Abstract
This narrative review evaluates the suitability of conventional biopolymer-based microencapsulation strategies, originally developed for facultative and aerotolerant probiotics, for the protection and delivery of extremely oxygen-sensitive (EOS) next-generation probiotics (NGPs). With increasing interest in NGPs, there is a pressing need to establish whether conventional formulation approaches can be effectively translated for these highly oxygen-sensitive bioactives. We reviewed commonly used microencapsulation materials and techniques, assessing their suitability and potential to preserve EOS bacterial viability. Hydrated pectin- and gellan-based microcomposite systems, particularly when combined with xanthan gum or other polymers, exhibited the strongest oxygen-protection performance. In contrast, alginate alone demonstrated inconsistent barrier properties, though its performance improved when blended or coated with chitosan. Dehydrated microcomposite systems did not yield additional viability benefits compared to their hydrated counterparts. Importantly, none of the studies explicitly quantified oxygen exposure parameters or established threshold levels required for effective protection of EOS strains. Despite some microcomposite systems demonstrating potential for EOS colonic delivery, our findings highlight a critical gap in formulation science for these sensitive bioactives and underscore the need for the development of bespoke, tailored delivery systems that advance beyond conventional approaches designed for facultative or aerotolerant strains. Addressing these gaps will support the advancement of microencapsulation technologies, improve biotherapeutic NGP formulation, and ultimately facilitate the translation of exploratory clinical findings into rationally designed, accessible, and effective microbiome-based interventions.

Similar content being viewed by others
Introduction
Probiotics are traditionally defined as viable microorganisms that, when administered in adequate amounts, confer health benefits to the host1. Numerous studies have demonstrated their therapeutic potential, including improved gastrointestinal (GI) health and immune function2,3,4. These microorganisms have long been consumed via probiotic foods and supplements. Conventional probiotic bacteria are typically facultative or aerotolerant anaerobes (e.g. lactic acid-producing strains such as Lactobacillus spp. and Bifidobacterium spp.)5,6, and can therefore tolerate transient oxygen exposure during manufacture and delivery.
An increasing number of strictly anaerobic bacteria are now being identified as next-generation probiotics (NGPs), largely driven by insights from Faecal Microbiota Transplantation (FMT) research7,8. These NGPs are inherently susceptible to oxygen, and given that current formulation methods for colonic delivery were developed for more oxygen-tolerant strains, it remains unclear whether existing approaches are suitable for these newer EOS bioactives.
The colon presents a particularly challenging but essential therapeutic target site for EOS probiotic delivery, given the multiple stressors (pH changes, gastric acidity, bile salts, and oxygen (important for EOS strains) that can compromise their viability and subsequent engraftment/colonisation ability9. To address these challenges, numerous formulation strategies have been developed to enhance probiotic survival. Microencapsulation is among the most widely adopted approach to provide physical protection during processing, storage, and GI delivery. While commonly used polymers in microencapsulation (e.g. alginate and pectin) can provide gastric protection and aid colon-targeted delivery, their gas permeability raises uncertainty about their ability to maintain EOS bacterial viability during storage and GI transit.
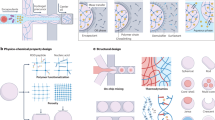
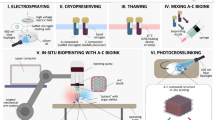
Microencapsulation formulation processes traditionally employ drying steps (such as lyophilisation, spray-drying, and fluid-bed drying) to improve the handling and shelf stability of probiotics10,11,12. However, while dehydration renders probiotics metabolically inactive (and likely insensitive to oxygen), minimising oxygen-related viability loss during initial processing still warrants consideration13. Encapsulating bacteria in a hydrated state offers a gentler alternative to dehydration-based methods12,14. Various hydrated microencapsulation techniques, either entrapping or coating bacteria in polymer matrices, have been developed, with extrusion and emulsification (Fig. 1) among the most widely used. These approaches are favoured for their affordability, versatility across biomaterials, and mild processing conditions compatible with live bioactives15. EOS oxygen exposure can occur during multiple stages of processing (e.g., homogenisation, gel hardening, or washing), and since most methods are performed in bulk liquid phases that are not typically deoxygenated, oxygen can diffuse freely and potentially inactivate EOS bioactives. Subsequent storage and ingestion can further expose probiotics to oxygen, particularly during transit through the relatively oxygenated upper GI tract. This underscores the need for both oxygen-free processing and materials that restrict oxygen ingress to the bioactive core.
Studies that directly assess the impact of oxygen exposure during encapsulation following formulation remain largely unexplored. Most available data can only provide indirect insights, for example, derived from simulated GI or storage studies performed under oxygen-rich conditions. These findings, though primarily aimed at evaluating acid or bile tolerance, offer useful insight into the protective capacity of encapsulation against oxidative stress. Ideally, oxygen effects would be decoupled from other stressors, (for example, performing GI simulation or storage experiments under oxygen-free conditions allows viability impacts from these stressors to be distinguished from those due to oxygen exposure)16, studying strain-specific oxygen sensitivity and thresholds before formulation17, or measuring inter-particle oxygen diffusion and/or concentrations. Recent studies have focused on improving the intrinsic oxygen tolerance of EOS strains, including the adaptive evolution of Faecalibacterium prausnitzii (F. prausnitzii), which extended air survival modestly but remains inadequate for colonic delivery without additional formulation protection8. In parallel, formulations containing antioxidants or oxygen scavengers improve survival, indirectly validating oxygen as a primary determinant of viability loss. To date, no studies have systematically assessed the impacts of oxygen during encapsulation or the potential for current microencapsulation formulations for preserving EOS viability.
Response of EOS bacteria following oxygen exposure
Bifidobacterium species, many of which are recognised as potential NGPs, exhibit varying degrees of oxygen sensitivity. Aerotolerant strains can withstand brief exposure to oxygen but do not proliferate under such conditions. Facultative strains can grow in both aerobic and anaerobic environments, in contrast to strictly anaerobic strains, which are unable to tolerate oxygen and only thrive under fully anaerobic conditions. Amongst Bifidobacterium species, there can also be degrees of strain specificity in oxygen sensitivity, with many industrial strains selected with high oxygen tolerability for their processing stability. Molecular adaptations (including oxidoreductases and oxygen scavenging systems) contribute to this variability13. For example, B. boum JCM1211 and B. thermophilum JCM1207 tolerate ~21% (atmospheric) oxygen through elevated NADH oxidase activity, in contrast to B. longum JCM1217 and B. bifidum JCM125518. Facultative anaerobe B. longum BBMN68 reached 8.5 log CFU/mL at 3% oxygen conditions, compared to 8.9 log CFU/mL in the absence of oxygen19. Strains of B. breve (77.7%), B. longum (83.8%), and B. bifidum (91.7%) tested for oxygen tolerability exhibited survival after 48 h exposure to ambient air, while all B. adolescentis strains remain highly sensitive20. It is also recognised that varying the oxygen concentration can help identify the threshold levels at which specific strains can tolerate (e.g., 5% oxygen compared with atmospheric levels)18, though others remain completely oxygen-sensitive21. Consequently, species-level trends are insufficient for probiotic formulation: oxygen tolerability must be verified at the strain level for NGPs20,22.
Scope of review
This review examines whether conventional biopolymer-based oral microcomposite colonic targeting formulations are effective in delivering EOS NGPs, with a particular focus on preserving bacterial viability in the presence of oxygen. To isolate the specific impact of oxygen exposure, we categorise microencapsulation methods into those involving a dehydration step and those that do not. As the viability effects of dehydration remain poorly defined for EOS strains, separating these approaches allows clearer interpretation of oxygen-specific impacts on bacterial survival.
The review focuses on widely used biopolymers (including alginate, chitosan, pectin, carrageenan, gellan gum and proteins) due to their prevalence in colon-targeted delivery systems and relevance for probiotic protection. Findings are discussed by material type to evaluate whether specific biopolymer materials demonstrate superior oxygen-restrictive properties. We concentrate on Bifidobacterium species as a representative model of EOS NGPs. Despite ongoing challenges in fully characterising their strain-specific oxygen sensitivity, their recognised vulnerability to oxygen exposure makes them an ideal group for assessing the protective potential of biopolymer-based encapsulation.
Critically, no studies have directly evaluated the specific impact of oxygen on EOS viability during microencapsulation. Studies used in our assessment here have indirectly exposed EOS bacteria to oxygen, such as during simulated gastric and intestinal treatments or stability assessments conducted under aerobic (~21% O2) conditions. While these experiments were primarily designed to assess these post-encapsulation environmental stressors, they nevertheless provide retrospective insight into the potential oxygen-protective benefits of biopolymer encapsulation. In this review, we adopt this indirect approach to examine whether current formulation strategies, developed for aerotolerant or facultative anaerobic strains, can be effectively translated to EOS NGPs.
To aid comparison across studies, encapsulation systems were qualitatively categorised according to the observed viability of encapsulated EOS bacteria following indirect oxygen exposure. Systems were described as highly protective when they maintained bacterial viability with less than a 1–2 log CFU/mL (or g) reduction, moderately protective when viability losses ranged between 3–4 log reductions, and poorly protective when reductions exceeded 4–5 logs.
Alginate
Chemical overview
Alginate, a polysaccharide derived from the cell walls of brown algae (Laminaria spp.), is widely employed for microencapsulation due to its mild gelling chemistry, biocompatibility, GRAS status, affordability, and water solubility. It comprises β-(1–4)-linked D-mannuronic acid and α-(1–4)-linked L-guluronic acid units, which cross-link with divalent cations (e.g., Ca²⁺) to form hydrogels with an ‘egg-box’ configuration14. Above its pKa (3.3–3.5), alginate carries a negative charge but remains stable at low pH through protonation of carboxyl groups, contributing to its efficacy in gastric protection and enteric probiotic delivery14,23. The porosity of Ca²⁺-cross-linked alginate gels may allow diffusion of digestive components, with permeability modulated by the mannuronic: guluronic acid ratio and pH-dependent gel swelling or shrinkage24. In addition to pH or chemical triggers (e.g., phosphate ions), intestinal release can also occur via enzymatic degradation25. Alginate-based microencapsulation has been extensively applied to the delivery of diverse therapeutic agents, including prokaryotic (e.g. probiotic bacteria) and eukaryotic (e.g. yeasts) microorganisms26, pharmaceutical agents27, functional proteins28 and even viral antigens in vaccine development29. Numerous studies report enhanced viability of facultative or aerotolerant bacteria following alginate encapsulation26,30. Facultative anaerobic and common industrial fermenting starter strains such as Lactobacillus (L.) plantarum, L. reuteri, L. acidophilus, L. casei, and L. rhamnosus have exhibited increased survival under GI conditions via alginate-based microencapsulation31,32,33,34,35. In some cases, these strains were initially cultured anaerobically before encapsulation was undertaken in oxygen-replete environments36,37, since oxygen limitation is assumed unnecessary for non-EOS strains.
Oxygen diffusion and permeability have been investigated in both hydrated and dry forms of alginate. In hydrated gels, oxygen readily penetrates the matrix, with diffusion coefficients comparable to those in pure water, indicating that small molecules such as O₂ diffuse easily through Ca-alginate beads38,39. Studies examining alginate gels as cell-immobilisation matrices have reported mixed trends: while some observed a marked decrease in oxygen diffusivity with increasing alginate concentration from 2% to 5%40, others found minimal dependence on alginate content38. Diffusivity may also decline with decreasing particle size, reflecting longer diffusion paths in smaller microcapsules41. In contrast, dry alginate films display excellent oxygen-barrier performance42,43. However, this property is highly moisture-sensitive: as humidity rises and the film rehydrates, permeability increases substantially, reducing oxygen-blocking capacity.
Alginate-based microencapsulation of EOS anaerobes
The use of alginate-based microencapsulation formulations for the GI delivery of EOS has been demonstrated, where alginate has been used as a sole core encapsulating matrix. These studies using alginate as an encapsulating matrix (in wet form) are summarised in Table 1, categorising their protective capacity.
Alginate-based microencapsulation has generally provided limited protection for viable EOS probiotics, underscoring the insufficiency of alginate formulations and their oxygen-limiting properties for these bioactives. For example, alginate encapsulation (via extrusion or emulsification) of various B. longum and B. lactis Bb-12 strains resulted in substantial viability losses during storage under refrigerated or frozen conditions (in atmospheric oxygen), with most B. longum strains becoming undetectable by day 21, and only 30% of B. lactis Bb-12 remaining viable after 10 days44,45. Frakolaki et al. reported improved ambient aerobic storage stability for B. animalis subsp. lactis Bb-12 by blending alginate with additional biopolymers such as inulin, proteins, glycerol, κ-carrageenan and nanocrystalline cellulose45. In another study, four Bifidobacterium strains (B. longum, B. lactis Bl-04 and Bi-07, B. bifidum) encapsulated in alginate via emulsion retained 7.4 log CFU/mL (3.2 log reduction) after 1 h and 5.77 log CFU/mL (4.8 log reduction) after 2 h simulated gastric fluid exposure under oxygen-rich (ambient) conditions, outperforming free cells or alternative biopolymer formulations (guar gum, locust bean gum, κ-carrageenan)46.
These findings have been challenged by studies reporting high survival of anaerobic bacteria encapsulated in alginate gels, even following prolonged oxygen exposure during stress assays under ambient conditions. Cook et al. demonstrated high recovery of viable B. breve NCIMB 8807 from alginate gels after 4 h sequential simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) exposure in open-air conditions47. Similarly, B. bifidum R0071 encapsulated in alginate beads exhibited only ~1.5 log viability loss after sequential SGF/SIF treatment under ambient conditions48. Although these results suggest improved EOS viability, the oxygen sensitivity of the strains was not directly validated, and strain-level variation is well recognised13.
Additional studies with alginate-based microencapsulation systems have also been conducted by Naklong et al. and Ullah et al. for B. breve TISTR 2130 and B. longum BL-10, respectively, observing moderate viability protection (~3 log CFU/mL reductions) following treatment in SGF and SIF (for 2 and 5 h in total) performed under atmospheric oxygen conditions49,50. Zhang et al. reported ~3.5 log CFU/mg reduction in viable B. pseudocatenulatum G7 cells after ambient air storage at 4 °C for 28 days. This stability was improved to ~ 2 log CFU/mg, with the incorporation of an antacid (e.g. CaCO3), which could reduce pore size and thus limit molecular diffusion51.
Machado et al.52 encapsulated Akkermansia muciniphila (A. muciniphila) DSM 22959 via extrusion, achieving significant improvements in aerobic (~21% O2) storage stability (4 °C, 28 days) and post-digestion viability (~0.2–1 log loss over 5 h). However, as A. muciniphila exhibits strain-specific oxygen tolerance53,54,55 and > 90% viability after 1 h atmospheric air exposure has been reported56, the specific oxygen protection afforded by alginate remains unclear. Additionally, Moghanjougi et al. reported > 92% encapsulation efficiency of B. animalis Bb-12, highlighting the role of particle size in enhancing bacterial protection during storage and simulated GI exposure57.
Overall, numerous studies have examined pure alginate-based gel formulations for encapsulating EOS Bifidobacterium strains. Although these formulations have been assessed for bacterial viability under stress conditions (e.g. storage, simulated GI digestion), the specific impact of oxygen exposure was often not directly addressed. Reported protective effects remain inconsistent, with some studies demonstrating poor survival and others indicating minimal viability loss. Incorporation of additional polymer coatings or excipients (e.g. prebiotics) generally enhanced EOS viability. The lack of prior oxygen sensitivity validation limits conclusions regarding the true oxygen barrier properties of alginate gels.
Chitosan
Chemical overview
Like alginate, chitosan is widely employed in oral delivery systems due to its non-toxicity, biocompatibility and biodegradability58. Its limited natural availability has led to the prevalent use of semi-synthetic forms derived from chitin30. Chitosan is a cationic polysaccharide composed of β-(1 → 4)-linked D-glucosamine and N-acetyl-D-glucosamine residues, with a pKa of ~6.5, rendering it soluble at acidic and neutral pH14. Its viscosity and charge density vary with molecular weight and degree of deacetylation59. Gelation can be achieved through ionotropic cross-linking with anions such as tripolyphosphate60, chemical cross-linking with glutaraldehyde61, or precipitation in NaOH solutions62. Chitosan also exhibits antimicrobial activity and high oxygen impermeability in dried films, supporting its use in wound dressings, anti-infectives, and food packaging63. Its strong mucoadhesive properties, via electrostatic interactions with mucin, enable localised and prolonged delivery64.
Chitosan microparticles have been investigated for the delivery of various agents, including drugs, proteins, vaccines and antivirals65, and for the treatment of gastric Helicobacter pylori infections66. In probiotic formulations, chitosan is typically applied as a coating on pre-formed anionic microparticles, rather than as a core scaffold, likely due to its broad-spectrum antimicrobial effects67. Given this usage trend, our review focuses on coated formulations to evaluate chitosan’s potential for oxygen protection.
Like alginate, dry chitosan films exhibit exceptionally low oxygen permeability42; again, this performance declines sharply with increasing humidity68. In hydrated gel systems, oxygen again diffuses rapidly through the aqueous network, and coating alginate beads with chitosan has been shown to make little difference to overall permeability41. Unlike alginate, however, quantitative studies of oxygen diffusivity in bulk chitosan gels or microparticles remain limited.
Chitosan-based microencapsulation of EOS anaerobes
Table 2 summarises studies employing chitosan in formulations for GI delivery of EOS anaerobic bacteria, categorising their effectiveness. Chitosan was commonly applied as a coating on alginate microcapsules via extrusion or emulsification. Krasaekoopt et al. reported moderate aerobic storage stability of B. bifidum ATCC 1994 in yoghurt (4 °C, 4 weeks, ~21% O2), with a ~3-log viability reduction to 5.3–5.5 log CFU/g using low-molecular-weight chitosan coatings, though no comparison with uncoated capsules was provided69. Around 3 log CFU/mL viability loss was also observed by Ullah et al. for B. longum BL-101 encapsulated in chitosan-coated alginate microcapsules after the incubation in either SGF or bile salt solution for 1 h under ambient air. In their study, the application of chitosan coatings conferred no significant improvement in viability protection compared with uncoated alginate50. Conversely, chitosan-coated alginate microcapsules offered only minimal improvement for B. infantis ATCC 15697 and B. breve ATCC 15700 under refrigerated aerobic storage (2-log reduction over 30 days, ~21% O2), with enhancement observed only when green tea extract was added70, potentially due to polyphenol oxygen scavenging or ROS-quenching effects71,72. B. infantis is known to be highly oxygen-sensitive73, while B. breve ATCC 15700 displays limited oxygen sensitivity74. Yeung et al. similarly reported no viability improvements with chitosan coatings, with no detectable survival after 21 days of ambient storage or following 30 min SGF/SIF exposure44. In contrast, Cook et al. observed higher recovery of B. breve NCIMB 8807 with chitosan-coated capsules versus uncoated controls after SGF and SIF exposure23,47.
To date, only one study has reported pure chitosan core-based encapsulation of EOS bacteria. Using a microfluidic approach, B. bifidum FL-276.1 exhibited high survival after 2 h SGF and sequential SGF/SIF exposure under ambient aerobic conditions75. However, the strain demonstrated pre-existing oxygen tolerance in pre-oxygenation tests, and no additional viability benefit was observed with cysteine-modified chitosan (likely owing to the oxygen-tolerance nature of the strain used).
Collectively, these findings suggest that chitosan coatings on alginate microcapsules offer inconsistent protection of EOS bacteria under oxygen exposure. Variability likely reflects strain-dependent oxygen tolerance and the challenges of coating formation under aerobic conditions or in acidic chitosan solutions.
Pectin
Chemical overview
Pectin, abundant in citrus and apple peels, is widely employed in microencapsulation owing to its structural diversity, GI stability and biocompatibility. Its complex structure comprises three domains: a homogalacturonan backbone of α-(1 → 4)-linked D-galacturonic acid units, and two branched regions, rhamnogalacturonan I and II. Through its carboxyl groups, pectin can cross-link with divalent cations (e.g. Ca²⁺), forming hydrogels similar to alginate. Gelling properties are strongly influenced by the degree of methylation of galacturonic acid residues, with higher methylation promoting gelation under acidic and high-sugar conditions. Homogalacturonan-rich pectins support gelling, while rhamnogalacturonan I regions hinder cross-linking76.
Alongside alginate, pectin-based encapsulation has been widely applied to preserve the viability of facultative anaerobes such as L. casei and L. acidophilus against acidic and bile salt conditions (also under oxygen conditions)77,78. Pectin beads form mechanically stable, acid-impermeable matrices that resist hydrolysis until reaching the colon, where degradation is mediated by microbiota79,80. Ghibaudo et al. demonstrated post-digestion recovery of viable bacteria from pectin beads. Beads deswell at low pH (pKa ~2.9)81, forming dense protective networks, though some bile salt permeability may reduce viability82. Protective effects are further modulated by methoxylation degree, molecular weight and viscosity of pectin83. In addition, pectin exhibits prebiotic properties favouring Bifidobacterium and Lactobacillus84, and modest mucoadhesive behaviour, supporting its potential for targeted delivery applications85.
Pectin-based dry films provide excellent oxygen barrier properties, though their hydrophilic nature limits performance under humid conditions86. In the dry state, pectin exhibits very low oxygen permeability, which can be further improved through Ca²⁺ cross-linking, tightening the network and reducing swelling87. In contrast, oxygen permeability data for hydrated pectin are scarce, but diffusion is expected to be high due to its water-rich matrix.
Pectin-based microencapsulation of EOS anaerobes
Compared with facultative anaerobes, there are relatively few studies evaluating pectin-based microencapsulation of strictly anaerobic bacteria (Table 3). Pectin encapsulation is typically performed via extrusion or emulsification. Several reports highlight its potential to preserve bacterial viability during ambient GI transit and storage, though bacterial oxygen sensitivity was not explicitly assessed.
Huang et al. encapsulated B. bifidum R0071 in low-methoxyl pectin using an emulsification/internal gelation method48, achieving high viability (8.6 and 7.72 log CFU/mL) after 1 h SGF and SIF treatments under ambient air conditions, with ≤1 log reduction (Fig. 2). Moghanjougi et al. demonstrated similar findings for B. animalis Bb-12 encapsulated in high-methoxyl pectin57, observing only 0.5 log viability loss after 1-month aerobic storage (4 °C, ~21% O2), and ≤2 log reduction after 4.5 h SGF/SIF treatment (Fig. 2). High encapsulation efficiency (>92%) was achieved via emulsification with 30 min gel hardening. Notably, B. animalis Bb-12 was selected for its known oxygen tolerance, attributed to genetic traits conferring resistance to environmental stresses88. Nualkaekul et al. observed high protection of B. longum NCIMB 8809 encapsulated in low-methoxyl pectin (formed by extrusion) when stored in pomegranate juice at 4 °C for a month, with only ~1.5 log CFU/mL loss. However, extending storage to 1.5 months, or storing for 1 month in the more acidic cranberry juice, significantly reduced stability, indicating that acidity and storage duration as key determinants of viability. Surface coatings with chitosan, gelatine, or glucomannan generally improved storage stability89.
Overall, pectin-based wet encapsulation appears promising for EOS protection under ambient air conditions, though high survival likely reflects strain-specific oxygen tolerance. Oxygen sensitivity testing remains essential to validate pectin’sprotective effects.
Carrageenan
Chemical overview
Carrageenan is an anionic high molecular-weight polysaccharide derived from red algae (primarily Kappaphycus alvarezii), composed of repeating disaccharide units of D-galactose and 3,6-anhydro-D-galactose. Based on the degree and position of sulphate ester groups, carrageenan is classified as κ- (monosulphated), ι- (bisulphated), or λ- (trisulphated)90. It is widely used as a gelling, thickening and stabilising agent in food, pharmaceutical and cosmetic applications. Only κ- and ι-carrageenan form gels via cation-mediated cross-linking (e.g. Ca²⁺, K⁺), with κ-carrageenan preferred for probiotic encapsulation due to its firmer gel30. κ-carrageenan dissolves in hot water and solidifies below 40–50 °C91. It is frequently combined with alginate to improve pH-dependent release, supporting the survival of L. acidophilus and L. casei under simulated GI, bile salt and storage conditions92.
Limited studies demonstrate the direct oxygen barrier performance for hydrated carrageenan gels. The oxygen permeability of dried film counterparts, however, is reported to be low, which can be further reduced by the integration of other biopolymers, including guar gum, locus bean gum and hydroxypropyl methylcellulose, into the casted film matrix93,94,95.
Carrageenan-based microencapsulation of EOS anaerobes
Few studies have examined κ-carrageenan-based microencapsulation of obligate anaerobes (Table 3), in contrast to its widespread use with facultative anaerobic Lactobacillus strains31,33,96,97,98. Frakolaki et al. blended alginate and glucose into a κ-carrageenan matrix, reporting ~50% viability loss for B. lactis Bb-12 after 30 days of ambient air storage (4 °C and −18 °C)45. L-cysteine HCl was added as an oxygen scavenger and nutrient but provided only minor stability improvement, possibly due to leaching, limited diffusion, antioxidant saturation, or strain-specific responses. Adhikari et al. reported high storage stability for B. longum strains (B6, ATCC 15708) encapsulated in carrageenan99, with ≤1 log viability loss over 30 days in yoghurt. However, strain oxygen sensitivity was not reported, and free cells also showed similar stability, likely reflecting intrinsic antioxidant properties100. Overall, limited data are available on pure κ-carrageenan scaffolds for EOS encapsulation, though some promising outcomes have been observed.
Gellan gum
Chemical overview
Gellan gum is a thermo-reversible anionic polysaccharide produced by Sphingomonas elodea, composed of repeating units of two β-D-glucose, one β-D-glucuronic acid, and one α-L-rhamnose residue. It exists in high-acyl and low-acyl forms: high-acyl gellan forms soft hydrogels upon cooling (~65 °C), while low-acyl forms rigid gels upon cooling (~40 °C) in the presence of cations (e.g. Ca²⁺)30. Low-acyl gellan gum exhibits anionic characteristics above its pKa value of 3.5101. Widely used in food applications, gellan gum also serves as a soluble fibre with potential prebiotic effects24. Its pH-dependent gelation allows for bacterial protection in gastric conditions with release in the intestine102. Several studies have explored gellan gum-based microencapsulation, often in combination with other biopolymers, for facultative anaerobes (e.g. L. rhamnosus, L. plantarum, L. casei), demonstrating positive GI protection outcomes103,104,105. There are few studies which report on the oxygen barrier properties of pure gellan gum. However, when gellan gum is blended with other materials, low-to-moderate oxygen diffusivity rates are observed for dried films and gel beads106,107.
Gellan and xanthan gum-based microencapsulation of EOS anaerobes
To date, no studies have reported effective viability protection of probiotic EOS bacteria encapsulated in pure gellan gum; reported applications involve blends with xanthan gum (Table 3). A single study used pure gellan gum for non-probiotic encapsulation of strictly anaerobic Thermosipho sp. AT1272 and Thermococcus kodakarensis KOD1 to study growth under extreme physicochemical stressors, though viability under aerobic conditions was not assessed108. The commonly added blending material, xanthan gum, an exopolysaccharide produced by Xanthomonas campestris, is widely used in food and pharmaceutical formulations due to its high viscosity, shear-thinning behaviour, and stability across broad pH and temperature ranges. Its polyanionic nature and ability to form synergistic gels with other polysaccharides, particularly gellan gum, in the presence of cations, create a more dense gel network than the individual biopolymers alone109,110. McMaster and Kokott encapsulated B. lactis in gellan-xanthan gum microcapsules using monoaxial extrusion with superposed airflow111. After aerobic encapsulation and 1 h gel hardening, encapsulated cells exhibited minimal viability loss (≤0.5 log) over 21 days of storage in phosphate buffer at 4 °C or 22 °C, with high encapsulation efficiency (10–12 log CFU/g) (Fig. 3). However, the oxygen sensitivity of the strain was not assessed, and B. lactis is considered aerotolerant, supported by enhanced NADH oxidase activity and antioxidant defences112. Thus, the protective capacity of gellan gum for strictly anaerobic strains remains unclear.
Blended microcomposites were highly protective for EOS strains during prolonged oxygen-exposed storage111
Proteins
Proteins are polymers whereby amino acids are polymerised via peptide bonds into polypeptide chains; these chains can fold into three-dimensional structures stabilised by hydrogen and other noncovalent interactions, thereby providing substantial structural and functional variety. These properties can vary with the biological source (e.g., animal, plant, or microbial)113. Proteins in dry film form exhibit low oxygen permeability due to their dense molecular structure and strong intramolecular interactions (hydrogen bonds, disulfide bridges, hydrophobic forces)114,115,116,117. These properties make them ideal for food packaging applications118. Barrier properties depend on protein type (e.g. whey, soy, β-lactoglobulin) and cross-linking. Shah et al. highlighted how crosslinking improves both mechanical strength and barrier function119, while Martins et al. emphasised strong oxygen barrier performance despite the hydrophilic nature of protein films120. Composite films incorporating antioxidants into gelatine-sodium caseinate (e.g. black elderberry extract) improved oxygen and moisture barrier properties121. Soy protein films demonstrate 260–670-fold lower oxygen permeability than plastics117, with cross-linking enzymes such as transglutaminase improving their gas barrier function114.
Protein films cast from solution generally show moderate to high oxygen barrier properties under standard (~50% relative humidity room-temperature) conditions. Gelatine, and in particular fish gelatine, is more permeable, while milk-protein films (e.g. casein, whey protein concentrate (WPC), and isolate (WPI)) typically provide stronger barriers. However, this oxygen barrier performance can vary widely with plasticiser type/concentration used, relative humidity, and film preparation122,123,124. Notably, oxygen barrier data have been mostly reported for dry-casted films, compared to measurements on hydrated protein gels (especially in microparticle form).
The number of studies reporting quantitative oxygen permeability data for protein-based microcomposite matrices is limited in comparison to their use in film forms (similar to polysaccharides). In addition, the influence of protein cross-linking (ionic, covalent, or enzymatic) on oxygen barrier properties is understudied in these microcapsule studies. However, protein-based micro-encapsulation has been consistently demonstrated to delay the oxidation process of lipids and fats (and thus oxygen diffusion)125,126,127. Yang et al. compared whey, pea and soy protein encapsulated systems and observed that soy protein-based microencapsulation provided the lowest peroxide formation of ω-3 medium- and long-chain triacylglycerols during aerobic storage for 10 weeks (either at 20 °C and 60 °C); associated with its smaller particle size and continuous surface films around the oil droplets126.
Protein-based microencapsulation of EOS anaerobes
For EOS probiotics, Annan et al. used a gelatine-based emulsion system to encapsulate B. adolescentis 15703 T (43% encapsulation efficiency) under atmospheric conditions (Table 3)128. During 5 h simulated (atmospheric) GI exposure, encapsulated cells exhibited a 3-log viability reduction, partially improved with an alginate coating. B. adolescentis is considered oxygen sensitive129. Furthermore, in transglutaminase-gelled caseinate microcapsules (formed via emulsification), B. lactis Bb-12 showed markedly high viability in SGF over 90 min in ambient air conditions, with encapsulated cells demonstrating a ~0.5 log CFU/g loss. The loading capacity for the viable bacteria was high (with 9.6 log CFU/g)130. In a rennet-gelled skim-milk-protein system, the same authors reported that encapsulated B. lactis Bb-12 survived ambient gastric challenge after 90 min, with only ~1 log CFU/g viability loss. Viable encapsulation yield was found around 9 log CFU/g. In terms of storage stability, casein-based microencapsulation provided high Bb-12 survival up to 90 days of ambient storage across 11–33% relative humidity at 4 °C or 25 °C131.
Using WPI microcapsules formed by extrusion, B. bifidum stored in yoghurt at 4 °C for 28 days declined by around 0.83 log (from 8.54 to 7.71 log CFU/mL), outperforming that with alginate microcapsules. Under SGF and SIF, WPI microcapsules exhibited ~0.30 and ~0.15 log viability reductions after 4 h each, respectively, with 95% viable encapsulation efficiency132. High storage stability of B. bifidum F-35 in set-yoghurt was also observed in similarly gelled WPI microparticles (using an emulsion approach), with a viable loss of 1.66 log CFU/g after 14 days at 4 °C133.
Overall, current protein-based wet encapsulation approaches (excluding those with post-encapsulation dehydration steps) provide varied protection for EOS strains under oxidative stress, with changes influenced by the source of protein used. Across pure protein systems, enzymatically gelled casein (transglutaminase or rennet) and WPI matrices provide substantial protection for bifidobacteria against indirect oxygen exposure during ambient air storage and GI simulation, whereas lower viability protection was achieved with gelatine.
Combined materials
Several studies have explored combining biopolymers to enhance probiotic encapsulation and stability (Table 4). Chen et al. encapsulated B. bifidum CCRC 11844 using alginate–gellan gum blends with peptides and fructooligosaccharides (FOS), achieving > 90% survival after 8 weeks at 4 °C under aerobic agitation (~21% O2), superior to alginate alone (Fig. 4)134. Similarly, Frakolaki et al. tested alginate blends with xanthan gum, whey, pectin, κ-carrageenan, cellulose nanocrystals and milk, supplemented with glucose, inulin, or L-cysteine-HCl45. These combinations improved B. animalis subsp. lactis Bb-12 stability under ambient air storage (50– 60% survival at −18 °C, 40–50% at 4 °C) and GI challenge compared to alginate alone. Inulin modestly enhanced survival, and L-cysteine-HCl provided only minor benefit.
Combined material formulations were highly protective for EOS strains when incorporating fructooligosaccharide (FOS) and peptide excipients during prolonged oxygen-exposed storage134
Xanthan gum-tea protein Pickering emulsions also provided strong protection for B. lactis during aerobic storage (11 days, 4 °C, ~21% O2) and post-SGF exposure, attributed to enhanced network structure and oil droplet barriers135. Chen et al. encapsulated B. bifidum BB01 in xanthan-chitosan microcapsules (78-92% encapsulation efficiency)136, achieving moderate aerobic (~21% O2) stability during 2 h SGF and bile exposure (with 3–4 log CFU/g reduction), further improved with an outer xanthan coating.
High storage stability at 4 °C for 14–28 days for B. bifidum F-35 and B. infantis ATCC 15697 has also been demonstrated via alginate micro-encapsulation when reinforced with proteins into the gel composite (i.e. WPI or PPI), with losses of 1.37 and 1.56 log CFU/g in viable counts for B. bifidum and B. infantis, respectively133,137. Similar viability protection (~1.16 log CFU/g reduction) of B. animalis Bb-12 has also been reported with alginate-chitosan blends of up to 21 days during refrigerated storage in fermented (acidic) beverages138. In addition, although no post-encapsulation viability assessments were conducted, the efficiency of viable B. lactis Bb-12 cells encapsulation via extrusion was found to be 52% using alginate and agar (reduced to 39% when casein was incorporated into the gel matrix)139.
While the oxygen sensitivity of strains was not explicitly assessed, overall, multi-material biopolymer composites appear to enhance EOS stability under 21% (atmospheric) O2 exposure compared to single-polymer formulations.
Dehydrated biopolymeric microformulations for EOS bacteria
Several studies aiming for EOS colonic delivery have incorporated a drying step post-microencapsulation (usually via extrusion or emulsification) to enhance stability and handling (Table 5). Many assessed viability impacts post-drying and during subsequent aerobic GI simulation or storage, again offering indirect insights into potential oxygen impacts on viability.
High encapsulation yields have been reported despite initial aerobic processing: e.g. B. animalis ATCC 25527 (~92–95% after 30 min hardening)140; B. longum CICC 6259 (~70% after 30 min phase separation of emulsion and gel formation)141. Cook et al. found no major difference in SGF protection between wet vs. fluid-bed dried chitosan-coated alginate microcapsules containing B. breve NCIMB 8807 (~2.6–2.9 log loss after 2 h SGF), with coated forms outperforming alginate-only ones under ambient air conditions23. Similarly, freeze-dried alginate microcapsules for B. longum strains showed variable SGF/SIF survival: ~5 log CFU/mL for CICC 6259 and BIOMA 5920 after 60 min SGF141,142, with no detectable counts for DD98143. However, it is unclear whether this detrimental effect was primarily due to the oxygen exposure, the GI environment itself, or the freeze-drying step. Survival in SIF also varied, with 3.6 log CFU/mL reported for CICC 6259 after 240 min and undetectable for DD98 after 15 min. Protection was improved with chitosan, N₂O-carboxymethyl chitosan coatings, or alginate oligosaccharide incorporation141,142,143. These modifications could increase the oxygen protection by improving structural integrity and reducing pore size. Viability of B. animalis ATCC 25527 under aerobic (~21% O2) GI and storage conditions exhibited reductions from ~9.8 to ~7 log CFU/mL during 2 h SGF/bile exposure and from ~9.3 to ~6 log CFU/mL following 1 month refrigerated storage140. Co-encapsulation with prebiotic ciceritol improved these survival rates. Schofield et al. also used a freeze-dried alginate-based carrier for B. lactis PA-BL21 and observed a moderate shelf-life protection. After 12 weeks of storage (at high humidity), viability losses were ~2.05 log CFU/g at 4 °C and ~2.45 log CFU/g at 30 °C. This viability was enhanced through blending with other materials, such as hydroxypropyl methyl cellulose and gellan gum144.
When alginate microcapsules were coated with chitosan and freeze-dried, several studies demonstrated improved EOS viability under aerobic (~21% O2) conditions: Chávarri et al.145 and Ji et al.143 reported high survival of B. bifidum and B. longum strains after 2 h SGF and SIF exposure without anaerobic handling, suggesting possible oxygen protection. Ji et al. further demonstrated that chitosan-coated alginate provided better protection during open-air GI simulation than uncoated alginate143. Chang et al. found no viability loss for B. longum CICC 6259 after 4 h aerobic SIF in chitosan oligosaccharide-coated, freeze-dried microcapsules141; however, SGF exposure still resulted in ~3.4 log reduction to 6.2 log CFU/mL. Mi et al. observed high survival (~7 log CFU/mL) for B. longum BIOMA 5920 after 120 min SGF treatment142. For long-term ambient air storage, Ji et al. reported strong stability of B. longum in chitosan-coated alginate capsules (~8 log CFU/g after 180 days at 25 °C)143, clearly outperforming both uncoated alginate and free cells. By contrast, Chávarri et al. found greater viability loss for B. bifidum (~7 log CFU/mL after 28 days at 4 °C)145. Together, these findings suggest that chitosan-coated, freeze-dried alginate formulations may offer superior oxygen protection for EOS strains during GI transit and long-term storage, compared to wet formulations.
Pectin-based freeze-dried microcapsules also demonstrated high GI protection for B. breve CICC 6182146, with >99% encapsulation yield and strong survival during 4.5 h of sequential SGF, bile, and SIF exposure (with ~2 log CFU/g reduction), and after 13 weeks of aerobic storage at both refrigerated and frozen temperatures (as re-hydrated microparticles in an EDTA buffer). Marcial-Coba et al147,148. demonstrated promising aerobic and anaerobic stability for A. muciniphila encapsulated in freeze-dried gellan–xanthan gum blends. Encapsulation efficiency ranged from 64 to 83% (depending on the cryoprotectant used, following 30 min gel hardening and freeze-drying under aerobic conditions), and viability was maintained (with ~ 1 log reduction) across storage conditions for 1 month with sucrose or trehalose. Around 0.3 log CFU/mL reduction was also reported after 1 h treatment in simulated gastric conditions (at pH 4). Survival of A. muciniphila was not significantly lower than that of the facultative anaerobe L. plantarum in these formulations during ambient air storage at 4 °C for 30 days, whereas significantly lower stability was reported at 25 °C.
In contrast, B. longum, encapsulated in soy protein isolate/ι-carrageenan complexes, in freeze-dried form, exhibited poor ambient air GI protection (~4 log remaining), although this was better than unencapsulated bacterial controls149. Aerobic storage stability at 4 °C was improved (with ≤2-log loss), but strain oxygen sensitivity was not assessed.
Across studies, freeze-dried alginate and chitosan-coated alginate formulations showed inconsistent EOS protection under oxygen-replete (~21% O2) conditions, similar to wet forms, though coating with chitosan generally enhanced viability. Pectin-based dehydrated systems mirrored those of wet forms, with high protection following open-air GI simulation. Gellan gum-xanthan gum blends provided high viability retention for A. muciniphila. Dehydrated protein- and multimaterial-based encapsulation remains poorly explored for EOS. No data were found for carrageenan systems which incorporate a drying step for EOS delivery.
Conclusions and future directions
This review examined whether conventional biopolymer-based microcomposite formulations can effectively deliver EOS NGPs to the colon. As no published studies have directly assessed the impact of oxygen exposure during microencapsulation on EOS viability, we systematically reviewed existing literature, focusing on studies that formulated EOS strains, applied microencapsulation, and assessed aerobic survival during GI simulation or storage. Most evidence derives from Bifidobacterium spp., used here as a model EOS NGPs.
Most studies employed extrusion or emulsification as encapsulation methods, but no consistent differences were observed in viability outcomes based on technique alone. Instead, the material composition of microcomposites appeared more critical. Pectin and gellan gum (especially when blended with xanthan gum) consistently supported viability under oxygen-replete conditions, as did microcomposites formulations that included proteins (WPI, casein, soy and skim-milk), or multi-material biopolymer blends. Conversely, alginate-based systems demonstrated a mixed performance, with viability generally improving when blended with other polymers or coated with chitosan. Pure chitosan was only tested once (with an aerotolerant Bifidobacterium). While freeze-drying is commonly used to improve storage and handling, viability outcomes for freeze-dried formulations were largely comparable to their wet counterparts (Fig. 5).
A key gap is the near-universal omission of anaerobic conditions during the encapsulation process itself. Even though bacteria were typically cultured anaerobically, atmospheric oxygen exposure during formulation was often unavoidable and unmeasured. Raise et al. are among the few to conduct encapsulation entirely under anaerobic conditions16, yet still observed 3–4 log reductions in F. prausnitzii viability after simulated GI exposure, suggesting that even in oxygen-free processing, other factors may compromise EOS survival.
Bifidobacterium species dominated the current literature, but many of these strains display partial aerotolerance. As such, findings cannot be reliably extended to fastidious EOS candidates like F. prausnitzii or A. muciniphila, which are increasingly proposed as NGPs. Future studies must include a broader range of true EOS organisms, systematically evaluate their oxygen sensitivity at the strain level, and align formulation strategies with their specific oxygen tolerance thresholds. Growth phase effects also remain underexplored; identifying growth resilience windows (e.g. exponential vs stationary phase) could inform optimal encapsulation strategies. Spore formation, while outside the scope of this review, also warrants further exploration for oxygen-sensitive delivery.
Hydrogels are chemically expected to provide limited oxygen barriers due to high water content and porosity; studies examining oxygen diffusion across such materials confirmed this. However, material packing, crosslinking, and layering may influence diffusion and have been overlooked. Variables such as particle size, surface morphology, and matrix density also require investigation.
Notably, many studies report substantial viability loss over time (>50%), yet surviving EOS bacteria remain detectable, suggesting some long-term buffering, possibly through slowed diffusion or metabolic dormancy. Whether survival arises from protective microenvironments or physiological adaptations remains unclear.
Most current formulations rely on natural polymers for regulatory simplicity. Future advances will likely require engineered materials forming denser matrices or incorporating oxygen-scavenging properties. Direct evaluation of polymer oxygen permeability, improved standardisation of oxygen exposure reporting, and systematic design of composite materials will be essential for next-generation EOS formulations. Although not discussed in detail in this review, consideration of how EOS microencapsulation could enable delivery through different formulation formats is important, as each presents distinct oxygen exposure challenges. Most commercial probiotics are currently delivered in dry (e.g., capsules, powders, or foods) or wet formats (e.g., yoghurts and drinks). Dry systems are generally more suitable for EOS strains due to lower oxygen diffusion in the dehydrated state and the potential use of barrier materials; however, exposure during processing, storage, and rehydration, alongside viability losses from drying, remain key limitations. EOS NGPs are not yet commercially available in wet formulations, likely due to the high oxygen permeability of such systems. Conventional probiotic formats are optimised for aerotolerant strains, whereas successful EOS formulations must be designed to maintain strict oxygen restriction throughout manufacture, storage, and use.
The literature provides very limited direct comparisons of the oxygen barrier performance between protein- and polysaccharide-based microcapsule systems, particularly for the case of ionically cross-linked matrices. In film form, proteins have often been reported to exhibit superior performance compared to polysaccharides in inhibiting oxygen ingress, especially under dry conditions. This advantage is generally attributed to their ability to form denser and more compact networks, as well as the presence of hydrophobic amino acid residues that provide additional resistance to both moisture and oxygen solubility, characteristics that polysaccharides inherently lack150,151,152. However, proteins also hold certain limitations for barrier applications, such as their potential structural changes (including denaturation) over long-term storage or under thermal conditions, which may affect oxygen permeability over time, as well as their higher sourcing costs and the risks of intolerances or incompatibility with specific dietary restrictions153,154,155.
Moving forward, key recommendations for future research and formulation development include:
-
Assess the oxygen tolerance of each strain prior to formulation, as this dictates the threshold of oxygen protection required; tailored colonic delivery formulations for NGPs are key.
-
Define whether oxygen protection is needed during brief processing windows or longer-term storage and GI transit.
-
Formulations intended to provide oxygen protection should be systematically evaluated for oxygen-barrier efficiency, with post-formulation testing under both aerobic and anaerobic conditions considered essential to fully validate protective performance.
-
For strains amenable to dehydration, layered or composite microencapsulation with freeze-drying may offer scalable solutions.
-
For highly sensitive strains, fully anaerobic encapsulation processes using wet microcomposites formulations may remain necessary.
-
Standardise and transparently report oxygen exposure across all stages of the encapsulation workflow.
Future research should prioritise direct measurement of oxygen permeability in encapsulating materials and the development of novel polymer systems with superior barrier properties. The methodological gaps identified in this review significantly affect the interpretation of EOS strain viability within current formulation materials, particularly for those with uncharacterised oxygen sensitivity. Addressing these limitations will enable more rational, evidence-based design of microencapsulation systems capable of delivering viable and therapeutically effective EOS probiotics.
References
Hill, C. et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
Chandrasekaran, P., Weiskirchen, S. & Weiskirchen, R. Effects of probiotics on gut microbiota: an overview. Int. J. Mol. Sci. 25, 6022 (2024).
Mazziotta, C., Tognon, M., Martini, F., Torreggiani, E. & Rotondo, J. C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 12, 184 (2023).
Petrariu O. A. et al. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front. Microbiol. 14, 1296447 (2024).
Feng, T. & Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes 12, 1801944 (2020).
Ibrahim, S. A. et al. A review and comparative perspective on health benefits of probiotic and fermented foods. Int. J. Food Sci. Technol. 58, 4948–4964 (2023).
El Hage, R., Hernandez-Sanabria, E. & Van De Wiele, T. Emerging trends in “smart probiotics”: functional consideration for the development of novel health and industrial applications. Front. Microbiol. 8, 1889 (2017).
Khan, M. T. et al. Synergy and oxygen adaptation for development of next-generation probiotics. Nature 620, 381–385 (2023).
Luo, Y. et al. Precise oral delivery systems for probiotics: a review. J. Control. Release 352, 371–384 (2022).
Iaconelli, C. et al. Drying process strongly affects probiotics viability and functionalities. J. Biotechnol. 214, 17–26 (2015).
Bircher, L., Geirnaert, A., Hammes, F., Lacroix, C. & Schwab, C. Effect of cryopreservation and lyophilization on viability and growth of strict anaerobic human gut microbes. Microb. Biotechnol. 11, 721–733 (2018).
Rajam, R. & Subramanian, P. Encapsulation of probiotics: past, present and future. Beni Suef Univ. J. Basic Appl. Sci. 11, 46 (2022).
Ladero, V. & Sánchez, B. Molecular and technological insights into the aerotolerance of anaerobic probiotics: examples from bifidobacteria. Curr. Opin. Food Sci. 14, 110–115 (2017).
Cook, M. T., Tzortzis, G., Charalampopoulos, D. & Khutoryanskiy, V. V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release Off. J. Control. Release Soc. 162, 56–67 (2012).
Song, H., Yu, W., Gao, M., Liu, X. & Ma, X. Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydr. Polym. 96, 181–189 (2013).
Raise, A. et al. Comparison of two encapsulation processes to protect the commensal gut probiotic bacterium Faecalibacterium prausnitzii from the digestive tract. J. Drug Deliv. Sci. Technol. 56, 101608 (2020).
Ta, L. P., Corrigan, S. & Horniblow, R. D. Novel pectin-carboxymethylcellulose-based double-layered mucin/chitosan microcomposites successfully protect the next-generation probiotic Akkermansia muciniphila through simulated gastrointestinal transit and alter microbial communities within colonic ex vivo bioreactors. Int. J. Pharm. 665, 124670 (2024).
Kawasaki, S., Mimura, T., Satoh, T., Takeda, K. & Niimura, Y. Response of the Microaerophilic Bifidobacterium Species, B. boum and B. thermophilum, to Oxygen. Appl. Environ. Microbiol. 72, 6854–6858 (2006).
Xiao, M. et al. Oxidative stress-related responses of Bifidobacterium longum subsp. longum BBMN68 at the proteomic level after exposure to oxygen. Microbiology 157, 1573–1588 (2011).
Andriantsoanirina, V., Allano, S., Butel, M. J. & Aires, J. Tolerance of Bifidobacterium human isolates to bile, acid and oxygen. Anaerobe 21, 39–42 (2013).
Marcos-Fernández, R. et al. Towards the isolation of more robust next generation probiotics: The first aerotolerant Bifidobacterium bifidum strain. Food Res. Int. 165, 112481 (2023).
Simpson, P. J., Stanton, C., Fitzgerald, G. F. & Ross, R. P. Intrinsic tolerance of Bifidobacterium species to heat and oxygen and survival following spray drying and storage. J. Appl. Microbiol. 99, 493–501 (2005).
Cook, M. T., Tzortzis, G., Charalampopoulos, D. & Khutoryanskiy, V. V. Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules 12, 2834–2840 (2011).
Ta, L. P. et al. Gastrointestinal-inert prebiotic micro-composites improve the growth and community diversity of mucosal-associated bacteria. J. Controlled Release 375, 495–512 (2024).
Wang, X. et al. Microencapsulating Alginate-Based Polymers for Probiotics Delivery Systems and Their Application. Pharm. Basel Switz. 15, 644 (2022).
Vivek, K. et al. A comprehensive review on microencapsulation of probiotics: technology, carriers and current trends. Appl. Food Res. 3, 100248 (2023).
He, L., Shang, Z., Liu, H. & Yuan, Z. Alginate-Based Platforms for Cancer-Targeted Drug Delivery. BioMed Res. Int. 2020, 1487259 (2020).
Yu, L. et al. Microfluidic formation of core-shell alginate microparticles for protein encapsulation and controlled release. J. Colloid Interface Sci. 539, 497–503 (2019).
Xu, C., Qiao, M., Huo, X., Liao, Z. & Su, J. An oral microencapsulated vaccine loaded by sodium alginate effectively enhances protection against GCRV infection in grass carp (Ctenopharyngodon idella). Front. Immunol. 13, 848958 (2022).
Pech-Canul, A. et al. A brief review of edible coating materials for the microencapsulation of probiotics. Coatings 10, 197 (2020).
Muthukumarasamy, P., Allan-Wojtas, P. & Holley, R. A. Stability of lactobacillus reuteri in different types of microcapsules. J. Food Sci. 71, M20–M24 (2006).
Li, X. Y., Chen, X. G., Sun, Z. W., Park, H. J. & Cha, D.-S. Preparation of alginate/chitosan/carboxymethyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydr. Polym. 83, 1479–1485 (2011).
Afzaal, M. et al. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci. Nutr. 7, 3931–3940 (2019).
Shoaei, F., Heshmati, A., Mahjub, R., Garmakhany, A. D. & Taheri, M. The assessment of microencapsulated Lactobacillus plantarum survivability in rose petal jam and the changes in physicochemical, textural and sensorial characteristics of the product during storage. Sci. Rep. 12, 6200 (2022).
Ali, U. et al. Stability and survivability of alginate gum-coated Lactobacillus rhamnosus GG in simulated gastrointestinal conditions and probiotic juice development. J. Food Qual. 2023, 3660968–13 (2023).
Iyer, C., Phillips, M. & Kailasapathy, K. Release studies of Lactobacillus casei strain Shirota from chitosan-coated alginate-starch microcapsules in ex vivo porcine gastrointestinal contents. Lett. Appl. Microbiol. 41, 493–497 (2005).
Qi, X., Simsek, S., Ohm, J.-B., Chen, B. & Rao, J. Viability of Lactobacillus rhamnosus GG microencapsulated in alginate/chitosan hydrogel particles during storage and simulated gastrointestinal digestion: role of chitosan molecular weight. Soft Matter 16, 1877–1887 (2020).
Mehmetoglu, Ü, Ateş, S. & Berber, R. Oxygen diffusivity in calcium alginate gel beads containing gluconobacter suboxydans. Artif Cells Blood Substit Biotechnol. 24, 91–106 (1996).
Han, P. & Bartels, D. M. Temperature dependence of oxygen diffusion in H2O and D2O. J. Phys. Chem. 100, 5597–5602 (1996).
Orimi, H. E. et al. An accessible platform to quantify oxygen diffusion in cell-laden hydrogels and its application to alginate-immobilized pancreatic beta cells. Biotechnol. Bioeng. 123, 348–362 (2025).
Zhao, W. et al. Oxygen diffusivity in alginate/chitosan microcapsules. J. Chem.Technol. Biotechnol. 88, 449–455 (2013).
Mayrhofer, A., Kopacic, S. & Bauer, W. Extensive characterization of alginate, chitosan and microfibrillated cellulose cast films to assess their suitability as barrier coating for paper and board. Polymers 15, 3336 (2023).
Phùng, T. T. et al. Sodium Alginate as a promising encapsulating material for extremely-oxygen sensitive probiotics. Food Hydrocoll 160, 110857 (2025).
Yeung, T. W., Üçok, E. F., Tiani, K. A., McClements, D. J. & Sela, D. A. Microencapsulation in alginate and chitosan microgels to enhance viability of Bifidobacterium longum for oral delivery. Front. Microbiol. 7, 494 (2016).
Frakolaki, G., Giannou, V. & Tzia, C. Encapsulation of Bifidobacterium animalis subsp. Lactis Through Emulsification Coupled with External Gelation for the Development of Synbiotic Systems. Probiotics Antimicrob. Proteins 15, 1424–1435 (2023).
Ding, W. & Shah, N. Effect of homogenization techniques on reducing the size of microcapsules and the survival of probiotic bacteria therein. J. Food Sci. 74, M231–M236 (2009).
Cook, M. T. et al. CLSM method for the dynamic observation of ph change within polymer matrices for oral delivery. Biomacromolecules 14, 387–393 (2013).
Huang, Y. et al. Osteopontin associated Bifidobacterium bifidum microencapsulation modulates infant fecal fermentation and gut microbiota development. Food Res. Int. 197, 115211 (2024).
Naklong, K. et al. Microencapsulation of Bifidobacterium breve to enhance microbial cell viability in green soybean Yogurt. Fermentation 9, 296 (2023).
Ullah, F. S., Saeed, M., Shabbir, M. A. & Israr, B. Microencapsulation of Lactobacillus acidophilus LA-832 and Bifidobacterium longum BL-101 by using sodium alginate and chitosan to improve the survival in simulated gastrointestinal conditions. J. Food Sci. Technol. https://doi.org/10.1007/s13197-025-06345-5 (2025).
Zhang, Z. et al. Encapsulation of bifidobacterium in alginate microgels improves viability and targeted gut release. Food Hydrocoll 116, 106634 (2021).
Machado, D. et al. Akkermansia muciniphila encapsulated in calcium-alginate hydrogelated matrix: viability and stability over aerobic storage and simulated gastrointestinal conditions. Gels Basel Switz 9, 869 (2023).
Derrien, M., Vaughan, E. E., Plugge, C. M. & De Vos, W. M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 54, 1469–1476 (2004).
Zhang, T., Li, Q., Cheng, L., Buch, H. & Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 12, 1109–1125 (2019).
Ouwerkerk, J. P. et al. Adaptation of Akkermansia muciniphila to the Oxic-Anoxic Interface of the Mucus Layer. Appl. Environ. Microbiol. 82, 6983–6993 (2016).
Zhao, Y. et al. Akkermansia muciniphila: a promising probiotic against inflammation and metabolic disorders. Virulence 15, 2375555 (2024).
Motalebi Moghanjougi, Z., Rezazadeh Bari, M., Alizadeh Khaledabad, M., Amiri, S. & Almasi, H. Microencapsulation of Lactobacillus acidophilus LA-5 and Bifidobacterium animalis BB-12 in pectin and sodium alginate: a comparative study on viability, stability, and structure. Food Sci. Nutr. 9, 5103–5111 (2021).
Azmana, M. et al. A review on chitosan and chitosan-based bionanocomposites: promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 185, 832–848 (2021).
Aranaz, I. et al. Chitosan: an overview of its properties and applications. Polymers 13, 3256 (2021).
Giraldo, J. D., Campos-Requena, V. H. & Rivas, B. L. Chitosan–tripolyphosphate bead: the interactions that govern its formation. Polym. Bull. 76, 3879–3903 (2019).
Kildeeva, N. R., Perminov, P. A., Vladimirov, L. V., Novikov, V. V. & Mikhailov, S. N. About mechanism of chitosan cross-linking with glutaraldehyde. Russ. J. Bioorganic Chem. 35, 360–369 (2009).
Enache, A. A., David, L., Puaux, J.-P., Banu, I. & Bozga, G. Kinetics of chitosan coagulation from aqueous solutions. J. Appl. Polym. Sci. 135, 46062 (2018).
Jiang, A. et al. Chitosan based biodegradable composite for antibacterial food packaging application. Polymers 15, 2235 (2023).
Ways, T. M. M., Lau, W. M. & Khutoryanskiy, V. V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 10, 267 (2018).
Haider, A. et al. Advances in chitosan-based drug delivery systems: a comprehensive review for therapeutic applications. Eur. Polym. J. 210, 112983 (2024).
Gonçalves, I. C. et al. Bacterial-binding chitosan microspheres for gastric infection treatment and prevention. Acta Biomater. 9, 9370–9378 (2013).
Kong, M., Chen, X. G., Xing, K. & Park, H. J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 144, 51–63 (2010).
Cazón, P. & Vázquez, M. Mechanical and barrier properties of chitosan combined with other components as food packaging film. Environ. Chem. Lett. 18, 257–267 (2020).
Krasaekoopt, W., Bhandari, B. & Deeth, H. C. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT- and conventionally treated milk during storage. LWT Food Sci. Technol. 39, 177–183 (2006).
Vodnar, D. C. & Socaciu, C. Green tea increases the survival yield of Bifidobacteria in simulated gastrointestinal environment and during refrigerated conditions. Chem. Cent. J. 6, 61 (2012).
Al-Awaida, W. et al. Assessing the protective role of Epigallocatechin Gallate (EGCG) against water-pipe smoke-induced toxicity: a comparative study on gene expression and histopathology. Molecules 28, 7502 (2023).
Shah, N. P., Ding, W. K., Fallourd, M. J. & Leyer, G. Improving the stability of probiotic bacteria in model fruit juices using vitamins and antioxidants. J. Food Sci. 75, M278–M282 (2010).
Tanaka, K. et al. O2-inducible H2O2-forming NADPH oxidase is responsible for the hyper O2 sensitivity of Bifidobacterium longum subsp. infantis. Sci. Rep. 8, 10750 (2018).
Bolduc, M.-P., Raymond, Y., Fustier, P., Champagne, C. P. & Vuillemard, J.-C. Sensitivity of bifidobacteria to oxygen and redox potential in non-fermented pasteurized milk. Int. Dairy J. 16, 1038–1048 (2006).
Luo, Y. et al. Microfluidic fabrication of encapsulated probiotic microspheres using cysteine-modified chitosan with dual functions of bacterial adhesion and intestinal mucosal adhesion. Food Hydrocoll 149, 109602 (2024).
Chen, P. et al. Enhance the resistance of probiotics by microencapsulation and biofilm construction based on rhamnogalacturonan I-rich pectin. Int. J. Biol. Macromol. 258, 128777 (2024).
Sun, Q. & Wicker, L. Hydrogel encapsulation of lactobacillus casei by block charge modified pectin and improved gastric and storage stability. Foods 10, 1337 (2021).
Raddatz, G. C. et al. Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Res. Int. 130, 108902 (2020).
Liu, L., Fishman, M. L., Kost, J. & Hicks, K. B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 24, 3333–3343 (2003).
Das, S. Pectin based multi-particulate carriers for colon-specific delivery of therapeutic agents. Int. J. Pharm. 605, 120814 (2021).
Ghibaudo, F., Gerbino, E., Campo Dall’ Orto, V. & Gómez-Zavaglia, A. Pectin-iron capsules: novel system to stabilise and deliver lactic acid bacteria. J. Funct. Foods 39, 299–305 (2017).
He, C., Sampers, I., Van de Walle, D., Dewettinck, K. & Raes, K. Encapsulation of Lactobacillus in Low-Methoxyl Pectin-Based Microcapsules Stimulates Biofilm Formation: Enhanced Resistances to Heat Shock and Simulated Gastrointestinal Digestion. J. Agric. Food Chem. 69, 6281–6290 (2021).
Larsen, N., Cahú, T. B., Isay Saad, S. M., Blennow, A. & Jespersen, L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol 74, 11–20 (2018).
Manuel, S. G. Fruit waste pectin in enhancing the establishment of probiotic bacteria. J. Nutr. Health Food Eng. 1, 3 (2014).
Gottesmann, M., Goycoolea, F. M., Steinbacher, T., Menogni, T. & Hensel, A. Smart drug delivery against Helicobacter pylori: pectin-coated, mucoadhesive liposomes with antiadhesive activity and antibiotic cargo. Appl. Microbiol. Biotechnol. 104, 5943–5957 (2020).
Panahirad, S. et al. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 110, 663–673 (2021).
Çavdaroğlu, E., Büyüktaş, D., Farris, S. & Yemenicioğlu, A. Novel edible films of pectins extracted from low-grade fruits and stalk wastes of sun-dried figs: Effects of pectin composition and molecular properties on film characteristics. Food Hydrocoll 135, 108136 (2023).
Sarıyer, S., Duranoğlu, D., Doğan, Ö & Küçük, İ pH-responsive double network alginate/kappa-carrageenan hydrogel beads for controlled protein release: Effect of pH and crosslinking agent. J. Drug Deliv. Sci. Technol. 56, 101551 (2020).
Nualkaekul, S., Cook, M. T., Khutoryanskiy, V. V. & Charalampopoulos, D. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res. Int. 53, 304–311 (2013).
Kalsi, G., Hazarika, U., Baruah, L. D., Bordoloi, P. L. & Gogoi, M. Comprehensive review of carrageenan’s multifaceted role in health and food systems. Discov. Food 5, 115 (2025).
Koh, W. Y., Lim, X. X., Tan, T.-C., Kobun, R. & Rasti, B. Encapsulated probiotics: potential techniques and coating materials for non-dairy food applications. Appl. Sci. 12, 10005 (2022).
Saeed, M. et al. Viability of free and alginate–carrageenan gum coated Lactobacillus acidophilus and Lactocaseibacillus casei in functional cottage cheese. ACS Omega 9, 13840–13851 (2024).
Wang, R., Zhang, S., Liu, S., Sun, Y. & Xu, H. A contribution to improve barrier properties and reduce swelling ratio of κ-carrageenan film from the incorporation of guar gum or locust bean gum. Polymers 15, 1751 (2023).
Guo, J. et al. Effects of hydroxypropyl methylcellulose on physicochemical properties and microstructure of κ-carrageenan film. Foods 11, 3023 (2022).
Phùng, T. T. et al. Comprehensive approach to the protection and controlled release of extremely oxygen sensitive probiotics using edible polysaccharide-based coatings. Int. J. Biol. Macromol. 218, 706–719 (2022).
Cheow, W. S. & Hadinoto, K. Biofilm-like lactobacillus rhamnosus probiotics encapsulated in alginate and carrageenan microcapsules exhibiting enhanced thermotolerance and freeze-drying resistance. Biomacromolecules 14, 3214–3222 (2013).
Shi, L.-E. et al. Encapsulation of Lactobacillus bulgaricus in carrageenan-locust bean gum coated milk microspheres with double layer structure. LWT Food Sci. Technol. 54, 147–151 (2013).
Dafe, A., Etemadi, H., Zarredar, H. & Mahdavinia, G. R. Development of novel carboxymethyl cellulose/k-carrageenan blends as an enteric delivery vehicle for probiotic bacteria. Int. J. Biol. Macromol. 97, 299–307 (2017).
Adhikari, K., Mustapha, A., Grün, I. U. & Fernando, L. Viability of microencapsulated bifidobacteria in set yogurt during refrigerated storage. J. Dairy Sci. 83, 1946–1951 (2000).
Mishra, V. et al. Probiotics as potential antioxidants: a systematic review. J. Agric. Food Chem. 63, 3615–3626 (2015).
Fasolin, L. H., Picone, C. S. F., Santana, R. C. & Cunha, R. L. Production of hybrid gels from polysorbate and gellan gum. Food Res. Int. 54, 501–507 (2013).
Machado, M., Silva, S. & Costa, E. Uses of gellan gum for nutrient delivery. in Application of Gellan Gum as a Biomedical Polymer (eds Nayak, A. K. & Hasnain, M. S.) 309-321 https://doi.org/10.1016/B978-0-323-91815-2.00025-9 (Academic Press, 2024).
Nag, A., Han, K.-S. & Singh, H. Microencapsulation of probiotic bacteria using pH-induced gelation of sodium caseinate and gellan gum. Int. Dairy J. 21, 247–253 (2011).
Jiménez-Pranteda, M. L. et al. Stability of lactobacilli encapsulated in various microbial polymers. J. Biosci. Bioeng. 113, 179–184 (2012).
Picone, C. S. F., Bueno, A. C., Michelon, M. & Cunha, R. L. Development of a probiotic delivery system based on gelation of water-in-oil emulsions. LWT 86, 62–68 (2017).
Teoh, R. W., Ting, A. S. Y. & Thoo, Y. Y. Characterization and modeling of diffusion kinetics of rosemary oleoresin extract from gellan gum-based film. J. Food Sci. Technol. 60, 2978–2989 (2023).
Hulst, A. C., Hens, H. J. H., Buitelaar, R. M. & Tramper, J. Determination of the effective diffusion coefficient of oxygen in gel materials in relation to gel concentration. Biotechnol. Tech. 3, 199–204 (1989).
Landreau, M. et al. Entrapment of anaerobic thermophilic and hyperthermophilic marine micro-organisms in a gellan/xanthan matrix. J. Appl. Microbiol. 120, 1531–1541 (2016).
Zhang, N. et al. Effect of gellan gum and xanthan gum synergistic interactions and plasticizers on physical properties of plant-based enteric polymer films. Polymers 12, 121 (2020).
Brunchi, C.-E., Morariu, S., Iftime, M.-M. & Stoica, I. Xanthan gum in solution and solid-like state: Effect of temperature and polymer concentration. J. Mol. Liq. 387, 122600 (2023).
McMaster, L. D. & Kokott, S. A. Micro-encapsulation of Bifidobacterium lactis for incorporation into soft foods. World J. Microbiol. Biotechnol. 21, 723–728 (2005).
Ruiz, L. et al. Molecular clues to understand the aerotolerance phenotype of Bifidobacterium animalis subsp. lactis. Appl. Environ. Microbiol. 78, 644–650 (2012).
Lopez, M. J. & Mohiuddin, S. S. Biochemistry, essential amino acids. in StatPearls (StatPearls Publishing, Treasure Island (FL), 2025).
Lim, L.-T., Mine, Y. & Tung, M. A. Transglutaminase cross-linked egg white protein films: tensile properties and oxygen permeability. J. Agric. Food Chem. 46, 4022–4029 (1998).
Purewal, S. S., Kaur, A., Bangar, S. P., Singh, P. & Singh, H. Protein-based films and coatings: an innovative approach. Coatings 14, 32 (2024).
Zink, J., Wyrobnik, T., Prinz, T. & Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: an extensive review. Int. J. Mol. Sci. 17, 1376 (2016).
Chen, H. et al. Application of protein-based films and coatings for food packaging: a review. Polymers 11, 2039 (2019).
Dissanayake, T., Vidanarachchi, J. K., Narvaez-Bravo, C., Mekonnen, T. & Bandara, N. Advances in protein-based materials for functional food packaging. ACS Food Sci. Technol 5, 2604–2617 (2025).
Shah, Y. A. et al. Mechanical properties of protein-based food packaging materials. Polymers 15, 1724 (2023).
Martins, V. G., Romani, V. P., Martins, P. C. & Nogueira, D. Protein-based materials for packaging applications. in Bio-based Packaging 27–49 https://doi.org/10.1002/9781119381228.ch2 (John Wiley & Sons, Ltd, 2021).
Choi, J., Lee, J.-S., Han, J. & Chang, Y. Development of gelatin–sodium caseinate high-oxygen-barrier film containing elderberry (Sambucus nigra L.) extract and its antioxidant capacity on pork. Food Biosci 53, 102617 (2023).
Tyuftin, A. A. & Kerry, J. P. Gelatin films: study review of barrier properties and implications for future studies employing biopolymer films. Food Packag. Shelf Life 29, 100688 (2021).
Wagh, Y. R., Pushpadass, H. A., Emerald, F. M. E. & Nath, B. S. Preparation and characterization of milk protein films and their application for packaging of Cheddar cheese. J. Food Sci. Technol. 51, 3767–3775 (2014).
Hong, S.-I. & Krochta, J. M. Oxygen barrier properties of whey protein isolate coatings on polypropylene films. J. Food Sci. 68, 224–228 (2003).
Kontopidis, G., Holt, C. & Sawyer, L. Invited review: β-Lactoglobulin: binding properties, structure, and function. J. Dairy Sci. 87, 785–796 (2004).
Yang, Z. et al. Encapsulation and characterization of ω-3 medium- and long-chain triacylglycerols microencapsulated with different proteins as wall materials. Food Chem. X 22, 101363 (2024).
Wang, H.-H., Li, M.-Y., Dong, Z.-Y., Zhang, T.-H. & Yu, Q.-Y. Preparation and characterization of ginger essential oil microcapsule composite films. Foods 10, 2268 (2021).
Annan, N. T., Borza, A. D. & Hansen, L. T. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res. Int. 41, 184–193 (2008).
Boyanova, L., Boyanova, L., Hadzhiyski, P., Gergova, R. & Markovska, R. Oxygen tolerance in anaerobes as a virulence factor and a health-beneficial property. Anaerobe 89, 102897 (2024).
Heidebach, T., Först, P. & Kulozik, U. Transglutaminase-induced caseinate gelation for the microencapsulation of probiotic cells. Int. Dairy J. 19, 77–84 (2009).
Heidebach, T., Först, P. & Kulozik, U. Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Food Hydrocoll 23, 1670–1677 (2009).
Afzaal, M. et al. Influence of encapsulation on the survival of probiotics in food matrix under simulated stress conditions. Saudi J. Biol. Sci. 29, 103394 (2022).
Mousa, A. H. et al. Microencapsulation of Bifidobacterium bifidum F-35 via modulation of emulsifying technique and its mechanical effects on the rheological stability of set-yogurt. J. Food Sci. Technol. 60, 2968–2977 (2023).
Chen, M., Chen, K. & Kuo, Y. Optimal thermotolerance of Bifidobacterium bifidum in gellan–alginate microparticles. Biotechnol. Bioeng. 98, 411–419 (2007).
Xu, D. et al. Incorporation of probiotics into 3D printed Pickering emulsion gel stabilized by tea protein/xanthan gum. Food Chem. 409, 135289 (2023).
Chen, L., Yang, T., Song, Y., Shu, G. & Chen, H. Effect of xanthan-chitosan-xanthan double layer encapsulation on survival of Bifidobacterium BB01 in simulated gastrointestinal conditions, bile salt solution and yogurt. LWT Food Sci. Technol. 81, 274–280 (2017).
Khan, W. A. et al. Protein-polysaccharide based double network microbeads improves stability of Bifidobacterium infantis ATCC 15697 in a gastro-Intestinal tract model (TIM-1). Int. J. Pharm. 652, 123804 (2024).
Naemeh, K., Ali, M. S., Elham, M. & Akram, A. Production of the whey protein-based probiotic beverages incorporated with Bifidobacterium bifidum, Lactobacillus acidophilus, and peppermint essence nanoliposomes. J. Food Meas. Charact. 17, 2708–2717 (2023).
Vinceković, M. et al. Development of alginate composite microparticles for encapsulation of Bifidobacterium animalis subsp. lactis. Gels 10, 752 (2024).
Azam, M. et al. Microencapsulation and invitro characterization of Bifidobacterium animalis for improved survival. J. Food Meas. Charact. 15, 2591–2600 (2021).
Chang, L., Wang, P., Sun, S., Shen, Z. & Jiang, X. A synbiotic marine oligosaccharide microcapsules for enhancing Bifidobacterium longum survivability and regulatory on intestinal probiotics. Int. J. FOOD Sci. Technol. 56, 362–373 (2021).
Mi, Y., Su, R., Fan, D.-D., Zhu, X.-L. & Zhang, W.-N. Preparation of N,O-carboxymethyl chitosan coated alginate microcapsules and their application to Bifidobacterium longum BIOMA 5920. Mater. Sci. Eng. C 33, 3047–3053 (2013).
Ji, R. et al. Extending viability of bifidobacterium longum in chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Front. Microbiol. 10, 1389 (2019).
Schofield, T. et al. Microencapsulation of Bifidobacterium lactis and Lactobacillus plantarum within a novel polysaccharide-based core–shell formulation: improving probiotic viability and mucoadhesion. ACS Biomater. Sci. Eng. 10, 6903–6914 (2024).
Chávarri, M. et al. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 142, 185–189 (2010).
Li, M. et al. Preparation of Bifidobacterium breve encapsulated in low methoxyl pectin beads and its effects on yogurt quality. J. Dairy Sci. 102, 4832–4843 (2019).
Marcial-Coba, M. S. et al. Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food Funct. 9, 5868–5879 (2018).
Marcial-Coba, M. S., Saaby, L., Knøchel, S. & Nielsen, D. S. Dark chocolate as a stable carrier of microencapsulated Akkermansia muciniphila and Lactobacillus casei. FEMS Microbiol. Lett. 366, 0 (2019).
Mao, L., Pan, Q., Yuan, F. & Gao, Y. Formation of soy protein isolate-carrageenan complex coacervates for improved viability of Bifidobacterium longum during pasteurization and in vitro digestion. Food Chem. 276, 307–314 (2019).
Pires, A. F., Díaz, O., Cobos, A. & Pereira, C. D. A review of recent developments in edible films and coatings-focus on whey-based materials. Foods 13, 2638 (2024).
Benbettaïeb, N., Gay, J., Karbowiak, T. & Debeaufort, F. Tuning the functional properties of polysaccharide–protein bio-based edible films by chemical, enzymatic, and physical cross-linking. Compr. Rev. Food Sci. Food Saf. 15, 739–752 (2016).
Bhaskar, R. et al. Recent development of protein-based biopolymers in food packaging applications: a review. Polym. Test. 124, 108097 (2023).
Hong, S.-I. & Krochta, J. M. Oxygen barrier performance of whey-protein-coated plastic films as affected by temperature, relative humidity, base film and protein type. J. Food Eng. 77, 739–745 (2006).
Hammann, F. & Schmid, M. Determination quantification of molecular interactions in protein films: a review. Materials 7, 7975–7996 (2014).
Gennadios, A., Weller, C. L. & Testin, R. F. Temperature effect on oxygen permeability of edible protein-based films. J. Food Sci. 58, 212–224 (1993).
Frakolaki, G., Giannou, V., Kekos, D. & Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food Sci. Nutr. 61, 1515–1536 (2021).
Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) Healthcare Technologies Award (EP/V027743). The study was also delivered through the National Institute for Health and Care Research (NIHR) Birmingham Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the EPSRC, the NIHR, or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
R.H. conceived the review design and approach. L.T., S.C., and H.A. conducted the literature searches and review. L.T. and R.H. drafted the manuscript. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ta, L.P., Corrigan, S., Abeysekera, H. et al. Extremely oxygen-sensitive next-generation probiotics: can current microcomposite formulations ensure effective colonic delivery?. Microsyst Nanoeng 12, 37 (2026). https://doi.org/10.1038/s41378-025-01151-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41378-025-01151-7