Abstract
Hand gesture deficits are common in schizophrenia predicting poor social functioning with no treatment currently available. We used 10-sessions of repetitive transcranial magnetic stimulation (rTMS; 2-weeks) over the right inferior parietal lobe (IPL) in combination with 16-sessions of social cognitive remediation group therapy (SCRT; 8-weeks) to examine improvements in hand gesture performance in schizophrenia. In this 3-arm, double-blind, randomized, sham-controlled trial, 89 patients were randomized and 73 received at least one session of rTMS/SCRT: 19 patients were allocated to the real rTMS + real SCRT treatment, 26 to the sham rTMS + real SCRT treatment, and 28 to sham SCRT treatment. Hand gesture performance along with socio-cognitive and functional assessments were examined at 2-weeks, 8-weeks, and 32-weeks follow-up. Of 73 patients analyzed, (57% male), 53 completed the intervention and week-8 assessments. At week-8 no difference in overall hand gesture performance accuracy was observed across treatments. However, at week-32 follow-up the real rTMS + real group SCRT treatment showed significant improvements in novel unlearned gestures (F(6, 210) = 2.2; p-value = 0.04), and significant gains in social functioning/personal performance at week-8 and sustained at week-32 follow-up (all F-values > 2.6; all p-values < 0.05). No treatment effects were found for overall hand gesture performance accuracy. However, improvements in secondary outcomes such as novel unlearned gestures and social/personal functioning hold promise for testing optimized rTMS + group SCRT combinations. Future studies should explore the neural effects of rTMS over right IPL + group SCRT.
Similar content being viewed by others
Introduction
Schizophrenia is a severe mental-illness with multiple clinical characteristics including impairments in social-cognitive abilities, which greatly affect overall functioning and quality of life [1]. Central to the social challenges patients with schizophrenia encounter, is nonverbal communication and in particular gestures [2,3,4]. Hand gestures are movements used alone or in conjunction with speech to convey a meaning or idea [5]. Not only do patients with schizophrenia use fewer gestures during social interactions [6, 7], their performance accuracy of hand gestures is subpar [8,9,10]. This is true for tool-based, communicative, and novel meaningless gestures, with the deficit being more pronounced in the pantomime meaningless gestures [9, 10], all of which predict poor social and occupational functioning [11]. Such impairments also affect nonverbal cue interpretation and body-knowledge, indicating a broader communication deficit with no specific treatment [8].
Correct performance of gestures is highly dependent on the interplay of parietal, motor, and language areas of the praxis network. In schizophrenia, the functional and structural integrity of the praxis network is compromised [4, 12,13,14,15,16]. For example, altered activation in the left inferior parietal lobe (IPL) [17] was observed during an imitation finger-task [13], while planning of gestures showed neural alterations in the left inferior frontal gyrus (IFG) [18], supplementary motor area and IPL [12]. Therefore, neural modulation of key areas of the praxis network might be beneficial in improving gesture performance in schizophrenia. Early evidence suggests that a single-session of transcranial direct-current stimulation (tDCS) over the left frontal lobe enhances gesture interpretation, while a single-session of repetitive transcranial magnetic stimulation [19] over the right IPL improves accuracy [18, 20].
Varying rTMS protocols affect neural activity differently. For example, continuous theta burst stimulation [cTBS] is inhibitory while intermittent theta burst stimulation ([iTBS] is facilitatory [21], and induce enduring neural changes through long-term potentiation/depression that may elicit permanent and specific changes in neural circuits. In fact, evidence from previous studies show that rTMS improves symptom severity in depression and schizophrenia [22,23,24,25], including psychomotor slowing [26] with effects lasting weeks or even months post-treatment. Specific to gesture abilities, our previous single-session randomized, double-blind, sham-controlled trial found that a single cTBS session on the right IPL may enhance gesture accuracy in schizophrenia by inhibiting interhemispheric rivalry [20]. To explore this positive effect further we aimed at testing this treatment with repeated administration of cTBS on right IPL in 10-sessions over 2-weeks. Additionally, we included 16-sessions over 8-weeks of a tailored integrative broad-based social-cognitive remediation group therapy [SCRT [27, 28]]. It combines social and neurocognitive domains and is an already well-established treatment for social impairments and community functioning in schizophrenia [29,30,31,32]. A combined rTMS and SCRT approach may enhance outcomes in schizophrenia by concurrently targeting both neural circuits of the praxis network and social-cognitive processes. While rTMS modulates brain dysfunction, SCRT reshapes maladaptive thoughts and behaviors through social learning and support, potentially boosting neuroplasticity [33]. Testing real SCRT across both real and sham TMS groups clarifies the added value of TMS, while a sham SCRT group helps isolating the effects of each intervention and their interaction. Growing evidence supports the synergistic benefits of combining non-invasive brain stimulation (NIBS), such as rTMS and tDCS, with therapeutic interventions across several neuropsychiatric disorders [34]. In depression, studies show that pairing rTMS or tDCS with cognitive behavioral therapy or cognitive control training may enhance symptom reduction and may accelerate remission, particularly when stimulation coincides with task engagement [17, 19, 35,36,37,38]. In obsessive compulsive disorders, rTMS paired with exposure therapy or cognitive treatment significantly improved obsessive-compulsive symptoms and insight [35, 39], while in PTSD combining rTMS with trauma-focused exposure can modulate fear-related circuits and enhance symptom relief, and improve hyperarousal [40, 41]. Together, these findings highlight the potential of NIBS to improve the efficacy of psychological therapies by targeting and modulating relevant neural circuits during active cognitive engagement.
To this end, the current clinical trial includes a comprehensive social-cognitive behavioral battery, one group with both rTMS and group SCRT treatments, one group with sham rTMS and group SCRT treatment, and one group with no rTMS and sham SCRT treatment to disentangle unspecific effects of add on rTMS and group SCRT treatment from group SCRT treatment alone or from treatment as usual (sham group SCRT). We expect that repetitive rTMS sessions modulating IPL neural activity will enhance training effects during the SCRT group sessions, and thus hypothesize that this combination will be superior to group SCRT treatment alone, or treatment as usual (sham SCRT) in improving gesture performance accuracy (primary outcome). In addition, we expect these improvements to be accompanied by gains in social functioning and social cognition (secondary outcomes). This is based on evidence linking gesture performance accuracy to social cognitive functioning, suggesting that enhancing gesture skills may support more effective nonverbal communication and interpretation of social cues [8, 11].
Methods
Trial design
This 3-arm double-blind randomized, sham-controlled clinical trial of add-on rTMS and group SCRT took place at the University Hospital of Psychiatry and Psychotherapy in Bern Switzerland [42]. Sample-size estimation can be found in SI 1 in Supplement 2. No significant changes to the study protocol were made after the trial commenced. The trial was registered on September 17, 2019 at clinical trials.gov (NCT04106427) before any patients were enrolled (SI 2).
Ethics approval and consent to participate
The study protocol (Supplement 1) was approved by the ethics committee of the canton of Bern (BASEC 2019-00798) and adhered to the Declaration of Helsinki. Written informed consent was obtained from all participants. The study methods adhered to the relevant guidelines.
Participants
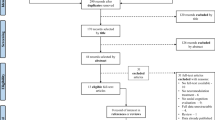
From October 30, 2019 to February 15, 2024, we screened 553 patients for eligibility, of which 89 were randomized: 29 to real rTMS and real SCRT, 30 to sham rTMS and real SCRT and 30 to sham SCRT (Fig. 1). We included patients aged between 18–65 years who were diagnosed with schizophrenia spectrum disorders according to DSM-5. Exclusion criteria included substance abuse (exception of nicotine), neurological conditions, epilepsy, head trauma, hearing problems, current pregnancy/breastfeeding, and no TMS/SCRT treatment in the past 3 months or 2 years respectively. The full inclusion/exclusion criteria can be found in Supplementary Table 1 in Supplement 2. Throughout the trial patients continued preexisting medication including antipsychotics and antidepressants. A total of 73 patients (59 patients with schizophrenia (81%) and 14 (19%) with schizoaffective disorder) received at least one rTMS/group SCRT session (modified intention-to-treat [ITT] group), however the number of completed assessments during follow-up varied. Follow-up was completed on April 24, 2024.
TMS Protocol
Stimulation was delivered using the MagPro x100 or MagPro R30 with theta burst option. Both are manufactured by Tonica Elektronik and dispersed by MagVenture. We used the MCF-B70 coil to deliver real TMS stimulation and the MCF-P-B65 to deliver sham stimulation. Application of rTMS protocols followed published guidelines [43, 44]. Both real and sham protocols were delivered in 10 daily sessions over 2-weeks targeting the right IPL located at P4/CP4 of the EEG 10–20 system. For the patients who underwent MRI (n = 20; 45%) we used the Brainsight Neuronavigation System dispersed by BrainBox Ltd to locate the right IPL using their structural T1-image. The coils and neuronavigation system used, as well as the duration for both real and sham protocols were identical (SI 3 in Supplement 2).
Two-sessions of cTBS included 801 pulses (1602 total) in 267 bursts at an intensity of 100% resting motor-thresholds (44-second duration each session). Each session was separated with a 15-minute break. This protocol was similar to our previous study [20]. For sham stimulation, the protocol matched the cTBS protocol, but a placebo coil was used, mimicking the real coil in sound and appearance without magnetic emissions.
SCRT Protocol
Group therapy was delivered using the Integrated Neurocognitive Therapy (INT) approach [28], in accordance to the MATRICS initiative [45], which includes modules tailored in improving both social cognition and neurocognition in patients with schizophrenia [2, 31, 33, 46,47,48]. For the real SCRT group only MATRICS dimensions relevant to gesture production were included, and the education-compensation-transfer process was shortened due to limited time (SI 4, Supplementary Table 2a in Supplement 2). In contrast, sham SCRT group (SI 4, Supplementary Table 2b in Supplement 2) primarily focused on psychoeducation, information on diet and sleep hygiene, exercise, stress-free environment, and the arts, while engaging them in leisure activities such as mindfulness, walking, and visits to museums. Patients in the sham group SCRT benefited from an interactive environment, but without the add on social-cognitive training. Both real and sham group SCRT were delivered biweekly for 8-weeks, totaling 16 sessions. Each session lasted for 90-minutes and was led by a head-therapist (V.C.) and a co-therapist both supervised by an INT-expert (D.M.).
Outcomes
The primary outcome was change in gesture performance accuracy following interventions over timepoints (Baseline, Week-2, and Week-8). We also investigated if any changes occurred or remained at Week-32 follow-up. Gesture performance accuracy was measured using the Test of Upper-Limb Apraxia (TULIA) [49] which contains two domains and three categories of gestures. TULIA is videorecorded and assessed by an independent examiner blinded to the treatment arms. (SI 5 in Supplement 2).
Secondary outcomes included changes over timepoints (Baseline, Week-2, and Week-8) in social-cognition (Mini-Profile of Nonverbal Sensitivity [Mini-PONS] [50, 51]), gestural knowledge (Postural Knowledge Test [PKT] [52, 53]), managing emotions (Mayer-Salovey-Caruso Emotional Intelligence test [MSCEIT] [54]; SI 6 in Supplement 2) and expert ratings covering illness severity (Positive and Negative Symptom Scale [PANSS] [55]; Brief Negative Symptom Scale [BNSS [56]]), social functioning (Social and Occupational Functioning Assessment Scale [SOFAS [57]]; SI 6 in Supplement 2]; Specific Level of Functioning [SLOF [58]]; Personal and Social Performance [PSP [57]]; SI 6 in Supplement 2) and functional capacity (University of California San Diego Performance-Based Skills Assessment [UPSA brief] [59]); In addition, we collected self-reported gesture perception and production (Brief Assessment of Gestures [BAG [60]] and negative symptoms (Self-evaluation of Negative Symptoms [SNS] [61]). Similarly to our primary outcome analyses, we investigate if the changes observed after completion of the interventions occur or carry-over to Week-32 follow-up.
Procedures
After providing informed consent and before any baseline assessments took place the real rTMS, SCRT, and sham groups were randomly allocated to treatment arms (Fig. 2). Safety outcomes included adverse stimulation effects after each rTMS session and after 10-sessions (Week-2). Adverse events and behavioral outcomes including experience and satisfaction were collected after 16 sessions (Week-8) of either real or sham SCRT (SI 9 in Supplement 2). At baseline, Week-2, Week-8, and Week-32 patients’ TULIA performance, social-cognition and symptom severity were measured; while managing emotions, social functioning and functional capacity were measured at Week-8 and Week-32. We summarized antipsychotic doses as mean olanzapine-equivalents (OLZ) [62].
Reproduced from Chapellier et al. [42] “Brain Stimulation and Group Therapy to Improve Gesture and Social Skills in Schizophrenia-The Study Protocol of a Randomized, Sham-Controlled, Three-Arm, Double-Blind Trial”, Frontiers in Psychiatry, under the terms of the Creative Commons Attribution License (CC BY 4.0). Both real and sham cTBS treatment arms underwent 10 sessions of neurostimulation, while all treatment arms participated in 16 sessions of SCRT. cTBS continuous theta burst stimulation. SCRT social cognitive remediation therapy.
Randomization
Organizational restrictions prevented simultaneous real and sham SCRT. Therefore, we divided the real and sham SCRT into separate time-blocks. Patients were randomized 1:1 to 1 of the 2 treatments with real SCRT using a research randomizer online-tool in one time-block. We did this after recruiting 6–8 patients. In a different time-block, we assigned another 6–8 patients to the sham SCRT group (SI 7 in Supplement 2). A total of 14 time-blocks were administered; 9 blocks with real SCRT and 5 blocks with sham SCRT. The randomization lists generated were accessible to two people (S.W. and A.P.), and treatment allocation was communicated only to the person administering rTMS and SCRT interventions (V.C.).
Blinding
Outcome assessors, clinicians, and patients were blinded to the treatment arms. Duration of treatment, setting, and TMS machinery were identical for all patients. In 53 patients, we assessed the suspected type of intervention (real or sham) received at Week-2.
Statistical analyses
Primary and secondary outcomes were analyzed by 2 researchers (S.W. and A.P.) with RStudio version 2024.12.0 + 467 (R-Foundation for Statistical Computing, Vienna, Austria). All analyses were done in the modified ITT sample (n = 73 with at least 1 rTMS/SCRT session) using the last-observation-carried-forward (LOCF) method to account for missing data. We compared improvement of gesture performance accuracy over timepoints (Baseline to Week-8 and Baseline to Week-32) between treatment arms using repeated-measure ANOVAs for the total TULIA score, domains, and categories. PostHoc-tests were corrected for multiple comparisons using false discovery rate. P-values < 0.05 were considered significant. Similarly, to test the effects of rTMS and SCRT on secondary outcomes (i.e. BNSS, Mini-PONS, SOFAS,) we also used repeated-measure ANOVAs (P-values < 0.05 significant). Frequencies of adverse events, blinding evaluation, and satisfaction ratings were calculated using binomial logistic regression [63] (SI 8 in Supplement 2) and Kruskal-Wallis tests (P-values < 0.05 significant).
Results
Recruitment
Of the 73 patients analyzed, 57% were male and 43% were female, with a mean age of 40.1 years (±SEM 2.5 years). A total of 53 patients completed the intervention period, as well as, the week-8 assessments (16 in the real rTMS and real group SCRT, 15 in the sham rTMS and real group SCRT, and 22 in the sham group SCRT). Reasons for discontinuation were withdrawal of consent (n = 12) and lost to follow-up (n = 8; Fig. 1; Supplementary Table 4 in Supplement 2).
Baseline demographic and clinical characteristics are given in Table 1. No differences between treatment arms were observed. The percentage of patients who completed or attended treatments in each arm is given in Supplementary Table 3 in Supplement 2.
Primary outcome
In the ITT analyses with the LOCF, repeated-measures ANOVAs from Baseline to Week-8 comparing the 3 treatment arms revealed significant effects of time in gesture performance accuracy in the total TULIA score, pantomime domain score as well as, in the pantomime meaningless and intransitive categories (all F(2140) > 4.3; p-value < 0.01; Supplementary Table 5, Fig. 3A). A similar pattern of results was observed for Baseline to Week-32, with the exception of the pantomime meaningless category, where a significant effect of time-by-treatment arm was observed (F(6, 210) = 2.2; p-value = 0.04; η2p = 0.06; Supplementary Table 6; Fig. 3B). Posthoc comparisons revealed an improvement in performance accuracy of pantomime meaningless gestures only for the real rTMS and real group SCRT treatment arm (Supplementary Table 6; Fig. 3B).
The line plots depict performance across timepoints for real rTMS + real SCRT (red), sham rTMS and real SCRT (blue) and sham SCRT (green). A shows performance of pantomime meaningless gestures from Baseline to Week-8. B shows performance of pantomime meaningless gestures from Baseline to Week-32. C shows social functioning performance from Baseline to Week-8. D shows social functioning performance from Baseline to Week-32. Analyses was done using last observation carried forward to account for missing values. Error bars represent SEMs. rTMS indicates repetitive transcranial magnetic stimulation; SCRT indicates social cognitive remediation therapy. *Significant effect at P < 0.05 for real rTMS + real SCRT from baseline.
Secondary outcomes
Repeated-measures ANOVAs of the secondary outcomes from Baseline to Week-8 revealed significant effects of time in PANSS positive, negative, total, BNSS Total, SOFAS, SLOF and PSP, while time-by-treatment arm interaction was exclusive to SOFAS (F(2, 70) = 6.2, p-value = 0.003; η2p = 0.15; eTable 5; Fig. 3C) and PSP (F(2, 70) = 3.5, p-value = 0.03; η2p = 0.09; Supplementary Table 5; Supplementary Figure 1). Posthoc comparisons revealed a pronounced improvement in social and occupational functioning only for the real rTMS and real group SCRT treatment arm. A similar pattern of results was observed for Baseline to Week-32 with an additional significant effect of time observed on UPSA-B (Supplementary Table 5). The time-by-treatment arm interaction on SOFAS (F(4, 140) = 4.2, p-value = 0.003; η2p = 0.11; Supplementary Table 6; Fig. 3D) and PSP (F(2, 70) = 2.6, p-value = 0.04; η2p = 0.07; Supplementary Table 5; Supplementary Figure 2) remained with real rTMS and real group SCRT again showing the most prominent improvement that endured at Week-32 follow-up.
Blinding efficacy
Patients receiving rTMS and group SCRT interventions were unable to identify their assigned treatment (X2 = 2.4, p-value = 0.3). Twenty-three patients (43%) correctly guessed that they received real or sham rTMS/SCRT.
Adverse events
Approximately half of the participants experienced adverse events at least once with no significant differences between treatment arms at any timepoint (all p-values > 0.09; Table 2a-c; SI 10 and Supplementary Tables 7-8 Supplement 2). Two serious adverse events occurred—one in the sham rTMS and real SCRT arm (severe headache and vomiting) and one in the sham SCRT arm (hospitalization due to psychotic relapse)—both determined to be unrelated to the treatment.
Discussion
Impairments in hand gesture performance occur frequently in schizophrenia and are associated with poor community functioning [4, 8, 11] for which no treatment is currently available. Neuroimaging research linked deficits in hand gesture performance in schizophrenia to altered functional and structural activity/connectivity of the praxis network, which comprises of parietal, motor and language areas [12, 15, 16]. This double-blind randomized sham-controlled clinical trial tested whether the combination of 10 sessions of add-on rTMS on the right IPL and 16 sessions of add-on group SCRT treatment would improve hand gesture performance in schizophrenia across different domains and categories [42]. Contrary to our hypothesis, add-on real rTMS and real SCRT did not improve overall hand gesture performance. However, we observed improvement of hand gesture performance in the pantomime meaningless category, when including Week-32 follow-up, and improvements in personal and social performance and functioning during Week-8 and Week-32 follow-up exclusively in the real rTMS and real SCRT treatment arm.
This result is in contrast to our previous clinical trial, which demonstrated immediate improvement in hand gesture performance following a single-rTMS session on right IPL [20]. Our previous trial aimed to assess the efficacy of different rTMS protocols on the praxis network. It did not include repeated rTMS, add-on SCRT treatment, or an analysis of specific hand gesture categories. However, the results from the current clinical trial reveal a more nuanced picture. In particular, we found unspecific time-effects across treatment arms, as all patients improved in symptom severity including negative symptoms, and overall hand gesture performance, in particular, pantomime gestures including the intransitive gesture category, which are highly-communicative gestures. Improved pantomime gestures may have meaningful clinical and functional implications. In daily life, pantomime gestures play a key role in nonverbal communication, particularly in situations where speech is limited (e.g., noisy environments or language barriers). Although assessed in TULIA through verbal command, these gestures, especially intransitive ones closely resemble spontaneous gestures used in real-world interactions. Because pantomime gestures engage motor planning, semantic knowledge, and executive functions, they reflect broader praxis abilities [64]. Their production relies on neural systems implicated in action understanding and planning such as IPL, IFG, and premotor cortex. Notably, impairments in both pantomime and spontaneous gestures are common in schizophrenia and are linked to poorer social functioning [8, 12, 65]. Thus, enhancing pantomime gestures may support better social communication and functional outcomes. From the current trial, it seems that the general group setting with biweekly sessions has strong benefits for schizophrenia. However, SCRT covered only part of integrated neurocognitive therapy (INT); key domains and session-time were reduced, possibly contributing to less consistent effects on social cognition and symptoms. As a trade-off, the sham SCRT arm proved to be a highly active control condition, far more effective than waiting list, often applied in psychological treatment trials [66,67,68]. The sham SCRT group participated in mindfulness and psychoeducation activities to maintain blinding while fostering a communal experience, reducing stress, enhancing comfort, and promoting well-being through self-awareness and presence [69, 70]. As such the group setting may have supported patients’ social-cognitive and emotional regulation indirectly, by fostering attentional focus, psychological containment, and a sense of shared purpose, even in the absence of explicit focused intervention.
We observed no specific time or time-by-treatment effects in imitative, or tool-based gestures, as well as social cognition or postural knowledge. While we could argue that other areas of the praxis network (i.e. IFG) in combination with our tailored group SCRT treatment might be more ideal for these specific outcomes, it might also be explained by insufficient statistical power. The sample size for all groups, especially for the real rTMS and real group SCRT treatment arm was suboptimal and the LOCF method is very conservative.
Notably, we observed beneficial time-by-treatment effects exclusively for pantomime meaningless gestures with real rTMS on right IPL and real group SCRT. The IPL is a key node in the brain’s praxis network, crucial for planning and executing gestures. In schizophrenia, gesture impairments are linked to grey matter loss in the IPL and connected regions, as well as disrupted network efficiency and white matter integrity [14, 71]. Similar patterns appear in stroke and Parkinson’s disease [72, 73]. cTBS to the right IPL was shown to improve gesture performance, suggesting that modulating overactive areas may rebalance network function [20]. This supports theories of state-dependent neuromodulation, where stimulation enhances learning by shifting brain state [74]. cTBS over the right IPL may facilitate left-hemispheric praxis networks via transcallosal disinhibition, improving gesturing in healthy and stroke populations [75, 76]. fMRI in Parkinson’s disease further links fine motor deficits to overactivation of the left praxis network and compensatory recruitment of temporal motor memory areas [77]. In this framework cTBS suppress maladaptive IPL activity and thereby enhance the effectiveness of SCRT. Future fMRI and connectivity studies could further clarify how IPLtargeted interventions support recovery in action planning networks.
Pantomime meaningless gestures are novel, unlearned gestures that are not tied to pre-existing motor patterns and external cues (i.e., imitation) and do not depend on semantic and symbolic processes [78, 79]. The improvement of pantomime meaningless gestures suggests the combination with rTMS and SCRT enhances cognitive flexibility, motor planning, and spatial awareness all of which are required to successfully execute novel, unlearned gestures [80,81,82]. The lack of significant improvement in transitive and intransitive gestures after combined rTMS and SCRT may reflect the complex cognitive and neural demands of these gestures. Unlike meaningless gestures, they rely on symbolic and semantic processing, engaging a broader network beyond the IPL, including the left IFG and temporal cortex [83, 84]. Stimulating the IPL alone may not sufficiently impact this network. These gesture types require integration across semantic and motor pathways, and deficits may persist unless regions like the IFG and middle temporal gyrus are also targeted [84, 85]. Additionally, SCRT may not have adequately addressed the symbolic aspects of gesture use. Future interventions might benefit from multisite neuromodulation or combining stimulation with semantic-action training to more directly support these processes. Alongside improvements in meaningless pantomime performance, we observed gains in personal and social functioning at Week-8 that were sustained through Week-32 (PSP, SOFAS), with similar trends in functional capacity (UPSA). This suggests a generalized carry-over effect of nonverbal communication skills transfer to general social functioning, improving patients’ ability to navigate novel cues [86].
Both rTMS and SCRT treatments where well tolerated. Two serious adverse events occurred but were unrelated to the study procedures. Mild and transient adverse events were noted in all treatment arms.
Ideally, this study should be replicated in larger multicenter trials. Both SCRT content and rTMS protocols can be readily optimized to enhance effects on social-cognitive skills, for example with accelerated rTMS [87] or more targeted training-sessions [88]. Other praxis network targets using non-invasive brain stimulation with SCRT could be tested including transdiagnostic studies [89,90,91,92,93]. Moreover, future studies should consider rTMS in combination with virtual reality; a method that provides a highly controlled, immersive and interactive environment that simulates complex real-life scenarios could be explored [94,95,96]. Further, to better connect neural and behavioral changes with real-world functioning, future research should include outcomes like employment, social engagement, and interpersonal skills. Tools like ecological momentary assessment and digital phenotyping can capture real-time data on daily behaviors and emotions, helping to assess whether lab-based gains translate into everyday life [97, 98]. These methods can also reveal how neural states, cognitive training, and social functioning interact in natural settings, improving ecological validity and clinical impact.
Limitations
Using a 3-arm design we tested the combined effects of repeated rTMS and group SCRT on hand gesture performance in schizophrenia. The study has some limitations. First, although OLZ was similar across treatment arms other medications (i.e. antidepressants) might affect the outcome. Second, while training effects from repeated outcome exposure are possible, we minimized this by randomizing trial order and allowing sufficient time between follow-ups. Third, randomization was conducted before any baseline assessments took place, thus the ITT population included all patients who received at least one rTMS/SCRT session, in line with other rTMS studies in psychiatry but different from drug trials [26, 99, 100]. Finally, 20 patients (27% of ITT) dropped out before week-8 assessments. LOCF analysis accounted for dropouts but introduced a pessimistic estimation of outcomes.
Conclusions
In this randomized clinical trial, the combination of rTMS and SCRT did not improve overall hand gesture performance. However, improvements were observed in a specific category of gestures (pantomime meaningless), as well as social and personal functioning, suggesting important beneficial effects of the combined treatment. Both intervention components can be intensified in future trials with larger sample sizes.
Data availability
The dataset analysed during the current study is not publicly available as participants did not consent to broad data sharing of their health records. But deidentified parts of the data may be provided by the corresponding author on reasonable request.
References
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia—an overview. JAMA Psychiatry. 2020;77:201–10.
Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat. Rev. Neurosci. 2015;16:620–31.
Walther S, Mittal VA. Why we should take a closer look at gestures. Schizophr Bull. 2016;42:259–61.
Walther S, Mittal VA, Stegmayer K, Bohlhalter S. Gesture deficits and apraxia in schizophrenia. Cortex. 2020;133:65–75.
Goldin-Meadow S. The role of gesture in communication and thinking. Trends Cognit. Sci. 1999;3:419–29.
Lavelle M, Healey PG, McCabe R. Is nonverbal communication disrupted in interactions involving patients with schizophrenia? Schizophr. Bull. 2013;39:1150–8.
Mittal VA, Tessner KD, McMillan AL, Delawalla Z, Trotman HD, Walker EF. Gesture behavior in unmedicated schizotypal adolescents. J. Abnorm. Psychol. 2006;115:351–8.
Walther S, Stegmayer K, Sulzbacher J, Vanbellingen T, Müri R, Strik W, et al. Nonverbal social communication and gesture control in schizophrenia. Schizophr Bull. 2015;41:338–45.
Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired pantomime in schizophrenia: association with frontal lobe function. Cortex. 2013;49:520–7.
Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired gesture performance in schizophrenia: Particular vulnerability of meaningless pantomimes. Neuropsychologia. 2013;51:2674–8.
Walther S, Eisenhardt S, Bohlhalter S, Vanbellingen T, Müri R, Strik W, et al. Gesture performance in schizophrenia predicts functional outcome after 6 months. Schizophr Bull. 2016;42:1326–33.
Stegmayer K, Bohlhalter S, Vanbellingen T, Federspiel A, Wiest R, Müri RM, et al. Limbic interference during social action planning in schizophrenia. Schizophr. Bull. 2018;44:359–68.
Thakkar KN, Peterman JS, Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am. J. Psychiatry. 2014;171:539–48.
Viher PV, Abdulkadir A, Savadijev P, Stegmayer K, Kubicki M, Makris N, et al. Structural organization of the praxis network predicts gesture production: Evidence from healthy subjects and patients with schizophrenia. Cortex. 2020;132:322–33.
Viher PV, Stegmayer K, Kubicki M, Karmacharya S, Lyall AE, Federspiel A, et al. The cortical signature of impaired gesturing: findings from schizophrenia. NeuroImage: Clin. 2018;17:213–21.
Wüthrich F, Viher PV, Stegmayer K, Federspiel A, Bohlhalter S, Vanbellingen T, et al. Dysbalanced resting-state functional connectivity within the praxis network is linked to gesture deficits in schizophrenia. Schizophr Bull. 2020;46:905–15.
Bajbouj M, Aust S, Spies J, Herrera-Melendez AL, Mayer SV, Peters M, et al. PsychotherapyPlus: augmentation of cognitive behavioral therapy (CBT) with prefrontal transcranial direct current stimulation (tDCS) in major depressive disorder-study design and methodology of a multicenter double-blind randomized placebo-controlled trial. Eur. Arch. Psychiatry Clin. Neurosci. 2018;268:797–808.
Schülke R, Straube B. Transcranial direct current stimulation improves semantic speech-gesture matching in patients with schizophrenia spectrum disorder. Schizophr. Bull. 2019;45:522–30.
Donse L, Padberg F, Sack AT, Rush AJ, Arns M. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11:337–45.
Walther S, Kunz M, Muller M, Zurcher C, Vladimirova I, Bachofner H, et al. Single session transcranial magnetic stimulation ameliorates hand gesture deficits in schizophrenia. Schizophr. Bull. 2020;46:286–93.
Jannati A, Oberman LM, Rotenberg A, Pascual-Leone A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology. 2023;48:191–208.
Blumberger DM, Mulsant BH, Thorpe KE, McClintock SM, Konstantinou GN, Lee HH, et al. Effectiveness of standard sequential bilateral repetitive transcranial magnetic stimulation vs bilateral theta burst stimulation in older adults with depression: the four-d randomized noninferiority clinical trial. JAMA Psychiatry. 2022;79:1065–73.
Cole EJ, Phillips AL, Bentzley BS, Stimpson KH, Nejad R, Barmak F, et al. Stanford Neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am. J. Psychiatry. 2022;179:132–41.
Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch. Gen. Psychiatry. 2003;60:49–56.
Hua Q, Wang L, He K, Sun J, Xu W, Zhang L, et al. Repetitive transcranial magnetic stimulation for auditory verbal hallucinations in schizophrenia: a randomized clinical trial. JAMA Netw. Open. 2024;7:e2444215.
Walther S, Alexaki D, Weiss F, Baumann-Gama D, Kyrou A, Nuoffer MG, et al. Psychomotor slowing in psychosis and inhibitory repetitive transcranial magnetic stimulation: a randomized clinical trial. JAMA Psychiatry. 2024;81:563–71.
Mueller DR, Schmidt SJ, Roder V. One-year randomized controlled trial and follow-up of integrated neurocognitive therapy for schizophrenia outpatients. Schizophr. Bull. 2015;41:604–16.
Roder V, Müller DR INT-integrated neurocognitive therapy for schizophrenia patients. Cham, Switzerland: Springer International Publishing AG; 2015, p. 141.
Kambeitz-Ilankovic L, Betz LT, Dominke C, Haas SS, Subramaniam K, Fisher M, et al. Multi-outcome meta-analysis (MOMA) of cognitive remediation in schizophrenia: Revisiting the relevance of human coaching and elucidating interplay between multiple outcomes. Neurosci. Biobehav. Rev. 2019;107:828–45.
Lejeune JA, Northrop A, Kurtz MM. A meta-analysis of cognitive remediation for schizophrenia: efficacy and the role of participant and treatment factors. Schizophr Bull. 2021;47:997–1006.
Vita A, Barlati S, Ceraso A, Nibbio G, Ariu C, Deste G, et al. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2021;78:848–58.
Mueller DR, Khalesi Z, Benzing V, Castiglione CI, Roder V. Does integrated neurocognitive therapy (INT) reduce severe negative symptoms in schizophrenia outpatients? Schizophr. Res. 2017;188:92–97.
Salahuddin NH, Schütz A, Pitschel-Walz G, Mayer SF, Chaimani A, Siafis S, et al. Psychological and psychosocial interventions for treatment-resistant schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry. 2024;11:545–53.
Sathappan AV, Luber BM, Lisanby SH. The dynamic duo: combining noninvasive brain stimulation with cognitive interventions. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;89:347–60.
Huang Y, Yang H, Zhu C, Jiang X, Zhu W, Liang Y, et al. An exploratory study of a novel combined therapeutic modality for obsessive-compulsive disorder. Brain Sci. 2022;12:1309.
Neacsiu AD, Luber BM, Davis SW, Bernhardt E, Strauman TJ, Lisanby SH. On the concurrent use of self-system therapy and functional magnetic resonance imaging-guided transcranial magnetic stimulation as treatment for depression. J. ect. 2018;34:266–73.
Segrave RA, Arnold S, Hoy K, Fitzgerald PB. Concurrent cognitive control training augments the antidepressant efficacy of tDCS: a pilot study. Brain Stimul. 2014;7:325–31.
Vedeniapin A, Cheng L, George MS. Feasibility of simultaneous cognitive behavioral therapy and left prefrontal rTMS for treatment resistant depression. Brain Stimul. 2010;3:207–10.
Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11:158–65.
Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder-a pilot study. Brain Stimul. 2013;6:377–83.
Osuch EA, Benson BE, Luckenbaugh DA, Geraci M, Post RM, McCann U. Repetitive TMS combined with exposure therapy for PTSD: a preliminary study. J. Anxiety Disord. 2009;23:54–59.
Chapellier V, Pavlidou A, Mueller DR, Walther S. Brain stimulation and group therapy to improve gesture and social skills in schizophrenia-the study protocol of a randomized, sham-controlled, three-arm, double-blind trial. Front. Psychiatry. 2022;13:909703.
Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014;125:2150–206.
Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–39.
Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72:1–3.
Solmi M, Croatto G, Piva G, Rosson S, Fusar-Poli P, Rubio JM, et al. Efficacy and acceptability of psychosocial interventions in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. Mol. Psychiatry. 2023;28:354–68.
Green MF, Leitman DI. Social cognition in schizophrenia. Schizophrenia Bulletin 2008;34:670–72.
Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39.
Vanbellingen T, Kersten B, Van Hemelrijk B, Van de Winckel A, Bertschi M, Müri R, et al. Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA). Eur. J. Neurol. 2010;17:59–66.
Rosenthal R, Archer D, Hall JA, DiMatteo MR, Rogers PL. Measuring sensitivity to nonverbal communication: the PONS test. nonverbal behavior. 1979:67–98.
Chapellier V, Pavlidou A, Maderthaner L, von Känel S, Walther S. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front. Psychol. 2022;13:804093.
Mozaz M, Rothi LJG, Anderson JM, Crucian GP, Heilman KM. Postural knowledge of transitive pantomimes and intransitive gestures. J. Int. Neuropsycholo Soc. 2002;8:958–62.
Pavlidou A, Chapellier V, Maderthaner L, von Känel S, Walther S. Using dynamic point light display stimuli to assess gesture deficits in schizophrenia. Schizophrenia Research: Cognition. 2022;28:100240.
Mayer JD, Salovey P, Caruso DR. Mayer-Salovey-Caruso emotional intelligence test (MSCEIT) users manual. 2002.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The brief negative symptom scale: psychometric properties. Schizophr. Bull. 2011;37:300–5.
Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV social and occupational functioning assessment scale (SOFAS) to assess routine social functioning. Acta Psychiatr. Scand. 2000;101:323–9.
Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Soc. Work. Res. Abstr. 1983;19:9–21.
Mausbach BT, Depp CA, Bowie CR, Harvey PD, McGrath JA, Thronquist MH, et al. Sensitivity and specificity of the UCSD performance-based skills assessment (UPSA-B) for identifying functional milestones in schizophrenia. Schizophr. Res. 2011;132:165–70.
Nagels A, Kircher T, Steines M, Grosvald M, Straube B. A brief self-rating scale for the assessment of individual differences in gesture perception and production. Learn. Individ. Differences. 2015;39:73–80.
Dollfus S, Mach C, Morello R. Self-evaluation of negative symptoms: a novel tool to assess negative symptoms. Schizophr. Bull. 2016;42:571–8.
Leucht S, Samara M, Heres S, Patel MX, Furukawa T, Cipriani A, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr. Bull. 2015;41:1397–402.
Hosmer Jr DW, Lemeshow S, Sturdivant RX Applied logistic regression. John Wiley & Sons; 2013.
Goldenberg G. Apraxia and beyond: life and work of hugo liepmann. Cortex. 2003;39:509–24.
Kupper Z, Ramseyer F, Hoffmann H, Tschacher W. Nonverbal synchrony in social interactions of patients with schizophrenia indicates socio-communicative deficits. PLOS ONE. 2016;10:e0145882.
Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJH. How effective are cognitive behavior therapies for major depression and anxiety disorders? a meta-analytic update of the evidence. World Psychiatry. 2016;15:245–58.
Zhu Z, Zhang L, Jiang J, Li W, Cao X, Zhou Z, et al. Comparison of psychological placebo and waiting list control conditions in the assessment of cognitive behavioral therapy for the treatment of generalized anxiety disorder: a meta-analysis. Shanghai Arch. Psychiatry. 2014;26:319–31.
Roder V, Mueller DR, Schmidt SJ. Effectiveness of integrated psychological therapy (IPT) for schizophrenia patients: a research update. Schizophr. Bull. 2011;37(Suppl 2(Suppl 2)):S71–9.
Reangsing C, Wongsuraprakit S, Punsuwun S, Oerther S. Effects of mindfulness-based interventions (MBIs) on psychotic symptoms and psychological outcomes in patients with schizophrenia spectrum disorders: a systematic review and meta-analysis. Psychiatry Res. 2024;342:116272.
Sabé M, Kohler R, Perez N, Sauvain-Sabé M, Sentissi O, Jermann F, et al. Mindfulness-based interventions for patients with schizophrenia spectrum disorders: a systematic review of the literature. Schizophr Res. 2024;264:191–203.
Stegmayer K, Bohlhalter S, Vanbellingen T, Federspiel A, Moor J, Wiest R, et al. Structural brain correlates of defective gesture performance in schizophrenia. Cortex. 2016;78:125–37.
Matt E, Foki T, Fischmeister F, Pirker W, Haubenberger D, Rath J, et al. Early dysfunctions of fronto-parietal praxis networks in parkinson’s disease. Brain Imaging Behav. 2017;11:512–25.
Frontzkowski L, Fehring F, Frey BM, Wróbel PP, Reibelt A, Higgen F, et al. Frontoparietal structural network disconnections correlate with outcome after a severe stroke. Hum. Brain Mapp. 2024;45:e70060.
Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008;12:447–54.
Pastore-Wapp M, Gyurkó DM, Vanbellingen T, Lehnick D, Cazzoli D, Pflugshaupt T, et al. Improved gesturing in left-hemispheric stroke by right inferior parietal theta burst stimulation. Front. Neurosci. 2022;ume 16:2022.
Vanbellingen T, Pastore-Wapp M, Kübel S, Nyffeler T, Schüpfer A-C, Kiefer C, et al. Interhemispheric facilitation of gesturing: a combined theta burst stimulation and diffusion tensor imaging study. BraStimul: Basic, Translational, Clin. Res. Neuromodulation. 2020;13:457–63.
Kübel S, Stegmayer K, Vanbellingen T, Pastore-Wapp M, Bertschi M, Burgunder J-M, et al. Altered praxis network underlying limb kinetic apraxia in parkinson’s disease - an fMRI study. NeuroImage: Clin. 2017;16:88–97.
Brown S, Mittermaier E, Kher T, Arnold P. How pantomime works: implications for theories of language origin. Front. Commun. 2019;4:9.
Haviland JB. Pointing, gesture spaces, and mental maps. Lang. gesture. 2000;2:13.
Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr. Opin. Neurobiol. 2005;15:626–31.
Numssen O, Bzdok D, Hartwigsen G. Functional specialization within the inferior parietal lobes across cognitive domains. Elife. 2021;10:e63591.
Yu S, Stock AK, Münchau A, Frings C, Beste C. Neurophysiological principles of inhibitory control processes during cognitive flexibility. Cereb. Cortex. 2023;33:6656–66.
Buxbaum LJ, Kyle K, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: evidence from stroke and corticobasal degeneration. Cortex. 2007;43:411–23.
Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–31.
Buxbaum LJ, Kalénine S. Action knowledge, visuomotor activation, and embodiment in the two action systems. Ann. N. Y. Acad. Sci. 2010;1191:201–18.
Lee LY, Healy MP, Fischer NL, Tong K, Chen ASH, Sahakian BJ, et al. Cognitive flexibility training for impact in real-world settings. Curr. Opin. Behav. Sci. 2024;59:101413.
van Rooij SJH, Arulpragasam AR, McDonald WM, Philip NS. Accelerated TMS - moving quickly into the future of depression treatment. Neuropsychopharmacology. 2024;49:128–37.
Riedl L, Nagels A, Sammer G, Choudhury M, Nonnenmann A, Sütterlin A, et al. Multimodal speech-gesture training in patients with schizophrenia spectrum disorder: effects on quality of life and neural processing. Schizophr. Res. 2022;246:112–25.
Gillissie ES, Lui LM, Ceban F, Miskowiak K, Gok S, Cao B, et al. Deficits of social cognition in bipolar disorder: systematic review and meta‐analysis. Bipolar Disord. 2022;24:137–48.
Pavlidou A, Viher PV, Bachofner H, Weiss F, Stegmayer K, Shankman SA, et al. Hand gesture performance is impaired in major depressive disorder: a matter of working memory performance? J. Affect. Disord. 2021;292:81–8.
Suffel A, Nagels A, Steines M, Kircher T, Straube B. Feeling addressed! The neural processing of social communicative cues in patients with major depression. Hum. brain Mapp. 2020;41:3541–54.
Han YM, Chan MM, Shea CK, Mo FY, Yiu KW, Chung RC, et al. Effects of prefrontal transcranial direct current stimulation on social functioning in autism spectrum disorder: a randomized clinical trial. Autism. 2023;27:2465–82.
Lozano-Goupil J, Shankman SA, Walther S, Wuethrich F, Maher RE, Grzelak LN, et al. Automatic quantification of hand gestures in current and remitted major depressive disorder during oral expression. J. Affect. Disord. 2025;389:119684.
Gainsford K, Fitzgibbon B, Fitzgerald PB, Hoy KE. Transforming treatments for schizophrenia: virtual reality, brain stimulation and social cognition. Psychiatry Res. 2020;288:112974.
Pavlidou A, Gorisse G, Banakou D, Walther S. Using virtual reality to assess gesture performance deficits in schizophrenia patients. Front. Psychiatry. 2023;14:1191601.
Pavlidou A, Walther S. Using virtual reality as a tool in the rehabilitation of movement abnormalities in schizophrenia. Front. Psychol. 2021;11:607312.
Lopez-Morinigo JD, Barrigón ML, Porras-Segovia A, Ruiz-Ruano VG, Escribano Martínez AS, Escobedo-Aedo PJ, et al. Use of ecological momentary assessment through a passive smartphone-based app (eb2) by patients with schizophrenia: acceptability study. J. Med. Internet Res. 2021;23:e26548.
Lozano-Goupil J, Mittal VA Capturing motor signs in psychosis: how the new technologies can improve assessment and treatment? Schizophr Bulletin 2025; sbaf010.
George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch. Gen. Psychiatry. 2010;67:507–16.
Wobrock T, Guse B, Cordes J, Wölwer W, Winterer G, Gaebel W, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol. Psychiatry. 2015;77:979–88.
Acknowledgements
We thank all the students who assisted with data acquisition, with special thanks to Ynès Bouamond and Carla Schiltknecht. We also thank Drs. Danai Alexaki, Evin Demir, Daniel Baumann-Gama, Alexandra Kyrou, and Alexios Malifatouratzis for assisting with some of the clinical assessments, as well as Florence Ibrahim, Linda Grossenbacher and Drs Florian Weiss and Irena Vladimirova for assisting with the SCRT.
Funding
This study was funded by the Swiss National Science Foundation (184717 to Dr Walther). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Open access funding provided by University of Bern.
Author information
Authors and Affiliations
Contributions
Drs Walther and Pavlidou had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Walther. Acquisition of data: Maderthaner, Chapellier, von Känel, Baer Interpretation of results: Walther, Pavlidou SCRT: Chapellier, Müller Drafting of the manuscript: Walther, Pavlidou. Critical review of the manuscript for important intellectual content: all authors Statistical analysis: Walther, Pavlidou. Obtained funding: Walther. Administrative, technical, or material support: Walther, Maderthaner, Chapellier, von Känel, Müller, Bohlhalter, Baer, Pavlidou. Supervision: Walther, Pavlidou.
Corresponding author
Ethics declarations
Competing interests
Dr Walther reported honoraria for medical education events from Neurolite outside the submitted work. No other disclosures were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walther, S., Maderthaner, L., Chapellier, V. et al. Gesture deficits in psychosis and the combination of group psychotherapy and transcranial magnetic stimulation: A randomized clinical trial. Mol Psychiatry 30, 5790–5799 (2025). https://doi.org/10.1038/s41380-025-03303-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03303-7