Yanovski SZ, Pomp A, et al. Mortality after bariatric surgery: findings from a 7-year multicenter cohort study. Surg Obes Relat Dis. 2019;15:1755–65. https://linkinghub.elsevier.com/retrieve/pii/S155072891930382X.
Wadhawan R. Bariatric surgery in the era of obesity management medication: a paradigm shift or a complementary tool?. J Bariatr Surg. 2024;3:87–8. https://journals.lww.com/10.4103/jbs.jbs_17_24.
Ri M, Aikou S, Seto Y. Obesity as a surgical risk factor. Ann Gastroenterol Surg. 2018;2:13–21. https://doi.org/10.1002/ags3.12049.
Rookes N, AL-Asadi O, Yeluri S, Vasas P, Samuel N, Balchandra S, et al. Causes of death after bariatric surgery: long-term study of 10 years. Obes Surg. 2025;35:47–58. https://doi.org/10.1007/s11695-024-07466-0.
Koisser L, Czypionka T, Institut für Höhere Studien. Mindestfallzahlen bei medizinischen Leistungen. Health Systems Watch. https://irihs.ihs.ac.at/id/eprint/6189/1/hsw-health-system-watch-II-2022-mindestfallzahlen-bei-medizinischen-leistungen-koisser-czypionka.pdf.
Swiss Society for the Study of Morbid Obesity and Metabolic Disorders. Richtlinien zur operativen Behandlung von Übergewicht. 2021. https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.bag.admin.ch/dam/bag/de/dokumente/kuv-leistungen/referenzdokumente-klv-anhang-1/01.1_SMOB%2520Richtlinien_vom_01.01.2021_DE.pdf.download.pdf/01.1_SMOB%2520Richtlinien_vom_01.01.2021_DE.pdf&ved=2ahUKEwiGgYC7mfuKAxXd_7sIHYVZObIQFnoECBYQAQ&usg=AOvVaw0fxijJiUz5368Eej-vGyQh.
Hollenbeak CS, Rogers AM, Barrus B, Wadiwala I, Cooney RN. Surgical volume impacts bariatric surgery mortality: a case for centers of excellence. Surgery. 2008;144:736–43. https://linkinghub.elsevier.com/retrieve/pii/S0039606008003577.
Torrente JE, Cooney RN, Rogers AM, Hollenbeak CS. Importance of hospital versus surgeon volume in predicting outcomes for gastric bypass procedures. Surg Obes Relat Dis. 2013;9:247–52. https://linkinghub.elsevier.com/retrieve/pii/S155072891200094.
Kauppila JH, Santoni G, Tao W, Lynge E, Koivukangas V, Tryggvadóttir L, et al. Reintervention or mortality within 90 days of bariatric surgery: population-based cohort study. Br J Surg. 2020;107:1221–30. https://academic.oup.com/bjs/article/107/9/1221/6120724.
Hernandez-Boussard T, Downey JR, McDonald K, Morton JM. Relationship between patient safety and hospital surgical volume. Health Serv Res. 2012;47:756–69. https://doi.org/10.1111/j.1475-616.6773.2011.01310.x.
Svarts A, Anders T, Engwall M. Volume creates value: the volume–outcome relationship in Scandinavian obesity surgery. Health Serv Manag Res. 2022;35:229–39. https://doi.org/10.1177/09514848211048598.
Tsui ST, Yang J, Nie L, Altieri MS, Talamini M, Pryor AD, et al. Association of revisions or conversions after sleeve gastrectomy with annual bariatric center procedural volume in the state of New York. Surg Endosc. 2020;34:3110–7. https://doi.org/10.1007/s00464-019-07068-3.
Celio AC, Kasten KR, Burruss MB, Pories WJ, Spaniolas K. Surgeon case volume and readmissions after laparoscopic Roux-en-Y gastric bypass: more is less. Surg Endosc. 2017;31:1402–6. https://doi.org/10.1007/s00464-016-5128-y.
Chadwick C, Burton PR, Brown D, Holland JF, Campbell A, Cottrell J, et al. The length of hospital stay following bariatric surgery in Australia: the impact of patient, procedure, system and surgeon. ANZ J Surg. 2023;93:2833–42. https://doi.org/10.1111/ans.18575.
Altieri MS, Pryor AD, Yang J, Nie L, Talamini MA, Spaniolas K. Bariatric peri-operative outcomes are affected by annual procedure-specific surgeon volume. Surg Endosc. 2020;34:2474–82. https://doi.org/10.1007/s00464-019-07048-7.
Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204. https://doi.org/10.1186/s13643-016-0376-4.
Padwal R, Klarenbach S, Wiebe N, Hazel M, Birch D, Karmali S, et al. Bariatric surgery: a systematic review of the clinical and economic evidence. J Gen Intern Med. 2011;26:1183–94. https://doi.org/10.1007/s11606-011-1721-x.
Klarenbach S, Padwal R, Wiebe N, Hazel N, Manns B, Karmali S, et al. Bariatric surgery for severe obesity: systematic review and economic evaluation. CADTH Technology Report; 2010. https://www.cda-amc.ca/sites/default/files/pdf/H0485_Bariatric_Surgery_for_Severe_Obesity_tr_e.pdf.
Zevin B, Aggarwal R, Grantcharov TP. Volume-outcome association in bariatric surgery: a systematic review. Ann Surg. 2012;256:60–71. https://journals.lww.com/00000658-201207000-00010.
Hsu JL, Ismail S, Hodges MM, Agala CB, Farrell TM. Bariatric surgery: trends in utilization, complications, conversions and revisions. Surg Endosc. 2024;38:4613–23. https://doi.org/10.1007/s00464-024-10985-7.
Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KGMM, Zimmet PZ, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Diab Care. 2016;39:861–77. https://diabetesjournals.org/care/article/39/6/861/29305/Metabolic-Surgery-in-the-Treatment-Algorithm-for.
Ugale S, Vennapusa A, Katakwar A, Ugale A. Laparoscopic bariatric surgery-current trends and controversies. Ann Laparosc Endosc Surg. 2017;2:154. http://ales.amegroups.com/article/view/4181/5022.
Bundesregierung. Entwurf eines Gesetzes zur Verbesserung der Versorgungsqualität im Krankenhaus und zur Reform der Vergütungsstrukturen (Krankenhausversorgungsverbesserungsgesetz – KHVVG). Bundesgesundheitsministerium. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Gesetze_und_Verordnungen/GuV/K/KHVVG_GE_Kabinett.pdf.
Vogel J, Polin K, Pross C, Geissler A. Qualitätsmonitor 2019 - Kapitel 5: Implikationen von Mindestmengen und Zertifizierungsvorgaben: Auswirkungen verschiedener Vorgaben auf den deutschen Krankenhaussektor. Medizinisch Wissenschaftliche Verlagsgesellschaft. https://www.wido.de/publikationen-produkte/buchreihen/qualitaetsmonitor/2019/.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:n71. https://doi.org/10.1136/bmj.n71.
Garritty C, Hamel C, Trivella M, Gartlehner G, Nussbaumer-Streit B, Devane D, et al. Updated recommendations for the Cochrane rapid review methods guidance for rapid reviews of effectiveness. BMJ. 2024:e076335. https://doi.org/10.1136/bmj-2023-076335.
Eckhardt H, Nimptsch U, Henschke C. Rapid reviews of volume-outcome relationship in hepatic resection, ovariectomy, hysterectomy, bariatric and non-bariatric gastric surgeries. National Institute for Health and Care Research; 2023. https://www.crd.york.ac.uk/PROSPERO/view/CRD42023398566.
Carlsson LMS, Carlsson B, Jacobson P, Karlsson C, Andersson-Assarsson JC, Kristensson FM, et al. Life expectancy after bariatric surgery or usual care in patients with or without baseline type 2 diabetes in Swedish Obese Subjects. Int J Obes]. 2023;47:931–8. https://www.nature.com/articles/s41366-023-01332-2.
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020:l6890. https://doi.org/10.1136/bmj.l6890.
Motheral B, Brooks J, Clark MA, Crown WH, Davey P, Hutchins D, et al. A checklist for retrospective database studies—report of the ISPOR Task Force on Retrospective Databases. Value Health. 2003;6:90–7. https://linkinghub.elsevier.com/retrieve/pii/S1098301510601374.
Higgins JPT, Morgan RL, Rooney AA, Taylor KW, Thayer KA, Silva RA, et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ Int. 2024;186:108602. https://linkinghub.elsevier.com/retrieve/pii/S0160412024001880.
Smith MD, Patterson E, Wahed AS, Belle SH, Bessler M, Courcoulas AP, et al. Relationship between surgeon volume and adverse outcomes after RYGB in Longitudinal Assessment of Bariatric Surgery (LABS) study. Surg Obes Relat Dis. 2010;6:118–25. https://linkinghub.elsevier.com/retrieve/pii/S1550728909006844.
Smith MD, Patterson E, Wahed AS, Belle SH, Courcoulas AP, Flum D, et al. Can technical factors explain the volume-outcome relationship in gastric bypass surgery?. Surg Obes Relat Dis. 2013;9:623–9. https://linkinghub.elsevier.com/retrieve/pii/S1550728912003917.
Krell RW, Finks JF, English WJ, Dimick JB. Profiling hospitals on bariatric surgery quality: which outcomes are most reliable?. J Am Coll Surg. 2014;219:725–34.e3. https://journals.lww.com/00019464-201410000-00015.
Murr MM, Martin T, Haines K, Torrella T, Dragotti R, Kandil A, et al. A state-wide review of contemporary outcomes of gastric bypass in Florida: does provider volume impact outcomes?. Ann Surg. 2007;245:699–706. https://journals.lww.com/00000658-200705000-00007.
Morino M, Toppino M, Forestieri P, Angrisani L, Allaix ME, Scopinaro N. Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg. 2007;246:1002–9. https://journals.lww.com/00000658-200712000-00012.
Bouchard P, Demyttenaere S, Court O, Franco EL, Andalib A. Surgeon and hospital volume outcomes in bariatric surgery: a population-level study. Surg Obes Relat Dis. 2020;16:674–81. https://linkinghub.elsevier.com/retrieve/pii/S1550728920300289.
Carbonell AM, Lincourt AE, Matthews BD, Kercher KW, Sing RF, Heniford BT. National study of the effect of patient and hospital characteristics on bariatric surgery outcomes. Am Surg. 2005;71:308–14.
Gould JC, Kent CK, Wan Y, Rajamanickam V, Leverson G, Campos GM. Perioperative safety and volume: outcomes relationships in bariatric surgery: a study of 32,000 patients. J Am Coll Surg. 2011;213:771–7. https://journals.lww.com/00019464-201112000-00011.
Kelles SMB, Barreto SM, Guerra HL. Mortality and hospital stay after bariatric surgery in 2,167 patients: influence of the surgeon expertise. Obes Surg. 2009;19:1228–35. https://doi.org/10.1007/s11695-009-9894-7.
Kohn GP, Galanko JA, Overby WD, Farrell TM. High case volumes and surgical fellowships are associated with improved outcomes for bariatric surgery patients: a justification of current credentialing initiatives for practice and training. J Am Coll Surg. 2010;210:909–18. https://journals.lww.com/00019464-201006000-00004.
Nguyen NT, Paya M, Stevens CM, Mavandadi S, Zainabadi K, Wilson SE. The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Ann Surg. 2004;240:586–94. https://journals.lww.com/00000658-200410000-00004.
Varban OA, Reames BN, Finks JF, Thumma JR, Dimick JB. Hospital volume and outcomes for laparoscopic gastric bypass and adjustable gastric banding in the modern era. Surg Obes Relat Dis. 2015;11:343–9. https://linkinghub.elsevier.com/retrieve/pii/S1550728914003967.
Dimick JB, Osborne NH, Nicholas L, Birkmeyer JD. Identifying high-quality bariatric surgery centers: hospital volume or risk-adjusted outcomes?. J Am Coll Surg. 2009;209:702–6. https://journals.lww.com/00019464-200912000-00004.
Doumouras AG, Saleh F, Anvari S, Gmora S, Anvari M, Hong D. A longitudinal analysis of short-term costs and outcomes in a regionalized center of excellence bariatric care system. Obes Surg. 2017;27:2811–7. https://doi.org/10.1007/s11695-017-2707-5.
Stenberg E, Szabo E, Ågren G, Näslund E, Boman L, Bylund A, et al. Early complications after laparoscopic gastric bypass surgery: results from the Scandinavian Obesity Surgery Registry. Ann Surg. 2014;260:1040–7. https://journals.lww.com/00000658-201412000-00018.
Chiu CC, Wang JJ, Chen YS, Chen JJ, Tsai TC, Lai CC, et al. Trends and predictors of outcomes after surgery for hepatocellular carcinoma: a nationwide population-based study in Taiwan. Eur J Surg Oncol. 2015;41:1170–8. https://linkinghub.elsevier.com/retrieve/pii/S074879831500431X.
Chao GF, Yang J, Thumma J, Chhabra KR, Arterburn DE, Ryan A, et al. Volume–outcome relationships for Roux-en-Y gastric bypass patients in the sleeve gastrectomy era. Surg Endosc. 2022;36:3884–92. https://doi.org/10.1007/s00464-021-08705-6.
Hunt KD, Doumouras AG, Lee Y, Gmora S, Anvari M, Hong D. The effect of surrogate procedure volume on bariatric surgery outcomes: do common laparoscopic general surgery procedures matter?. Surg Endosc. 2020;34:1278–84. https://doi.org/10.1007/s00464-019-06897-6.
Lopez J, Sung J, Anderson W, Stone J, Gallagher S, Shapiro D, et al. Is bariatric surgery safe in academic centers?. Am Surg. 2002;68:820–3. https://doi.org/10.1177/000313480206800918.
Luft HS, Hunt SS, Maerki SC. The volume-outcome relationship: practice-makes-perfect or selective-referral patterns?. Health Serv Res. 1987;22:157–82.
Kumbhani DJ, Fonarow GC, Heidenreich PA, Schulte PJ, Lu D, Hernandez A, et al. Association between hospital volume, processes of care, and outcomes in patients admitted with heart failure: insights from get with the guidelines-heart failure. Circulation. 2018;137:1661–70. https://doi.org/10.1161/CIRCULATIONAHA.117.028077.
Oria HE, Moorehead MK. Updated bariatric analysis and reporting outcome system (BAROS). Surg Obes Relat Dis. 2009;5:60–6. https://linkinghub.elsevier.com/retrieve/pii/S1550728908007739.
Pradarelli JC, Varban OA, Ghaferi AA, Weiner M, Carlin AM, Dimick JB. Hospital variation in perioperative complications for laparoscopic sleeve gastrectomy in Michigan. Surgery. 2016;159:1113–20. https://linkinghub.elsevier.com/retrieve/pii/S0039606015008065.
Markar SR, Santoni G, Holmberg D, Kauppila JH, Lagergren J. Bariatric surgery volume by hospital and long-term survival: population-based NordOSCo data. Br J Surg. 2023;110:177–82. https://academic.oup.com/bjs/article/110/2/177/6828839.
El Amrani M, Lenne X, Clement G, Delpero JR, Theis D, Pruvot FR, et al. Specificity of procedure volume and its association with postoperative mortality in digestive cancer surgery: a nationwide study of 225,752 patients. Ann Surg. 2019;270:775–82.
Sinclair P, Vijgen GHEJ, Aarts EO, Van Nieuwenhove Y, Maleckas A. First inventory of access and quality of metabolic surgery across Europe. Obes Surg. 2021;31:5196–206.
Celio AC, Kasten KR, Brinkley J, Chung AY, Burruss MB, Pories WJ, et al. Effect of surgeon volume on sleeve gastrectomy outcomes. Obes Surg. 2016;26:2700–4. https://doi.org/10.1007/s11695-016-2190-4.
Nussbaumer-Streit B, Sommer I, Hamel C, Devane D, Noel-Storr A, Puljak L, et al. Rapid reviews methods series: guidance on team considerations, study selection, data extraction and risk of bias assessment. BMJ EBM. 2023;28:418–23. https://doi.org/10.1136/bmjebm-2022-112185.
Encinosa WE, Bernard DM, Du D, Steiner CA. Recent improvements in bariatric surgery outcomes. Med Care. 2009;47:531–5. https://journals.lww.com/00005650-200905000-00005.
Ibrahim AM, Ghaferi AA, Thumma JR, Dimick JB. Variation in outcomes at bariatric surgery centers of excellence. JAMA Surg. 2017;152:629–36.
Jafari MD, Jafari F, Young MT, Smith BR, Phalen MJ, Nguyen NT. Volume and outcome relationship in bariatric surgery in the laparoscopic era. Surg Endosc. 2013;27:4539–46. https://doi.org/10.1007/s00464-013-3112-3.
Liu JH, Zingmond D, Etzioni DA, O’Connell JB, Maggard MA, Livingston EH, et al. Characterizing the performance and outcomes of obesity surgery in California. Am Surg. 2003;69:823–8. https://doi.org/10.1177/000313480306901001.
Wilson GC, Cutler Quillin R, Sutton JM, Wima K, Shaw JJ, Hoehn RS, et al. Factors related to readmission after major elective surgery. Dig Dis Sci. 2015;60:47–53. https://doi.org/10.1007/s10620-014-3306-0.
Chiu CC, Wang JJ, Tsai TC, Chu CC, Shi HY. The relationship between volume and outcome after bariatric surgery: a nationwide study in Taiwan. Obes Surg. 2012;22:1008–15. https://doi.org/10.1007/s11695-012-0636-x.
Funding
This study was partially supported by a research grant from the German National Association of Statutory Health Insurance Funds. The broad research area for the research grant was provided, but the development of the methods, the conducting of the research, the analysis, and interpretation were all carried out by the authors independently. The manuscript was prepared solely based on the initiative of the author team. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CH, UN and HE conceptualized the study and developed the methodology. CH supervised the project. HE conducted the systematic literature search. AC and CH were responsible for title screening and extraction as well as table production. AC produced the figures, analyzed the data and drafted the original manuscript. All authors were involved with writing, editing and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Campione, A., Nimptsch, U., Eckhardt, H. et al. Volume‒outcome relationships in bariatric surgery: a rapid review. Int J Obes 50, 33–52 (2026). https://doi.org/10.1038/s41366-025-01931-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01931-1