Abstract
BCL-XL is a crucial anti-apoptotic protein that supports survival of intestinal cells during the progression and in established colorectal cancer (CRC). While targeting BCL-XL with BH3 mimetics is effective, its significant toxicity highlights the need for alternative approaches. Importantly, the early steps in intestinal transformation are marked by a competition between normal and transformed stem cells in which the mutant cells gain a supercompetitive advantage due to the secretion of WNT inhibitors. Using multiple human and murine CRC models, we revealed that GSK-3 inhibition strongly sensitized to BH3 mimetic-induced killing. As expected, GSK-3 inhibition significantly upregulated the WNT pathway, but also led to marked enhancement of BH3 mimetic-induced apoptosis, as measured by mitochondrial BAX aggregation, Caspase-3 activation and Propidium Iodide exclusion. Furthermore, GSK-3 inhibition provided an advantage to wild-type intestinal organoids in competition with APC-mutant counterparts due to reactivation of the WNT pathway. More strikingly, combining GSK-3 and BCL-XL inhibition profoundly affected the supercompetition APC-mutant intestinal cells exert over the wild-types. In effect, the combination therapy enhanced the competitive fitness of wild-type cells and resulted in the killing of APC-mutant organoids, pointing to a novel combination therapy that can be further exploited in the treatment of adenomas and CRC.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) development is a multi-step process, often initiated by mutations in the tumor suppressive gene APC, leading to constitutive WNT pathway activity [1]. In familial adenomatous polyposis (FAP) patients, germline APC mutations cause early onset polyps due to loss, methylation or inactivation of the second APC locus [2]. Similarly, sporadic CRC often begins with APC loss, followed by alterations in KRAS and TP53, driving malignancy [3].
APC loss is particularly impactful in intestinal stem cells (ISCs). We recently showed that APC loss in ISCs causes supercompetition with wild-type (WT) ISCs via secretion of WNT inhibitors like Notum [4, 5], promoting their clonal expansion and adenoma formation. Intriguingly, GSK-3 inhibitors, partially restore the competitive fitness of WT ISCs both in vitro and in vivo by boosting WNT signaling, thereby decreasing adenoma formation [6].
Besides WNT alterations, transformation of ISCs involves changes in apoptotic pathways. While early lesions rely on BCL-2 and BCL-XL for survival after APC loss [7, 8], BCL-2 dependency is quickly lost due to oncoMir17-92 activation, leaving BCL-XL critical survival factor [8]. However, BCL-XL inhibitors are limited by platelet toxicity, necessitating safer combination strategies.
Here, we aimed to combine the sensitizing effect of compounds towards BH3 mimetics and target the supercompetition exerted by APC-mutant cells. We identified that GSK-3 inhibitors selectively sensitized cancer cells to low doses of BCL-XL inhibitor A-1155463, while enhancing WT ISC fitness and reversing APC-mutant supercompetition. Our findings, therefore, reveal a novel combination strategy that can be especially exploited to treat early stages of CRC.
Methods and materials
Spheroids and organoids
Spheroid culture
Co01 spheroid cultures were maintained in supplemented advanced DMEM/F12 (Thermo Fisher Scientific, Bleiswijk, The Netherlands) (supplementary data) and grown in ultra-low adherent flasks (Corning, Amsterdam, The Netherlands) at 37 °C and 5% CO2.
Construction of Co01-BAX-KO-CFP-BAX-MitoDsRed line
Co01-BAX-KO-CFP-BAX-MitoDsRed line was generated as described [9]. BAX was knocked out using lentiCRISPRv2, followed by sequential lentiviral transduction with hBAX-ORF in frame with CFP (CFP-BAX) and mitochondria targeting DsRed (MitoDsRed) into Co01-BAX-KO cell line.
Mouse organoid/tumoroid construction and cultures
Mouse intestinal organoids were established from Lgr5-EGFP-IRES-CreERT2 mice (WT) as described [6]. WT organoids were transduced with an mCherry construct (LeGO-C2; #27399, Addgene). Apc-mutant organoids (Apc-/-) were generated by in vitro recombination using 1 μM 4OH-tamoxifen in Lgr5-EGFP-IRES-CreERT2; Apcfl/fl and transduced with Venus construct (LeGO-V2; #27340, Addgene). Tumoroids were derived from primary tumors in KPN (KrasLSL-G12D/+, Trp53fl/fl, Rosa26LSL-N1icd/+) mice via Villin-CreERT2-driven recombination, as previously described [10, 11].
Human organoid construction and cultures
CRISPR-Cas9-derived mutant colon organoids were generated as described [12]. Normal organoids were transfected with Cas9 and sgRNAs targeting APC, KRAS, or TP53. Clonal lines were selected and genotyped to confirm mutations.
All organoids and tumoroids were maintained in a 50 µl matrigel (Corning) droplet in base medium and supplements (supplementary data).
Cell lines
RCM-1 and SW620 cells (Wellcome Sanger Institute) were cultured in supplemented (supplementary data) RPMI 1640 (Gibco) and DMEM/F12 (Gibco) respectively, STR profiled and mycoplasma tested monthly.
Compounds
A-1155463, CHIR-98014 and CHIR-99021 were purchased from Selleck Chemicals (Huissen, The Netherlands) and dissolved in DMSO to a stock of 10 mM.
Viability assays
Cell Titer Blue
Co01, SW620 (4000 cells/well) and RCM-1 (9500 cells/well) cells were seeded in 96-well plates (Corning). After 24 h, treatments were applied for 48 h. Viability was assessed using Cell Titer Blue (Promega, Leiden, The Netherlands) after a 2 h incubation.
Cell Titer-Glo
Organoids were embedded in 6 μL matrigel domes per well in 96-well plates (Corning) and treated immediately. Viability was measured after 72 h using the CellTiter-Glo (Promega) according to manufacturer’s instructions.
Flow cytometry
Caspase-3 activation
Caspase-3 activation was measured by CaspaTag Caspase-3,7 In Situ Assay Kit, Sulforhodamine (Merck, Amsterdam, The Netherlands). Fluorescence reflects active Caspase-3 measured by flow cytometry.
Propidium Iodide staining
Propidium Iodide (PI, Sigma) was measured using 1 µg/ml PI incubated at room temperature for 10 min. PI fluorescence was measured and recorded in the FL-2 PE channel by flow cytometry.
Mitochondrial ROS measurement
Mitochondrial reactive oxygen species (ROS) levels were assessed using MitoSOX Mitochondrial Superoxide Indicator (Thermo Fisher Scientific). Cells were treated for 24 h, incubated with 1 μM MitoSOX for 30 min and analyzed by flow cytometry using FL-2 PE channel. Geometric mean fluorescence was analyzed using FlowJo v10 (BD Biosciences, Drachten, The Netherlands).
WNT signaling activity assay with TOP-GFP reporter
WNT signaling activity of Co01 cells was monitored by TOP-GFP reporter activity as described [13, 14]. Cells were treated for 24 h. TOP-GFP signal was measured by flow cytometry.
Quantitative PCR and RNA sequencing
Co01 cells were treated for 48 h before RNA extraction. cDNA was synthesized from 1 µg RNA. qPCR (primers listed in the supplementary section) was performed by LightCycler 480 Sybr Green I Master (Roche, Woerden, The Netherlands). For RNAseq, quality control of RNA was performed by Agilent 2100 Bioanalyzer before standard RNA sequencing. Sequencing and analysis were conducted as described [6].
Confocal fluorescence microscopy
Co01-BAX-KO-CFP-BAX-MitoDsRed cells were seeded into 4-well Nunc™ Lab-Tek™ II Chamber Slide™ and treated for 24 h. Imaging was performed with Leica TCS SP8 SMD. BAX localization was quantified and expressed as a percentage of positive cells.
Organoid competition assay
WT and Apc-/- organoids were co-cultured in 24-well plates as described [15] and treated immediately. Wells were imaged over time using the EVOS-FL Cell Imaging System (Thermo Fisher Scientific). Quantification was performed in ImageJ (supplementary data).
Statistics
All experiments were repeated at least three times. All data is represented as mean ± standard deviation, unless otherwise noted. Two-tailed unpaired Mann-Whitney tests were used for comparisons; significance was set at p ≤ 0.05 (∗p ≤ 0.05, ∗∗ p ≤ 0.01, ∗∗∗ p ≤ 0.001, ∗∗∗∗ p ≤ 0.0001). Synergy was calculated via SynergyFinder [16] applying the Bliss independence model, with scores >10 indicating synergy, −10 to 10 additive, and <−10 antagonism.
Results
GSK-3 inhibitors sensitize CRC to BCL-XL inhibition
To find a safe CRC-targeting strategy that would spare platelets, we previously screened a compound library with a pre-selected low dose of BCL-XL inhibitor A-1155463 and identified GSK-3 inhibitors as potent sensitizers [17]. Five GSK-3 inhibitors, targeting GSK-3α and GSK-3β, sensitized CRC cell line Co01 to 5 nM A-1155463 (Fig. 1A). To identify an optimal synergy dose, we titrated A-1155463 to identify a concentration causing minimal toxicity, selecting 10 nM for further combination studies (Fig. S1A). Subsequently, two representative GSK-3 inhibitors, CHIR-98014 and CHIR-99021 were titrated alone and in combination with fixed dose of A-1155463 (Fig. S1B, C), confirming a reduction in viability upon combination treatment. To comprehensively assess synergy, we performed matrix-based combinatorial titrations, revealing synergy at multiple concentrations for both compounds (Fig. 1B, D), with Bliss scores exceeding 10 and peaking above 30, indicating a very strong synergy with A-1155463 (Fig. 1C, E). To confirm that synergy reflected cell death we measured Caspase-3 activation (Fig. 1F, G) and Propidium Iodide influx (Fig. 1H, I). This showed that the observed reduction in Cell Titer Blue levels with GSK-3 inhibitors alone rather reflects inhibition of metabolism as these inhibitors alone did not affect caspase-activation or PI influx. Nevertheless, GSK-3 inhibition strongly sensitized cells to BH3-induced apoptosis and cell death (Fig. 1F–I).
A Screening assay. Co01 cells were treated with 1 µM compounds from the library with or without 5 nM A-1155463. Targets of the hits that showed synergy were summarized. The targeting efficacy of five GSK-3 inhibitors was presented in the table. B–E Matrix titration and Bliss synergy scores for each concentration in the most synergistic area of two GSK-3 inhibitors CHIR-98014 (B, C) and CHIR-99021 (D, E) with A-1155463. Cell viability was measured by Cell Titer Blue. Inhibition rate was related to the untreated control (0,0 nM). Synergy scores were calculated by the online tool Synergyfinder. F, G Activation of Caspase-3 induced by 1 µM CHIR-98014 (F) or 2.5 µM CHIR-99021 (G) with or without 5 nM A-1155463 in Co01 was measured by flow cytometry. Percentage of the cells with active Caspase-3 was plotted.* :p ≤ 0.05,**: p ≤ 0.01. H, I Percentage of dead cells with PI influx induced by 1 µM CHIR-98021 (H) or 2.5 µM CHIR-99021 (I) with or without 5 nM A-1155463 in Co01 was measured by flow cytometry. *:p ≤ 0.05.
To assess the broader applicability of our findings, we extended the analysis to additional CRC models. Two more CRC cell lines, RCM-1 and SW620, showed a strong synergistic response to the combination treatment (Fig. S2A–D).
We next tested whether the observed synergy between GSK-3 and BCL-XL inhibition also applied to more complex, genetically defined CRC models. Here, we used human colon-derived organoids engineered to carry APCKO, KRASG12D, and TP53KO mutations [12]. APC-mutant organoids strongly responded to the combination treatment (Fig. S2E, F), like CRC cell lines. Importantly, triple mutant (APCKO/KRASG12D/TP53KO) organoids (Fig. S2G, H) showed reduced synergy, suggesting that KRASG12D and TP53 mutations could dampen, although not abolish, the synergistic effect of GSK-3 and BCL-XL inhibition.
GSK-3 inhibitors induce upregulation of WNT signaling activity
To investigate the mechanism of synergy, we examined WNT signaling in APC-mutant Co01 cells using the TOP-GFP reporter system [13], where GFP fluorescence indicates WNT pathway activity. Treatment with CHIR-98014 and CHIR-99021 significantly increased GFP fluorescence, indicating elevated β-catenin-driven transcription (Fig. 2A). Gene set enrichment analysis of RNA-seq data confirmed WNT pathway activation beyond the baseline APC mutation-driven activity (Fig. 2B). Additionally, qPCR analysis revealed that treatment with GSK-3 inhibitors significantly upregulated WNT target gene LGR5 (Fig. 2C, D), while downregulating differentiation marker CK20 (Fig. 2E, F), indicating a shift toward a stem-like phenotype. Although previous studies linked elevated WNT signaling to BH3 mimetic resistance [18], our results show that overstimulation of this pathway can instead enhance sensitivity. To test whether WNT activation was required for the observed synergy, we used KPN tumoroids, derived from KrasLSL-G12D/+, Trp53fl/fl, Rosa26LSL-N1icd/+ (KPN) mice [10], and generated from these β-catenin knockout (Ctnnb1KO) lines. In KPN lines, GSK-3 inhibition leads to significant upregulation of WNT pathway activity [10], while this was effectively blocked in the Ctnnb1KO lines. Notably, β-catenin-deficient tumoroids showed complete reduction of synergy (Fig. S3B) to the combination treatment compared to the KPN controls (Fig. S3A), suggesting that WNT pathway activity is functionally required for this effect.
A Flow cytometry analysis of the TOP-GFP in Co01 cells treated with 1 µM CHIR-98021 or 2.5 µM CHIR-99021 for 24 h. * :p ≤ 0.05,**: p ≤ 0.01. B Gene Set Enrichment Analysis (GSEA) of WNT/β-catenin signaling pathways in Co01 cells treated with 1 µM CHIR-99021 for 48 h. p = 0.04338. C, D mRNA expression of LGR5 in Co01 cells treated with 1 µM CHIR-98014 (C) and 2.5 µM CHIR-99021 (D) for 24 h. E, F mRNA expression of CK20 in Co01 cells treated 1 µM CHIR-98014 (E) and 2.5 µM CHIR-99021 (F) for 24 h.
GSK-3 inhibition enhances localization of BAX to the mitochondria
To elucidate the molecular basis of sensitization and explore mitochondrial involvement, we measured mitochondrial ROS levels using the MitoSOX Red indicator. Although combination treatment showed a trend toward increased mitochondrial superoxide production, the difference was not statistically significant (Fig. S4A). Therefore, to confirm the mitochondrial role in sensitization, we used Co01 cells co-expressing CFP-BAX and MitoDsRed to trace co-localization of BAX and mitochondria after treatment. In the absence of an apoptotic stimulus, BAX remains mostly cytoplasmatic, while effective apoptosis induction causes its mitochondrial aggregation [19]. Treatment with CHIR-99021 alone induced slight mitochondrial BAX localization, while A-1155463 triggered minor localization in few Co01 cells. Strikingly, CHIR-99021 pretreatment dramatically enhanced BAX mitochondrial translocation upon A-1155463 treatment (Fig. 3A), raising the proportion of cells with strong BAX mitochondrial staining from approximately 10% to 40% (Fig. 3B), indicating GSK-3 inhibition facilitated BAX localization and sensitized CRC cells to BCL-XL inhibitor-induced apoptosis.
A Representative confocal microscopic images of Co01-BAX-KO-CFP-BAX-MitoDsRed. Cells were treated with 2.5 µM CHIR-99021 for 24 h before 5 nM A-1155463 was added to trigger CFP-BAX aggregation. The pictures were captured at 63× Oil magnification. B Statistics of the percentage of cells with BAX localization treated with 2.5 µM CHIR-99021 or/and 5 nM A-1155463, ***:p ≤ 0.001,****:p ≤ 0,0001.
Combination therapy provides competitive fitness to WT intestinal cells over APC-mutant cells
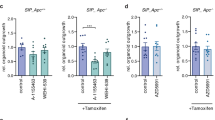
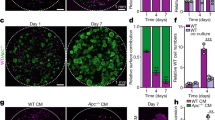
Previously, we showed that APC-mutant intestinal organoids exhibit supercompetition towards WT neighboring cells by secreting WNT antagonists [6]. Due to their intrinsic constitutive WNT pathway activation, these APC-mutant cells are resistant to these antagonists and thus outcompete WT cells. GSK-3 inhibitors were shown to partly restore WNT pathway function in WT intestinal cells, boosting their competitive fitness and mitigating the advantage of APC-mutant cells. Intriguingly, we recently showed that BCL-XL inhibition prevents adenoma formation in mice after APC loss [8]. Here, we tested whether combining GSK-3 and BCL-XL inhibitors could also impact the competition between WT and APC-mutant organoids, labeled with mCherry and Venus respectively and co-cultured as illustrated in Fig. 4A. In line with the previous results, APC-mutant cells (green) repressed the growth of WT cells (red) and dominated the co-culture after 7 days (Fig. 4B left lane). In contrast, treatment with CHIR-99021 partially restored the fitness of WT cells, allowing both populations to expand (Fig. 4B right lane). Fluorescence quantification in Fig. 4D confirmed this shift in population dynamics, showing a marked reduction in the APC-mutant cells following combination treatment. Strikingly, the combination treatment completely reverted the competition in favor of WT cells, killing and outcompeting APC-mutant counterparts (Fig. 4C).
A Schematic illustration of the setting of co-culture assay. B Representative images of the time course co-culture experiment. Co-cultures were treated with or without 10 µM CHIR-99021. Pictures were captured at 4× magnification on Days 1, 3, and 7 after treatment. C Representative images of the co-cultures treated with 5 or 10 µM CHIR-99021 with or/and 1, 2.5, 5 µM A-1155463. Pictures were captured at 4× magnification at Day 7 after treatment. D Statistics of the relative surface contribution of wild-type organoids (Red) and APC-mutant organoids (Green). E Mechanistic Illustration of the synergistic effect of combination of GSK-3β inhibitor and BCL-XL inhibitor on the competition between WT and APC-mutant ISCs.
Discussion
In this study, we revealed that GSK-3 inhibitors synergized with BCL-XL inhibitor A-1155463 to target CRC. This combination restored the fitness of WT ISCs and eliminated APC-mutant cells, restraining their supercompetitive activity.
GSK-3 has context-dependent roles in cancer, acting as a tumor promoter and suppressor [20]. In WNT signaling, it functions within the β-catenin destruction complex, typically preventing oncogenic transcription [20, 21]. However, GSK-3 also supports pro-tumorigenic pathways, and its inhibition has shown anti-tumor effects in various cancers [20, 22].
In APC-mutant CRC cells, where WNT signaling is constitutively active due to a disrupted destruction complex [23], GSK-3 inhibition amplifies WNT activity beyond optimal levels, inducing oncogenic stress and priming cells for apoptosis [24], especially alongside BCL-XL inhibition.
Our findings support this mechanism, showing that GSK-3 inhibition sensitized CRC cells to BCL-XL inhibition, likely due to hyperactivation of oncogenic pathways triggering anti-tumor effects in genetically unstable tumor cells [25]. Importantly, most APC mutations retain partial β-catenin regulation [26]. The “just-right-signaling model” suggests APC mutations are selected to optimize WNT output [27] and GSK-3 inhibition may push signaling beyond the acceptable threshold, causing oncogenic stress [26, 27] related to DNA replication [28, 29], ER stress [30], and mitochondrial dysfunction [31], potentially increasing ROS [32]. These stresses sensitize cells to apoptosis [33], such as that induced by A-1155463. Although combination treatment showed a non-significant ROS increase (Fig. S4), this may reflect timing limitations or indicate ROS is not central to the apoptotic response. Instead, sensitization likely results from the combined stresses mentioned above [22, 28, 29, 31].
Accordingly, GSK-3 and BCL-XL inhibition triggered apoptosis in APC-mutant organoids but had reduced impact in more advanced organoids harboring additional KRASG12D and p53 mutations (Fig. S2). This suggests that once WNT signaling is no longer the predominant driver, likely due to additional oncogenic alterations supporting survival and reducing apoptosis [23], stress imposed by GSK-3 inhibition becomes less impactful. KRAS signaling may counterbalance WNT-driven stress through MAPK/ PI3K pathways [34, 35] and inhibit pro-apoptotic proteins like BAX [36], further dampening apoptosis. Moreover, p53 loss impairs apoptotic checkpoints [37] by suppressing key effectors such as PUMA and BAX [8, 37], reducing sensitivity to stress-induced death. These findings underscore that synergy relies on a window of WNT dependence and apoptotic competence, which may be diminished during tumor evolution [23]. Supporting this, β-catenin-deficient KPN tumoroids were markedly less sensitive to combination treatment than KPN counterparts (Fig. S3), indicating that active WNT signaling is functionally required for the observed synergy.
Beyond ISCs, GSK-3 and BCL-XL inhibition may also affect the tumor microenvironment. For instance, GSK-3β inhibition can suppress the endothelial-to-mesenchymal transition in endothelial cells, affecting angiogenesis and vascular integrity [38], while BCL-XL supports cancer-associated fibroblast survival, potentially contributing to therapy resistance [39].
However, resistance mechanisms like MCL-1 upregulation [17, 40, 41] or activation of survival pathways like PI3K/AKT, could reduce efficacy over time [42]. Sustained GSK-3 inhibition might also trigger feedback loops altering WNT [43] or other oncogenic pathways [44], enabling cells to bypass the induced oncogenic stress. Addressing these issues and evaluating clinical translation will require extensive in vivo studies.
Importantly, this combination therapy dramatically reverted the supercompetition bias between WT and APC-mutant ISCs (Fig. 4) [5, 6]. Although the exact mechanism is unclear, the combination therapy clearly promotes APC-mutant cell death and WT cell outgrowth. While the first is likely mediated by combination therapy the latter is the result of GSK-3 inhibition, which activates WNT signaling in WT ISCs, restoring their competitive fitness against APC-mutants [6]. In cancer cells, this pathway activation imposes substantial stress [25] as described above and has shown robust anti-tumor effects in APC-mutant organoids models [45].
Further investigation is needed to understand how this combination influences ISC interactions, but clearly tips the balance in favor of WT cells. We propose combining BH3 mimetics with GSK-3 inhibition as an effective strategy against early-stage CRC in high-risk FAP patients.
Given the promising results, ongoing in vivo studies will evaluate its therapeutic and preventive potential in relevant CRC models, including efforts to define a platelet-sparing dose of A-1155463 that retains efficacy against APC-driven adenomas.
References
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67.
Gomez Garcia EB, Knoers NV. Gardner’s syndrome (familial adenomatous polyposis): a cilia-related disorder. Lancet Oncol. 2009;10:727–35.
Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507.
Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science. 2013;342:995–8.
Flanagan DJ, Pentinmikko N, Luopajarvi K, Willis NJ, Gilroy K, Raven AP, et al. NOTUM from Apc-mutant cells biases clonal competition to initiate cancer. Nature. 2021;594:430–5.
van Neerven SM, de Groot NE, Nijman LE, Scicluna BP, van Driel MS, Lecca MC, et al. Apc-mutant cells act as supercompetitors in intestinal tumour initiation. Nature. 2021;594:436–41.
van der Heijden M, Zimberlin CD, Nicholson AM, Colak S, Kemp R, Meijer SL, et al. Bcl-2 is a critical mediator of intestinal transformation. Nat Commun. 2016;7:10916.
Ramesh P, Lannagan TRM, Jackstadt R, Atencia Taboada L, Lansu N, Wirapati P, et al. BCL-XL is crucial for progression through the adenoma-to-carcinoma sequence of colorectal cancer. Cell Death Differ. 2021;28:3282–96.
Zhang L, Ramesh P, Atencia Taboada L, Roessler R, Zijlmans DW, Vermeulen M, Picavet-Havik DI, et al. UGT8 mediated sulfatide synthesis modulates BAX localization and dictates apoptosis sensitivity of colorectal cancer. Cell Death Differ. 2024;32;657–71.
Wouters VM, Helderman R, Cameron K, van der Hooff SR, Torang A, van den Bergh S, et al. CDX2 downregulation regulates intrinsic WNT pathway activation, dictating metastasis in APC and CTNNB1 wildtype colorectal cancer. Oncogene. 2025;44;2091–2102.
Jackstadt R, van Hooff SR, Leach JD, Cortes-Lavaud X, Lohuis JO, Ridgway RA, et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell. 2019;36:319–36.e7.
Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521:43–7.
Vermeulen L, Melo FDSE, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature Cell Biol. 2010;12:468–U121.
Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–14.
van Neerven SM, Ramadan R, van Driel MS, Huels DJ, Vermeulen L. Intestinal organoid co-culture protocol to study cell competition in vitro. STAR Protoc. 2022;3:101050.
Ianevski A, He L, Aittokallio T, Tang J. SynergyFinder: a web application for analyzing drug combination dose-response matrix data. Bioinformatics. 2017;33:2413–5.
Ramesh P, Di Franco S, Atencia Taboada L, Zhang L, Nicotra A, Stassi G, et al. BCL-XL inhibition induces an FGFR4-mediated rescue response in colorectal cancer. Cell Rep. 2022;38:110374.
Colak S, Medema JP. Human colonic fibroblasts regulate stemness and chemotherapy resistance of colon cancer stem cells. Cell Cycle. 2016;15:1531–7.
Pawlowski J, Kraft AS. Bax-induced apoptotic cell death. Proc Natl Acad Sci USA. 2000;97:529–31.
Thapa R, Gupta G, Bhat AA, Almalki WH, Alzarea SI, Kazmi I, et al. A review of glycogen synthase kinase-3 (GSK3) inhibitors for cancers therapies. Int J Biol Macromol. 2023;253:127375.
Marchand B, Arsenault D, Raymond-Fleury A, Boisvert FM, Boucher MJ. Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem. 2015;290:5592–605.
Kuroki H, Anraku T, Kazama A, Bilim V, Tasaki M, Schmitt D, et al. 9-ING-41, a small molecule inhibitor of GSK-3beta, potentiates the effects of anticancer therapeutics in bladder cancer. Sci Rep. 2019;9:19977.
Dow LE, O’Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, et al. Apc restoration promotes cellular differentiation and reestablishes Crypt homeostasis in colorectal cancer. Cell. 2015;161:1539–52.
Dias MH, Friskes A, Wang S, Fernandes Neto JM, van Gemert F, Mourragui S, et al. Paradoxical activation of oncogenic signaling as a cancer treatment strategy. Cancer Discov. 2024;14:1276–301.
Dias MH, Papagianni C, Bernards R. The case for therapeutic overactivation of oncogenic signaling as a potential cancer treatment strategy. Cancer Cell. 2024;42:919–22.
Parker TW, Rudeen AJ, Neufeld KL Oncogenic serine 45-deleted beta-catenin remains susceptible to Wnt stimulation and APC regulation in human colonocytes. Cancers. 2020;12;2114.
Albuquerque C, Breukel C, van der Luijt R, Fidalgo P, Lage P, Slors FJ, et al. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet. 2002;11:1549–60.
da Costa A, Chowdhury D, Shapiro GI, D’Andrea AD, Konstantinopoulos PA. Targeting replication stress in cancer therapy. Nat Rev Drug Discov. 2023;22:38–58.
Gaillard H, Garcia-Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer. 2015;15:276–89.
Chen X, Cubillos-Ruiz JR. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer. 2021;21:71–88.
O’Malley J, Kumar R, Inigo J, Yadava N, Chandra D. Mitochondrial stress response and cancer. Trends Cancer. 2020;6:688–701.
Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel?. Nat Rev Cancer. 2014;14:709–21.
Tian X, Zhang S, Zhou L, Seyhan AA, Hernandez Borrero L, Zhang Y, et al. Targeting the integrated stress response in cancer therapy. Front Pharm. 2021;12:747837.
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22.
Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313.
Ferreira A, Manon S, Eyitayo AR, Chaves SR, Corte-Real M, Preto A, et al. Oncogenic KRAS mutations modulate BAX-mediated cell death. Biochim Biophys Acta Mol Cell Res. 2025;1872:119872.
Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression?. Cell Death Differ. 2018;25:104–13.
Kim SH, Song Y, Seo HR. GSK-3beta regulates the endothelial-to-mesenchymal transition via reciprocal crosstalk between NSCLC cells and HUVECs in multicellular tumor spheroid models. J Exp Clin Cancer Res. 2019;38:46.
Nocquet L, Roul J, Lefebvre CC, Duarte L, Campone M, Juin PP, et al. Low BCL-xL expression in triple-negative breast cancer cells favors chemotherapy efficacy, and this effect is limited by cancer-associated fibroblasts. Sci Rep. 2024;14:14177.
Frederick DT, Salas Fragomeni RA, Schalck A, Ferreiro-Neira I, Hoff T, Cooper ZA, et al. Clinical profiling of BCL-2 family members in the setting of BRAF inhibition offers a rationale for targeting de novo resistance using BH3 mimetics. PLoS One. 2014;9:e101286.
Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, et al. Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–9.
Opydo M, Mlyczynska A, Mlyczynska E, Rak A, Kolaczkowska E synergistic action of MCL-1 inhibitor with BCL-2/BCL-XL or MAPK pathway inhibitors enhances acute myeloid leukemia cell apoptosis and differentiation. Int J Mol Sci. 2023;24.
Cantoria MJ, Alizadeh E, Ravi J, Varghese RP, Bunnag N, Pond KW, et al. Feedback in the beta-catenin destruction complex imparts bistability and cellular memory. Proc Natl Acad Sci USA. 2023;120:e2208787120.
Li C, Furth EE, Rustgi AK, Klein PS When you come to a fork in the road, take it: Wnt signaling activates multiple pathways through the APC/Axin/GSK-3 complex. Cells. 2023;12.
Chang L, Jung NY, Atari A, Rodriguez DJ, Kesar D, Song TY, et al. Systematic profiling of conditional pathway activation identifies context-dependent synthetic lethalities. Nat Genet. 2023;55:1709–20.
Acknowledgements
Supported by Oncode Institute, L.Z. was supported by a CSC (China Scholarship Committee) scholarship.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: L.Z. and J.P.M. Experimental work: L.Z., L.A.T., S.B., M.K., C.E., P.R., R.F.C.P.A.H., A.T. and K.C. Data analysis and interpretation: L.Z., L.A.T. and J.P.M. Drafting of the manuscript: L.Z. L.A.T. and J.P.M. Final manuscript contribution: L.Z., L.A.T., S.B., M.K., C.E., P.R., R.F.C.P.A.H., A.T., K.C., M.S.v.D., V.M.W., S.M.v.N. and J.P.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were carried out in accordance with the relevant guidelines and regulations. Human organoids were derived with informed consent and ethical approval, as previously described [12]. Mouse organoids were obtained under approved animal protocols, as reported earlier [10].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Atencia Taboada, L., Baglamis, S. et al. GSK-3 and BCL-XL inhibition mitigates the competitive advantage of APC-mutant colorectal cancer cells. Oncogenesis 14, 25 (2025). https://doi.org/10.1038/s41389-025-00569-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41389-025-00569-y