Abstract
Fructose-1,6-bisphosphatase 1 (FBP1), a rate-limiting enzyme in gluconeogenesis, is important for cancer progression. The post-translational regulation of FBP1 in hypoxic environments is still unclear. Here, we report that FBP1 is down-regulated, and a low expression level of FBP1 predicts a poor prognosis in pancreatic cancer. A hypoxic environment makes FBP1 more prone to degradation, and this effect can be reversed by inhibiting global O-GlcNAcylation signalling. O-linked N-acetylglucosamine transferase (OGT) interacts with FBP1 and induces its O-GlcNAcylation at serine 47 residue (FBP1-S47) to modulate its protein function in pancreatic cancer cells. O-GlcNAcylation of FBP1-S47 promotes FBP1 degradation and also influences the expression of canonical HIF-1α target genes involved in glucose metabolism, resulting in an increase in glucose uptake and lactate secretion in pancreatic cancer cells. In addition, O-GlcNAcylation of FBP1-S47 facilitates FBP1 K48-linked polyubiquitination at lysine 51 residue (FBP1-K51), in which GlcNAc moiety can serve as a prerequisite for an FBP1 ubiquitin ligase. FBP1 (K51) K48-linked polyubiquitination mediated protein degradation can also promote cancer progression, similarly to the O-GlcNAcylation of FBP1-S47. Our data uncover a mechanism whereby FBP1 can be regulated by a protein O-GlcNAcylation-polyubiquitination axis, paving the way to cancer cell metabolic reprogramming.
Similar content being viewed by others
Introduction
Hypoxia and concurrent nutrient deficiencies are important factors that contribute to changes in tumour progression [1]. These factors stimulate the expression of HIF family genes to change the energy metabolism of cancer cells [2]. Moreover, interstitial cells, such as vascular endothelial cells and macrophages, are also influenced by these factors. Together, altered tumour metabolism, aberrant angiogenesis in the tumour microenvironment and immune escape can change the biological characteristics of tumours and their responses to therapy [3,4,5]. Pancreatic ductal adenocarcinomas (PDACs) are characterised by severe hypovascularity and an extensive desmoplastic stroma, resulting in an extremely hypoxic intratumor microenvironment [6]. O-GlcNAcylation is considered an important posttranslational modification controlled via various metabolic pathways. O-GlcNAcylation participates in the regulation of many protein functions, and its dysregulation is associated with processes related to tumour progression, including cancer metabolism, metastasis, chemoresistance and immunotherapeutic responses [7,8,9,10]. Previous work has shown that hypoxia can activate O-GlcNAcylation signalling in a HIF-1α-dependent manner in skeletal muscle [11]. The glutamate–glutamine cycle can also be triggered by hypoxia-induced O-GlcNAcylation in the mouse brain cells [12]. In addition, several glycolytic regulators, such as PFKFB3 and PFK1, are O-GlcNAcylated in response to hypoxia [13, 14]. Collectively, these observations indicate a potential relationship between O-GlcNAcylation and hypoxia.
Fructose-1,6-bisphosphatase 1 (FBP1) is a rate-limiting enzyme in gluconeogenesis, and its catalytic activity-independent function in cancer progression has been identified [15]. FBP1 is downregulated in numerous malignant tumours, and its downregulation predicts a poor prognosis [15,16,17,18]. FBP1 expression can be regulated at the DNA level, posttranscriptional level, or posttranslational level. The promoter of FBP1 (CpG island) is methylated by a Snail-G9a-Dnmt1 complex to repress its expression in breast cancer [16]. TET2 is recruited by the transcription factor HNF4α to activate FBP1 expression, and decreased TET2 expression can promote the Warburg effect via FBP1 [19]. At the posttranslational level, phosphorylation of FBP1 by PIM2 at serine 144 increases p65-regulated PD-L1 expression. In normal hepatocytes, glucose deprivation induces PERK-mediated FBP1 phosphorylation at serine 170, and O-GlcNAcylation of FBP1 at serine 124 can reverse this effect [20]. In addition, ubiquitin carboxyl-terminal hydrolase 7 (USP7) binds to and deubiquitinates FBP1, which prevents FBP1 from translocating to the nucleus [21]. The combined results of previous studies and our studies have shown that FBP1 expression is repressed in PDAC and that FBP1 repression predicts a poor prognosis in patients with PDAC [22]. Hypoxia can inhibit GBE1 expression, and FBP1 is a downstream target of GBE1, indicating that hypoxia might influence the expression of FBP1 posttranscriptionally [23]. However, whether hypoxia can influence the expression of FBP1 posttranslationally and whether O-GlcNAcylation signalling is involved in this pathway are still unknown. Therefore, we hypothesised that O-GlcNAcylation signalling is augmented in response to hypoxia and that FBP1 is O-GlcNAcylated in pancreatic cancer.
In this study, we demonstrated that O-GlcNAcylation of FBP1 at serine 47 induced by hypoxia promotes FBP1 protein degradation in pancreatic cancer. We further revealed that O-GlcNAcylation of FBP1 at S47 can facilitate its polyubiquitination at lysine 51 (K51). Moreover, abrogation of FBP1 polyubiquitination at K51 by base substitution partially antagonised the tumour-promoting effect of FBP1 O-GlcNAcylation.
Materials and methods
Immunohistochemistry staining and scoring standard
Immunohistochemistry (IHC) staining results combined with clinical parameter was used to analyze the expression of FBP1 in pancreatic cancer tissue microarrays (purchased from Shanghai Outdo Biotech Co., Ltd. (CAT: HPan-Ade120Su-02).). Tissue samples on the microarrays from 38 patients (unqualified cases were eliminated, details in Supplementary Table 1) with PDAC were examined and informed consent was obtained. The details of IHC methods and scoring standards have been described in our previous study [24]. The IHC results of FBP1 was determined separately by two double-blind pathologists. At last, the study was approved by the Second Affiliated Hospital of the School of Medicine of Zhejiang University Review Board and the ethics committees of Zhejiang University.
Proximity ligation assay
In the Proximity Ligation Assay, 1 × 104 cells were seeded in the 24-well culture plate and incubation with anti-FBP1 (1:1000, ab109732, Abcam) and anti-O-GlcNAc (1:500, MA1-072, CST). The proximity ligation assay (PLA) was performed according to the protocol in the Duolink In Situ kit (DUO92008, Sigma), and the image results were obtained using a fluorescence confocal microscope.
Cell culture
Human HEK293T, AsPC-1, SW1990 cells were obtained from the Cell Bank of the Chinese Academy of Science and grown at 37 °C supplied with 5% CO2. For the HEK293T cell line, we used DMEM medium supplemented with 10% foetal bovine serum (FBS) for cultivation. The AsPC-1 cell line was cultured in RPMI-1640 medium with 10% FBS, and the SW1990 cell line was cultured in L-15 medium supplemented with 10% FBS. Cell authentication was confirmed by the STR analysis and mycoplasma tests were weekly performed.
Cycloheximide (CHX) assay
A total of 3 × 105 pancreatic cancer cells (AsPC-1 and SW1990) were seeded in a 6-cm culture plate for 24 h. To measure the protein stability of FBP1 and its mutants, we collected cells at four specific time points, which are 0, 2, 4, and 6 h after 300 μg/ml CHX (S7418, Selleck) was added to block protein synthesis.
Plasmids and reagents
FBP1 was cloned from cDNA templated and then inserted into pcDNA 3.1(+) plasmid (Addgene). FBP1 mutants (S47A, S67A, S211A, K51R, K110R, K143R, K151R and K232R) were prepared using a sitedirected mutagenesis protocol (Hieff Mut™ Site-Directed Mutagenesis Kit, Yeasen). All the sequences of the FBP1 mutants were assessed by a DNA sequencing service. The primers above were designed with Primer Premier 5.0 (see Supplementary Tables 2–4). The siRNA sequences for OGT are listed in Supplementary Table 6.
Stable cell lines construction
We used short hairpin RNA (shRNA) (sequences are listed in Supplementary Table 5) to knockdown endogenous FBP1 expression and target cells that stably expressed the constructs were selected with puromycin (2 mg/ml). Then we transduced the treated cells with lentiviral vectors carrying the FBP1-wildtype (WT)/S47A/K51R plasmid for overexpression. To avoid knockdown by shRNA, we made a synonymous mutation (primers are listed in Supplementary Table 4). Then the cells were selected with blasticidin S (10 μg/ml) for approximately one week. ShOGT was similar to shFBP1. The targets and primers of shOGT are listed in Supplementary Table 5.
Western blot analysis
Cells were lysed in RIPA lysis buffer (Sigma), and the lysate was resolved on an 8–12% SDS-PAGE gel, transferred to a PVDF (polyvinylidenedifluoride) membrane and immunoblotted with the indicated antibodies (listed in Supplementary Table 9) overnight at 4 °C. The next day, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody and protein was detected by chemiluminescence [24].
Immunoprecipitation (IP) and Co-immunoprecipitation (co-IP)
Cells were lysed in Pierce IP buffer (Thermo Fisher). After incubation with the indicated antibody overnight at 4 °C, the lysate was mixed with protein A/G agarose (Thermo Fisher). Agarose was washed with TBST (0.1% Tween-20, 150 mM NaCl, 10 mM Tris-HCl pH 7.5) and eluted in SDS lysis buffer (100 mM NaCl, 1%SDS, 50 mM Tris-HCl pH 7.5) for further immunoblotting.
Quantitative real-time PCR (qRT-PCR)
TRIzol RNA isolation system (Invitrogen) was used to extract total RNA and then we reversely transcripted it to cDNA. Then qRT-PCR was performed using the Sensi Mix SYBR Kit (Bio-Rad) in a 7500 Fast™ System (Applied Biosystems). The mRNA level was calculated using the 2−ΔΔCt method and normalised to actin (The sequences of primers are listed in Supplementary Table 7).
Chromatin immunoprecipitation (ChIP)
AsPC-1 and SW1990 cell lines that had been stably transfected with lentivirus were harvested, and then ChIP assay was performed using Pierce Agarose ChIP Kit (No.26156, Thermo Fisher Scientific). (The sequences of the primers specific for each target gene promoter are listed in Supplementary Table 8) [25].
Liquid chromatograph-mass spectroscopy/mass spectroscopy (LC–MS/MS)
The gels (the mixture pulled down by anti-Flag immunomagnetic beads) containing O-GlcNAcylated FBP1 or ubiquitinated FBP1 proteins were cut and then sent for LC–MS/MS to identify the modification sites at Shanghai Applied Protein Technology [26].
Cell proliferation assay and colony formation assay
For cell proliferation assay, 1 × 103 AsPC-1 and SW1990 cell lines that had been stably transfected with lentivirus were seeded in a 96-well plate and incubated for the indicated hours. About 10 μl MTT reagent was added per well, and the mixture was incubated for 4 h until purple precipitate was visible. Then, 100 μl detergent reagent was added. The absorbance was recorded at 562 nm. For colony formation assay, a total of 5 × 103 stable cancer cells were transplanted into six-well plates. On the 14th day, the colonies were clearly visible to the naked eye and then stained with crystal violet for 5 min [24].
Glucose uptake assay and lactate production assay
A total of 2–5 × 103 AsPC-1 and SW1990 cell lines that had been stably transfected with lentivirus were seeded in a 96-well plate. Glucose uptake assay and lactate production assay were performed using the Glucose Uptake Fluorometric Assay Kit (ab136956) and l-Lactate Assay kit (Fluorometric, High Sensitivity) (ab169557) according to the manufacturer’s instructions, respectively.
Animal studies
The cells injected were AsPC-1 and SW1990 cell lines that had been stably transfected with lentivirus. Immunocompromised mice (BALB/c-nude, male, 6–8 weeks old) were injected subcutaneously with 2 × 106 cells each in the right flank (five mice per group). The growth of tumours was monitored every 3 days over a 6-week period. All mice were sacrificed in 6th week, and the tumours were harvested to detect tumour volume (Vtumour = 0.5 × L× W2; L = length, W = width) and weight. All animal experiments were approved by the Zhejiang University Institutional Animal Care and Use Committee.
Statistics
All results (biological replicates) reported in main and supplementary data were shown as the mean ± SD. P values were calculated based on two-tailed, unpaired t-tests or Chi-square (x2) tests. Kaplan–Meier survival analyses combined with the log-rank test were used to compare survival times among PDAC patients based on FBP1 expression. P < 0.05 was considered statistically significant (*).
Results
FBP1 is downregulated in pancreatic cancer and can be regulated by O-GlcNAcylation in hypoxic environments
First, we examined the expression of FBP1 in PDAC samples to obtain a comprehensive understanding of FBP1. An evaluation of three pairs of representative images revealed that FBP1 expression in the tumour tissues was lower than that in the matched adjacent tissues (Fig. 1A). Statistical analysis revealed that FBP1 expression was lower in PDAC tissues than in adjacent normal pancreatic tissues (P < 0.001) (Fig. 1B). Next, we demonstrated that high FBP1 expression in tumour tissues usually predicted a better prognosis in patients (P = 0.04) (Fig. 1C). Conversely, low FBP1 expression in PDAC tissues was negatively correlated with advanced T stage (P = 0.018), lymph node metastasis (P < 0.001), distant metastasis (P < 0.001) and late AJCC TNM stage (P = 0.002), suggesting that FBP1 downregulation contributed to PDAC tumorigenesis and progression in these 38 clinical cases (Fig. 1D). Therefore, additional pathways leading to FBP1 protein degradation need to be discovered. FBP1 protein expression levels were detected in pancreatic cancer lines, and we found that FBP1 was expressed in the AsPC-1 and SW1990 cell lines. Therefore, these two cell lines were used for further investigation (Fig. 1E). FBP1 knockdown in the sPC-1 and SW1990 cell lines accelerated cell growth, indicating that FBP1 is a tumour suppressor in these two cell lines (Fig. 1F). Because PDAC tissue is usually reported to be hypoxic, we investigated whether there is a relationship between hypoxia and FBP1. To validate the effect of hypoxia on FBP1, we exposed the pancreatic cancer cell lines AsPC-1 and SW1990 to hypoxia and found that FBP1 expression was downregulated (Fig. 1G). A cycloheximide (CHX) chase assay was subsequently performed, and the results revealed that the protein stability of FBP1 decreased under hypoxic conditions (Fig. 1H and Supplementary Fig. 1).
A Representative IHC staining of 38 pairs of pancreatic tumour and normal adjacent tissues. Scale bar as shown. B Statistical analysis of FBP1 expression from the IHC results. We defined high expression as two points and low expression as 1 point. C Kaplan–Meier overall survival curves for all 38 patients with pancreatic cancer stratified by high and low FBP1 expression. D Correlations of FBP1 expression with T stage, lymph node metastasis, distant metastasis and AJCC TNM stage in clinical patients with pancreatic cancer according to the χ2 test. E Screening of FBP1 expression in pancreatic cancer cell lines. F Downregulation of FBP1 accelerates tumour growth in AsPC-1 and SW1990 cells. G FBP1 is repressed under hypoxia in AsPC-1 and SW1990 cells. H A hypoxic environment triggered the degradation of FBP1 in AsPC-1 and SW1990 cells.
OGT binds to FBP1 and promotes FBP1 O-GlcNAcylation at serine 47
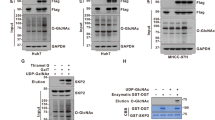
A previous study reported that hypoxia can induce hyper-O-GlcNAcylation. As the exposure time to the hypoxic environment increased, the O-GlcNAcylation level gradually increased, and FBP1 expression gradually decreased (Fig. 2A). Then, we used the siOGT plasmid and OSMI-1 (an OGT inhibitor) to treat AsPC-1 and SW1990 cells that had been subjected to hypoxia for 6 h. Treatment with either siOGT or OSMI-1 dramatically reduced O-GlcNAcylation. Moreover, FBP1 expression was elevated (Fig. 2B). Collectively, these findings suggest that O-GlcNAcylation signalling might participate in hypoxia-induced FBP1 degradation.
A Time course of FBP1 expression and hypoxia exposure time. B Treatment with siOGT or OSMI-1 reversed the degradation of FBP1 in AsPC-1 and SW1990 cells under hypoxia for 6 h. C A co-IP assay was performed to validate the interaction between exogenous FBP1/FBP1 (K908A) and OGT plasmids in 293 T cells. D A co-IP assay was performed to validate the interaction between endogenous FBP1 and OGT in AsPC-1 and SW1990 cells. E A PLA was performed to validate the interaction between endogenous FBP1 and OGT in AsPC-1 cells. The PLA signal was detected by fluorescence microscopy (red arrows). Scale bar as shown. F Four FBP1 truncations were constructed according to their structural domains. A co-IP assay was performed to determine the interacting domain. G Mass spectrometry results of the FBP1 serine 47 residue. H A co-IP assay between O-GlcNAc and the FBP1-WT/mutant was performed to validate the potential O-GlcNAcylation site. Endogenous FBP1 was knocked down and then rescued by Flag-tagged FBP1-WT, S47A, S67A, and S233A plasmids in AsPC-1 and SW1990 cells.
We hypothesised that there is a relationship between O-GlcNAcylation and FBP1. To test this hypothesis, we performed an exogenous IP assay, and the results revealed that FBP1 can interact with OGT in the 293T cell line. 293T cells were also transfected with the OGT mutant K908A (with loss of O-GlcNAcylation activity), and this mutation was found to disrupt FBP1 O-GlcNAcylation and increase FBP1 protein expression. However, the interaction between the two proteins was not disrupted (Fig. 2C). Next, endogenous IP assays were performed in AsPC-1 and SW1990 cells, and the results indicated that endogenous FBP1 could be O-GlcNAcylated via interaction with endogenous OGT (Fig. 2D). A proximity ligation assay (PLA) was performed to further validate the interaction between OGT and FBP1, and red fluorescent puncta were detected mainly in the cytoplasm (Fig. 2E). We next sought to determine which domain of FBP1 interacts with OGT. To this end, four truncations of FBP1 were constructed according to its functional domains. OGT mainly interacted with the Δ1 domain of FBP1 (exons 1 and 2) (Fig. 2F).
To identify the O-GlcNAcylation site(s) on FBP1, we performed protein purification and mass spectrometry analysis, and two potential O-GlcNAcylation sites (S47 and S211) on FBP1 were revealed by LC–MS/MS (Fig. 2G and Supplementary Fig. 2). A co-IP assay was subsequently performed with the wild-type (WT) FBP1 protein and three FBP1 site mutants (S47A, S211A and S67A (negative control); serine-to-alanine mutants), and the results revealed that the S47A mutation, but not the S211A or S67A mutation, significantly reduced the O-GlcNAcylation level of FBP1 (Fig. 2H). Therefore, we chose the S47A mutant for further investigation.
FBP1 O-GlcNAcylation at S47 can promote its degradation
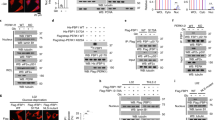
To determine whether the S47A mutation interferes with FBP1 protein degradation, we constructed two stably transfected AsPC-1 and SW1990 cell lines (FBP1-WT and FBP1-S47A). We subsequently downregulated O-GlcNAcylation in these two cell lines by transfecting two siOGT plasmids. The western blot results revealed that the downregulation of OGT in the FBP1-WT cell line increased the protein expression of FBP1. However, FBP1 expression did not change in the FBP1-S47A cell line under the same conditions (Fig. 3A). In contrast, OGT downregulation did not change the mRNA level of FBP1 in either cell line, suggesting the possibility of posttranslational regulation (Fig. 3B). To determine whether O-GlcNAcylation can affect the protein stability of FBP1, we performed a CHX chase assay in AsPC-1 and SW1990 cells, and the results revealed that OGT knockdown increased the protein stability of FBP1-WT. However, cells transfected with FBP1-S47A did not exhibit this effect (Fig. 3C and Supplementary Fig. 3). Similarly, forced O-GlcNAcylation by transfection of the OGT plasmid but not the OGT K908A mutant plasmid resulted in decreased FBP1 expression, although this effect was not observed in cells transfected with FBP1-S47A (Fig. 3D), suggesting that the OGT-mediated change in FBP1 protein expression was dependent on the catalytic function of OGT. In addition, OGT overexpression did not affect the FBP1 mRNA expression in either cell line (Fig. 3E). We also performed a CHX chase assay, and the results revealed that OGT overexpression decreased the protein stability of FBP1-WT. However, cells transfected with FBP1-S47A did not exhibit this effect (Fig. 3F and Supplementary Fig. 4). Next, we stimulated these two cell lines (FBP1-WT and FBP1-S47A) in a hypoxic environment and found that hypoxia dramatically downregulated FBP1 expression in the FBP1-WT cells compared with the FBP1-S47A cells (Fig. 3G). A CHX chase assay was performed, and the results revealed that a hypoxic environment decreased the protein stability of FBP1-WT to a greater extent (Fig. 3H and Supplementary Fig. 5). Together, these results indicate that FBP1 O-GlcNAcylation at serine 47 can promote FBP1 protein stability in a posttranslational manner.
A Silencing OGT with siRNAs regulates FBP1-WT/S47A protein expression levels in AsPC-1 and SW1990 cells. B Silencing OGT with siRNAs regulates FBP1-WT/S47A mRNA expression levels in AsPC-1 and SW1990 cells. C CHX assays were performed to determine the stability of the FBP1-WT/S47A proteins in OGT-knockdown AsPC-1 and SW1990 cells. D Hyper-O-GlcNAcylation by OGT or an OGT mutant (K908A) regulates FBP1-WT/S47A protein expression levels in AsPC-1 and SW1990 cells. E Hyper-O-GlcNAcylation by OGT or an OGT mutant (K908A) regulates FBP1-WT/S47A mRNA expression levels in AsPC-1 and SW1990 cells. F CHX assays were performed to determine the stability of the FBP1-WT/S47A proteins under hyper-O-GlcNAcylation by OGT in AsPC-1 and SW1990 cells. G Changes in FBP1-WT/S47A protein expression levels in AsPC-1 and SW1990 cells under hypoxic conditions. H CHX assays were performed to determine the stability of the FBP1-WT/S47A proteins in a hypoxic environment in AsPC-1 and SW1990 cells.
FBP1 O-GlcNAcylation at S47 influences the expression of its downstream target genes and promotes cancer progression
Because FBP1 can inhibit the onset of the Warburg effect by reducing the expression of canonical HIF-1α target genes (PDK1, LDHA, GLUT1 and VEGF), we performed qRT‒PCR to confirm whether FBP1 O-GlcNAcylation at serine 47 can affect the expression of downstream target genes under hypoxic conditions. Compared with the FBP1-WT group, the FBP1-S47A group presented decreased expression of target genes (PDK1, LDHA, GLUT1 and VEGF) induced by FBP1 overexpression. Furthermore, OGT knockdown via shRNA decreased the expression levels of these target genes in the AsPC-1 and SW1990 cell lines, but no significant difference was found between the FBP1-WT and FBP1-S47A groups (Fig. 4A). Chromatin immunoprecipitation (ChIP) assays of the AsPC-1 and SW1990 cell lines revealed that FBP1-WT was enriched at the hypoxia response elements (HREs) of the PDK1, LDHA, GLUT1 and VEGF promoters but not at the promoter of RPL13A, which is nonresponsive to hypoxia. The FBP1-S47A mutation or OGT knockdown increased FBP1 enrichment at the HREs of the PDK1, LDHA, GLUT1 and VEGF promoters, which may enhance the inhibitory effect of FBP1 on the expression of its target genes (Fig. 4B).
A RT‒qPCR results showing the expression levels of downstream target genes in AsPC-1 and SW1990 (FBP1-WT/S47A) cells with or without OGT knockdown. B ChIP‒qPCR results showing FBP1 occupancy at the promoters of downstream target genes in AsPC-1 and SW1990 (FBP1-WT/S47A) cells with or without OGT knockdown. C Glucose uptake assays were performed in AsPC-1 and SW1990 (FBP1-WT/S47A) cells with or without OGT knockdown. D Lactate secretion assays were performed in AsPC-1 and SW1990 (FBP1-WT/S47A) cells with or without OGT knockdown. E MTT assays were performed to measure the growth of AsPC-1 and SW1990 (FBP1-WT/S47A) cells with or without OGT knockdown. F Colony formation assays were performed in AsPC-1 and SW1990 (FBP1-WT/S47A) cells with or without OGT knockdown, and the statistical results were calculated. G Xenograft experiments in nude mice were performed in AsPC-1 and SW1990 (FBP1-WT/S47A) cells with or without OGT knockdown, and the statistical results (i.e., tumour weight and volume) were analysed.
Next, we measured glucose metabolism in pancreatic cancer cell lines. The FBP1-S47A mutation can reduce glucose uptake and lactate production. OGT knockdown also reduced glucose uptake and lactate production in the FBP1-WT group but had no effect on the FBP1-S47A mutant group (Fig. 4C, D), which was consistent with the results described above for the expression of FBP1 target genes. Compared with WT FBP1, the expression of the FBP1-S47A mutant suppressed AsPC-1 and SW1990 cell growth (Fig. 4E). Indeed, OGT knockdown promoted colony formation by FBP1-WT cells but did not further inhibit colony formation by FBP1-S47A mutant cells (Fig. 4F). Consistent with the in vitro results, OGT knockdown significantly reduced xenograft tumour growth (i.e. tumour volume and weight) in mice injected with FBP1-WT cells but had no significant effect on tumour growth in mice injected with the FBP1-S47A mutant cells, suggesting that the suppressive effect may be largely dependent on FBP1 O-GlcNAcylation at serine 47 in vivo (Fig. 4G).
FBP1 O-GlcNAcylation at S47 can promote its K48 polyubiquitination
FBP1 has been shown to be O-GlcNAcylated at serine 47 to promote its degradation and tumour progression under hypoxic conditions. Given that ubiquitination is usually involved in protein degradation, we sought to determine whether ubiquitination could play a role in the O-GlcNAcylation-mediated degradation of FBP1. First, we transfected plasmids expressing Ub and the E3 ligase TRIM28 into pancreatic cancer cells to increase the activity of the ubiquitination system, and we found that OGT (but not OGT 908 A) expression increased FBP1 polyubiquitination and that this effect can be abolished by S47 mutation (Fig. 5A). Because hypoxia can induce hyper-O-GlcNAcylation, we exposed cancer cell lines to hypoxia and found that hypoxia promoted both the polyubiquitination and the degradation of FBP1 in the FBP1-WT cell group (Fig. 5B). Next, we found that the E3 ligase TRIM28 catalysed FBP1 polyubiquitination in a K48-dependent manner and that O-GlcNAcylation at S47 promoted this process (Fig. 5C). However, the K63-dependent polyubiquitination process of FB1 was very weak in this system, and O-GlcNAcylation at S47 did not affect it (Fig. 5D). To determine the potential effect of TRIM28-mediated K48 polyubiquitination, TRIM28 was knocked down, and the results revealed that the K48 polyubiquitination level dramatically decreased in both the FBP1-WT and FBP1-S47 groups without affecting the O-GlcNAcylation level of FBP1 (Fig. 5E). We then replaced OGT overexpression with a hypoxic environment, and the results showed that silencing TRIM28 could partially reverse FBP1 O-GlcNAcylation-mediated degradation (Fig. 5F).
A Cotransfection of OGT and an OGT mutant (K908A) revealed that OGT facilitates TRIM28-mediated FBP1 polyubiquitination in AsPC-1 and SW1990 wild-type cells. B Hypoxia facilitates TRIM28-mediated FBP1 polyubiquitination in AsPC-1 and SW1990 wild-type cells. C OGT facilitates TRIM28-mediated FBP1 K48-linked polyubiquitination in AsPC-1 and SW1990 wild-type cells. D OGT fails to affect TRIM28-mediated FBP1 K63-linked polyubiquitination in AsPC-1 and SW1990 wild-type cells. E Silencing TRIM28 abolishes OGT-mediated protein degradation in AsPC-1 and SW1990 wild-type cells. F Silencing TRIM28 abolishes hypoxia-mediated protein degradation in AsPC-1 and SW1990 wild-type cells.
FBP1 polyubiquitination at K51 is dependent on S47 O-GlcNAcylation
To identify potential polyubiquitination sites in the FBP1 protein, the gel band containing ubiquitinated FBP1 was excised and analysed by LC–MS/MS. As shown in Supplementary Figs. 5A, 6, five potential ubiquitination sites in FBP1 were identified. We subsequently constructed five lysine (K)-to-arginine (R) mutants (K51R, K110R, K143R, K151R and K232R) and performed a co-IP assay to determine the functional sites. Mutation of only the K51R site attenuated FBP1 K48 polyubiquitination (Fig. 6B). The “SAVRK” sequence is highly conserved in many species (Fig. 6C). We subsequently performed a CHX chase assay to determine whether this mutant (K51R) can affect FBP1 protein stability, and the results revealed that, compared with FBP1-WT, FBP1-K51R was less prone to degradation (Fig. 6D and Supplementary Fig. 7). We next sought to determine whether there is a relationship between FBP1 O-GlcNAcylation at S47 and FBP1 polyubiquitination at K5. Interestingly, compared with the FBP1-WT group, OGT transfection changed the O-GlcNAcylation level in the FBP1-K51R group but not in the FBP1-S47A group. However, the polyubiquitination level increased in the FBP1-WT group but changed slightly in the FBP1-K51R and FBP1-S47A groups (Fig. 6E). In addition, hypoxia led to increased O-GlcNAcylation in the FBP1-WT and FBP1-K51R groups but not in the FBP1-S47A group, and this increase was partially reversed by siOGT transfection. Moreover, hypoxia resulted in greater K48 polyubiquitination in the FBP1-WT group than in the other groups, and this effect was inhibited in both the FBP1-S47A and FBP1-K51R groups (Fig. 6F). Collectively, these data suggest that O-GlcNAcylation at serine 47 might be a prerequisite for FBP1 polyubiquitination at lysine 51 in the context of hypoxia-induced protein degradation.
A Mass spectrometry results of the FBP1 serine 47 residue. B Co-IP between Ub and the FBP1-WT/lysine mutants was performed to validate the potential polyubiquitination site. C Conserved domains in the FBP1 protein in many species. D CHX assays were performed to determine the stability of the FBP1-WT/K51R proteins in AsPC-1 and SW1990 cells. E Cotransfection of OGT and Ub plasmids revealed that blocking FBP1 O-GlcNAcylation at the serine 47 residue can reverse its K51 polyubiquitination in AsPC-1 and SW1990 cells. F Blocking the O-GlcNAcylation of FBP1 at the serine 47 residue can reverse the K51 polyubiquitination of FBP1 in AsPC-1 and SW1990 cells induced by a hypoxic environment.
FBP1 polyubiquitination at K51 influences the expression of its downstream target genes and promotes cancer progression
Because FBP1 K51 polyubiquitination is the downstream effect of its O-GlcNAcylation, we measured the expression levels of canonical HIF-1α target genes (PDK1, LDHA, GLUT1 and VEGF) in the FBP1-WT and FBP1-K51R groups. Mutation of the K51 site increased the expression levels of these target genes under hypoxic conditions, and these increases were partially reversed by OGT knockdown in pancreatic cancer cells (Fig. 7A). Finally, to further confirm that the K51 polyubiquitination of FBP1 is downstream of its O-GlcNAcylation during pancreatic cancer progression, we repeated the functional experiments with FBP1-WT and FBP1-K51R cells. Consistent with the previous results, OGT knockdown in the FBP1-WT cells reduced glucose uptake and lactate production but had no such effect on the FBP1-K51R mutant cells (Fig. 7B, C). Compared with the expression of FBP1-WT, the expression of the FBP1-K5R mutant suppressed AsPC-1 and SW1990 cell growth (Fig. 7D). OGT knockdown also promoted colony formation by FBP1-WT cells but did not further inhibit colony formation by FBP1-K51R mutant cells (Fig. 7E). Consistent with the in vitro results, OGT knockdown significantly reduced xenograft tumour growth (i.e., tumour volume and weight) in mice injected with FBP1-WT cells but had no significant effect on tumour growth in mice injected with FBP1-K51R mutant cells, suggesting that the effect of K51 polyubiquitination may be dependent mainly on O-GlcNAcylation activity in vivo (Fig. 7F). In summary, we showed that hypoxia results in the deactivation and degradation of FBP1 in pancreatic cancer cells both in vivo and in vitro via the serine 47 O-GlcNAcylation-lysine 51 polyubiquitination axis (Fig. 7G).
A RT‒qPCR results showing the expression levels of downstream target genes in AsPC-1 and SW1990 (FBP1-WT/K51R) cells with or without OGT knockdown. B Glucose uptake assays were performed in AsPC-1 and SW1990 (FBP1-WT/K51R) cells with or without OGT knockdown. C Lactate secretion assays were performed in AsPC-1 and SW1990 (FBP1-WT/K51R) cells with or without OGT knockdown. D MTT assays were performed to measure the growth of AsPC-1 and SW1990 (FBP1-WT/K51R) cells with or without OGT knockdown. E Colony formation assays were performed in AsPC-1 and SW1990 (FBP1-WT/K51R) cells with or without OGT knockdown, and the statistical results were calculated. F Xenograft experiments in nude mice were performed in AsPC-1 and SW1990 (FBP1-WT/K51R) cells with or without OGT knockdown, and the statistical results (i.e. tumour weight and volume) were analysed. G Schematic model of how the FBP1 O-GlcNAcylation/polyubiquitination axis functions in pancreatic cancer cells in a hypoxic environment.
Discussion
A key gluconeogenic enzyme, FBP1, is also a novel tumour suppressor that antagonises anaerobic glycolysis [15]. FBP1 has been shown to be downregulated by various mechanisms in tumours [16]. In yeast, the gluconeogenic enzyme FBP1 can be degraded via the Gid4-dependent Pro/N-degron pathway [27]. However, in mammals, the orthologue of the yeast GID complex, called the CTLH complex, has not been shown to degrade FBP1, suggesting that the mechanism of FBP1 degradation is complex in mammals [28]. Here, we provide several pieces of evidence indicating that FBP1 is degraded in a novel posttranslational manner under hypoxic conditions.
First, hypoxia inhibited the expression of FBP1 in pancreatic cancer cells, and this effect was reversed by abolishing O-GlcNAcylation. Previous reports have shown that high-glucose concentrations can induce the O-GlcNAcylation of FBP1, mainly by antagonising its phosphorylation and nuclear translocation [20]. In this study, we found that hypoxia exposure can also induce the O-GlcNAcylation of FBP1 at serine 47, which leads to FBP1 degradation. O-GlcNAcylation has been proven to be closely related to protein stability, but the underlying mechanisms are diverse. Historically, O-GlcNAcylation has been believed to increase protein stability through competitive antagonism with phosphorylation, such as serine and threonine phosphorylation [29]. In addition, the SIRT7 protein can be degraded by interacting with the nucleolar protein REGγ, and our previous work confirmed that O-GlcNAcylation of SIRT7 at serine 136 can interrupt this interaction to promote SIRT7 protein stability [24]. However, researchers have shown that O-GlcNAcylation can also induce protein degradation. For example, O-GlcNAcylation of ryanodine receptor 1 (RYR1) can promote its K48-linked ubiquitination and further proteasomal degradation [11]. With respect to the FBP1 protein, published research has shown that the MAGE–TRIM28 E3 ligase complex can interact with FBP1 and lead to its degradation via the proteasome in hepatocellular carcinoma cells [30]. Moreover, the deubiquitinase USP44 can suppress pancreatic cancer progression by interacting with FBP1 (preventing its degradation) [31]. However, the patterns and sites of FBP1 ubiquitination have not been fully elucidated. In this study, we found that FBP1 can be polyubiquitinated in a K48-linked (but not K63-linked) manner by TRIM28 under hypoxic conditions, and multiple ubiquitination sites were identified. Only by blocking the K51 site can its K48-linked polyubiquitination and degradation be inhibited. Polyubiquitination has been associated with diverse proteasome-independent cellular functions, including intracellular protein translocation and interactions. For example, the ubiquitination of MALT1 at lysine 644 at the C-terminus is essential for activating its protease activity [32]. RLCK botrytis-induced kinase 1 (BIK1) is ubiquitinated following its phosphorylation, and its ubiquitination is essential for its subsequent release from the FLS2–BAK1 complex and immune signalling activation in plants [33]. Notably, K48-linked polyubiquitination always leads to protein degradation [34]. There have been many reports on the degradation mechanism of FBP1, and for the first time, we have explained this phenomenon from the perspective of O-GlcNAcylation and K48-linked polyubiquitination.
Second, we revealed that O-GlcNAcylation of FBP1 at serine 47 is a prerequisite for its K51 polyubiquitination. The transmission of protein modification signals is crucial for signal transduction. For example, MELK can increase EZH2 serine 220 phosphorylation along with the concomitant loss of EZH2 lysine 222 ubiquitination, suggesting the phosphorylation-dependent regulation of EZH2 ubiquitination [35]. Oestrogen stimulation of the pS2 promoter can induce arginine methylation following the acetylation of H3 [36]. Recently, the interplay between O-GlcNAcylation and other PTMs has emerged as an important area of investigation since O-GlcNAc was discovered in 1984, and O-GlcNAc modifications are very common in the initiation of histone signalling [37]. Serine 112 of histone subunit H2B can be O-GlcNAcylated, and its O-GlcNAcylation is an important prerequisite for H2A ubiquitination at lysine 120 [38]. With respect to nonhistone proteins, O-GlcNAcylation of MAVS (a signalling adaptor) at serine 366 is required for its ubiquitination and downstream signalling activation upon vesicular stomatitis virus (VSV) infection [39]. Therefore, our work is an important extension of previous research on the crosstalk between the O-GlcNAcylation and ubiquitination of a single protein.
Aerobic glycolysis is an important characteristic feature in PDAC, and the regulatory process and the underlying mechanism remains unexplained. Reportedly, the CDK4/6 inhibitor, PD0332991 stabilised FBP1 to hinder aerobic glycolysis by increasing MAGED1 expression in pancreatic cancer [40], suggesting that FBP1 could be a potential therapeutic target. Our results indicated that FBP1 might be stabilised by an O-GlcNAcylation antagonist in the treatment of PDAC. Our work has several limitations. First, O-GlcNAcylation at serine 124 was previously reported to antagonise FBP1 phosphorylation under high-glucose conditions. With our model, we identified another new site of O-GlcNAcylation under hypoxic conditions, but we did not identify an association between these two sites or determine whether O-GlcNAcylation at serine 124 is involved in FBP1 protein degradation. Next, FBP1 degradation via the O-GlcNAcylation— K48-linked polyubiquitination axis was confirmed. However, whether the O-GlcNAc group at S47 interferes with the connection of the Ub group at K51 through steric hindrance effects still needs to be verified in the future. Finally, we evaluated only effects on protein degradation; whether other functions of FBP1, such as its translocation or enzymatic activity, are affected is still unknown.
Conclusion
FBP1 is degraded by an O-GlcNAcylation‒polyubiquitination axis under hypoxia, which activates the Warburg effect by upregulating the expression of HIF-1α target genes to promote tumour progression in patients with PDAC.
Data availability
The data in the current study are available from the corresponding author on reasonable request.
References
Fu Z, Zhang P, Zhang R, Zhang B, Xiang S, Zhang Y, et al. Novel hypoxia-induced HIF1alpha-circTDRD3-positive feedback loop promotes the growth and metastasis of colorectal cancer. Oncogene. 2023;42:238–52.
Liu Q, Luo Q, Feng J, Zhao Y, Ma B, Cheng H, et al. Hypoxia-induced proteasomal degradation of DBC1 by SIAH2 in breast cancer progression. Elife. 2022;11:e81247.
Wicks EE, Semenza GL. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest. 2022;132:e159839.
Byrnes JR, Weeks AM, Shifrut E, Carnevale J, Kirkemo L, Ashworth A, et al. Hypoxia is a dominant remodeler of the effector T cell surface proteome relative to activation and regulatory T cell suppression. Mol Cell Proteom. 2022;21:100217.
Ma S, Zhao Y, Lee WC, Ong LT, Lee PL, Jiang Z, et al. Hypoxia induces HIF1alpha-dependent epigenetic vulnerability in triple negative breast cancer to confer immune effector dysfunction and resistance to anti-PD-1 immunotherapy. Nat Commun. 2022;13:4118.
Siddiqui I, Bilkey J, McKee TD, Serra S, Pintilie M, Do T, et al. Digital quantitative tissue image analysis of hypoxia in resected pancreatic ductal adenocarcinomas. Front Oncol. 2022;12:926497.
Zhou P, Chang WY, Gong DA, Xia J, Chen W, Huang LY, et al. High dietary fructose promotes hepatocellular carcinoma progression by enhancing O-GlcNAcylation via microbiota-derived acetate. Cell Metab. 2023;35:1961–1975.
Liu Y, Yu K, Kong X, Zhang K, Wang L, Zhang N, et al. FOXA1 O-GlcNAcylation-mediated transcriptional switch governs metastasis capacity in breast cancer. Sci Adv. 2023;9:eadg7112.
Everts GAH. B. O-GlcNAcylation at the center of antitumor immunity. Curr Opin Biotechnol. 2023;84:103009.
Le Minh G, Esquea EM, Young RG, Huang J, Reginato MJ. On a sugar high: role of O-GlcNAcylation in cancer. J Biol Chem. 2023;299:105344.
Yan W, Cao M, Ruan X, Jiang L, Lee S, Lemanek A, et al. Cancer-cell-secreted miR-122 suppresses O-GlcNAcylation to promote skeletal muscle proteolysis. Nat Cell Biol. 2022;24:793–804.
He QQ, Yang M, Huang J, Wu W, Tang K, Zhang Y, et al. Hypoxia-triggered O-GlcNAcylation in the brain drives the glutamate-glutamine cycle and reduces sensitivity to sevoflurane in mice. Br J Anaesth. 2022;129:703–15.
Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–80.
Lei Y, Chen T, Li Y, Shang M, Zhang Y, Jin Y, et al. O-GlcNAcylation of PFKFB3 is required for tumor cell proliferation under hypoxia. Oncogenesis. 2020;9:21.
Alderton GK. Tumorigenesis: FBP1 is suppressed in kidney tumours. Nat Rev Cancer. 2014;14:575.
Xiong X, Zhang J, Li A, Dai L, Qin S, Wang P, et al. GSK343 induces programmed cell death through the inhibition of EZH2 and FBP1 in osteosarcoma cells. Cancer Biol Ther. 2020;21:213–22.
Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–31.
Son B, Lee S, Kim H, Kang H, Jeon J, Jo S, et al. Decreased FBP1 expression rewires metabolic processes affecting aggressiveness of glioblastoma. Oncogene. 2020;39:36–49.
Zhang X, Li S, He J, Jin Y, Zhang R, Dong W, et al. TET2 suppresses VHL deficiency-driven clear cell renal cell carcinoma by inhibiting HIF signaling. Cancer Res. 2022;82:2097–109.
Lu C, Ren C, Yang T, Sun Y, Qiao P, Han X, et al. Fructose-1, 6-bisphosphatase 1 interacts with NF-kappaB p65 to regulate breast tumorigenesis via PIM2 induced phosphorylation. Theranostics. 2020;10:8606–18.
Cheng X, Zhang B, Guo F, Wu H, Jin X. Deubiquitination of FBP1 by USP7 blocks FBP1-DNMT1 interaction and decreases the sensitivity of pancreatic cancer cells to PARP inhibitors. Mol Oncol. 2022;16:1591–607.
Zhu Y, Shi M, Chen H, Gu J, Zhang J, Shen B, et al. NPM1 activates metabolic changes by inhibiting FBP1 while promoting the tumorigenicity of pancreatic cancer cells. Oncotarget. 2015;6:21443–51.
Li L, Yang L, Fan Z, Xue W, Shen Z, Yuan Y, et al. Hypoxia-induced GBE1 expression promotes tumor progression through metabolic reprogramming in lung adenocarcinoma. Signal Transduct Target Ther. 2020;5:54.
He X, Li Y, Chen Q, Zheng L, Lou J, Lin C, et al. O-GlcNAcylation and stablization of SIRT7 promote pancreatic cancer progression by blocking the SIRT7-REGgamma interaction. Cell Death Differ. 2022;29:1970–81.
Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–5.
Wang Y, Liu J, Jin X, Zhang D, Li D, Hao F, et al. O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc Natl Acad Sci USA. 2017;114:13732–7.
Melnykov A, Chen SJ, Varshavsky A. Gid10 as an alternative N-recognin of the Pro/N-degron pathway. Proc Natl Acad Sci USA. 2019;116:15914–23.
Mohamed WI, Park SL, Rabl J, Leitner A, Boehringer D, Peter M. The human GID complex engages two independent modules for substrate recruitment. EMBO Rep. 2021;22:e52981.
Liu X, Cai YD, Chiu JC. Regulation of protein O-GlcNAcylation by circadian, metabolic, and cellular signals. J Biol Chem. 2023;300:105616.
Jin X, Pan Y, Wang L, Zhang L, Ravichandran R, Potts PR, et al. MAGE-TRIM28 complex promotes the Warburg effect and hepatocellular carcinoma progression by targeting FBP1 for degradation. Oncogenesis. 2017;6:e312.
Yang C, Zhu S, Yang H, Deng S, Fan P, Li M, et al. USP44 suppresses pancreatic cancer progression and overcomes gemcitabine resistance by deubiquitinating FBP1. Am J Cancer Res. 2019;9:1722–33.
Pelzer C, Cabalzar K, Wolf A, Gonzalez M, Lenz G, Thome M. The protease activity of the paracaspase MALT1 is controlled by monoubiquitination. Nat Immunol. 2013;14:337–45.
Ma X, Claus LAN, Leslie ME, Tao K, Wu Z, Liu J, et al. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature. 2020;581:199–203.
Zhang Q, Li Y, Zhu Q, Xie T, Xiao Y, Zhang F, et al. TRIM65 promotes renal cell carcinoma through ubiquitination and degradation of BTG3. Cell Death Dis. 2024;15:355.
Li B, Yan J, Phyu T, Fan S, Chung TH, Mustafa N, et al. MELK mediates the stability of EZH2 through site-specific phosphorylation in extranodal natural killer/T-cell lymphoma. Blood. 2019;134:2046–58.
Daujat S, Bauer UM, Shah V, Turner B, Berger S, Kouzarides T. Crosstalk between CARM1 methylation and CBP acetylation on histone H3. Curr Biol. 2002;12:2090–7.
Ruan HB, Nie Y, Yang X. Regulation of protein degradation by O-GlcNAcylation: crosstalk with ubiquitination. Mol Cell Proteom. 2013;12:3489–97.
Fujiki R, Hashiba W, Sekine H, Yokoyama A, Chikanishi T, Ito S, et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–60.
Li T, Li X, Attri KS, Liu C, Li L, Herring LE, et al. O-GlcNAc transferase links glucose metabolism to MAVS-mediated antiviral innate immunity. Cell Host Microbe. 2018;24:791–803 e796.
Zhang B, Li D, Jin X, Zhang K. The CDK4/6 inhibitor PD0332991 stabilizes FBP1 by repressing MAGED1 expression in pancreatic ductal adenocarcinoma. Int J Biochem Cell Biol. 2020;128:105859.
Funding
This work was supported by grants from the National Natural Science Foundation of China: No.81702316 (Dr. Yi Zhu) and No.82303068 (Dr. Xiaoman He).
Author information
Authors and Affiliations
Contributions
YZ was responsible for designing and performing experiments and writing papers. XH was responsible for performing experiments. XM was responsible for data analysis. YZ and WF were responsible for data collection.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to publish
All authors have provided their consent to publish the manuscript.
Ethical approval
This study was approved by the Ethics Committee of Zhejiang University, in accordance with the Declaration of Helsinki. All animal experiments were approved by the Animal Ethics Committee of Zhejiang University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Y., He, X., Ma, X. et al. O-GlcNAcylation of FBP1 promotes pancreatic cancer progression by facilitating its Lys48-linked polyubiquitination in hypoxic environments. Oncogenesis 14, 11 (2025). https://doi.org/10.1038/s41389-025-00555-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41389-025-00555-4
This article is cited by
-
E2 ubiquitin-conjugating enzyme Ubc11 regulates Rst2 protein stability in the fission yeast Schizosaccharomyces pombe
Archives of Microbiology (2025)