Abstract
Neonatal Encephalopathy (NE) remains a major cause of death and long-term severe disabilities, including epilepsy and cerebral palsy in term and near-term infants. The single most common cause is hypoxic-ischemic encephalopathy (HIE). However, there are many other potential causes, including infection, intracranial hemorrhage, stroke, brain malformations, metabolic disorders, and genetic causes. The appropriate management depends on both the specific cause and the stage of evolution of injury. Key tools to expand our understanding of the timing and causes of NE include aEEG, or even better, video EEG monitoring, neuro-imaging including cranial ultrasound and MRI, placental investigations, metabolic, biomarker, and genetic studies. This information is critical to better understand the underlying causes of NE. Therapeutic hypothermia improves outcomes after HIE, but there is still considerable potential to do better. Careful clinical and pre-clinical studies are needed to develop novel therapeutics and to help provide the right treatment at the right time for this high-risk population.
Impact
-
Neonatal encephalopathy is complex and multifactorial.
-
This review seeks to expand understanding of the causes, timing, and evolution of encephalopathy in newborns.
-
We highlight key unanswered questions about neonatal encephalopathy.
Similar content being viewed by others
Introduction
Neonatal encephalopathy (NE) in term and near-term infants is defined as altered neurologic function in a newborn infant, characterized by altered consciousness and abnormal tone and reflexes. This broad definition does not specify etiology, or guide diagnosis and management, and the pragmatic definitions vary widely between studies. The single most common cause of NE is hypoxic-ischemic encephalopathy (HIE) but it can also be associated with infection, intracranial hemorrhage, stroke, brain malformations, metabolic disorders, genetic causes, and combinations thereof. This broad differential must be considered and carefully scrutinized to make the correct diagnosis and inform management and outcomes. NE and HIE have often been used interchangeably, leading to confusion around root causality. Here we will describe many etiologies of NE and ways of approaching the diagnosis of specific types of NE, focusing on recent updates and future research directions.
The current approach to the diagnosis of neonatal encephalopathy
A detailed review of the pregnancy, maternal medical and family history, and of labor and delivery should precede evaluation of the infant. However, given that newborn infants with encephalopathy must be considered for therapeutic hypothermia (TH) within 6 h of birth,1 it is not always immediately clear whether NE is due to HIE or whether another diagnosis might be involved. A focused history and examination to diagnose HIE is recommended. Perinatal sentinel events (e.g.,: placental abruption, shoulder dystocia, prolapse of the umbilical cord), fetal heart rate abnormalities consistent with exposure to hypoxemia in labor, need for resuscitation at birth, severe acidosis in the perinatal blood gas (cord blood pH <7.0 or base excess > 15 mmol/L), Apgar ≤5 at 10 min after birth and being encephalopathic soon after birth suggest hypoxia-ischemia (HI) as the likely cause for the neonatal encephalopathy, although it can still occur with less severe acidosis.2 The presence and severity of encephalopathy must be confirmed by clinical neurological exam (e.g., Sarnat neuro exam or Thompson score), ideally supported by electroencephalographic (EEG) or amplitude integrated EEG (aEEG) evidence of abnormal brain activity. Further evidence to support the diagnosis of moderate to severe HIE can include multi-organ impairment involving the liver (raised transaminases), kidney (oliguria, elevated creatinine), thrombocytopenia, or coagulopathy, although it is important to appreciate that it is possible to have HIE without systemic injury.3 HIE can be associated with acute or subacute or chronic placental lesions; however, pathology is typically only available in retrospect, and even if such lesions are present, at present they are neither necessary nor sufficient for a diagnosis of HIE.4 Further, even if HIE is confirmed, in principle it is still possible for additional factors to contribute to encephalopathy.5
Other factors contributing to NE can include maternal infection, medication or substance use, hypertensive disorders (pregnancy-induced hypertension, preeclampsia, chronic hypertension or HELLP syndrome), maternal obesity, birth trauma and abnormalities on antenatal ultrasound, or history of recurrent miscarriages or consanguinity or a family history of genetic disorders, neonatal illness, seizures, cerebral palsy or learning difficulties and importantly delayed onset of encephalopathy after birth.6,7 Specific causes of NE are described in the body of this review.
Search strategy and selection criteria
We searched Pubmed and the Cochrane Database of Systematic Reviews (CDSR) for publications in English from Jan 1, 2019, to July 1, 2024, using the search terms: Neonatal encephalopathy OR hypoxic ischemic encephalopathy OR (neonatal seizures AND encephalopathy) OR neonatal infection OR neonatal cerebral hemorrhage OR neonatal stroke AND epidemiology OR treatment OR neurodevelopmental follow-up OR magnetic resonance imaging OR (metabolic disorders OR inborn errors of metabolism including dysglycemia) OR genetic etiologies. Additional references were identified by searching the references in randomized controlled trials for NE.
Hypoxic-ischemic encephalopathy
Metabolic acidosis (pH < 7.2) at birth is an important, dose-related risk factor for neonatal outcomes in general, presumably as a marker of the extent of anaerobic metabolism.8 In many studies, severe acidosis with cord blood pH < 7.00 was used to help select infants at risk of HIE.9 However, in a recent population-based cohort study of 340 431 infants born from 2012 to 2018 in Denmark,10 severe adverse neonatal outcomes (including death, seizures, need for therapeutic hypothermia (TH), need for mechanical ventilation, and hypoglycemia) increased progressively compared to infant without acidosis as the umbilical pH fell below 7.2; 7.10 to 7.19 (259 of 73,244 (0.4%)), 7.00 to 7.09 (101 of 11 904 (0.8%)) and < 7.00 (171 of 1,743 (9.8%)), similarly to a previous meta-analysis.8 Neonatal death was only increased if the pH was < 7.10. Consistent with this, in a cohort study of 27028 infants, Vesoulis et al. found that although the rate of moderate to severe encephalopathy was highest for infants with cord blood pH <7.00 (35/85), there was a still significant risk of HIE in infants with pH 7.00–7.10 (34/327).2 These important studies suggest that our current threshold of acidosis for intensive observation and treatment is too low.
Originally, both HIE and its severity were defined in retrospect, based on evidence of a metabolic acidosis or need for resuscitation, combined with evolving encephalopathy based on clinical examination and aEEG/EEG and imaging, as detailed in Tables 1–3.11 Since TH became standard care in high-income countries, it is now essential to identify probable HIE as early as possible within the first 6 h of life in order to initiate treatment at a time when it is likely to be effective. Thus, most studies now use a staged combination of evidence of perinatal events followed by a modified Sarnat score and/or acute aEEG/EEG examination. Pragmatically, this approach does not seem to be associated with significant misdiagnosis. In a recent large trial, only 4% of infants had an additional neurological diagnosis that likely increased their ultimate level of disability.12 Interestingly, 5% had either a genetic diagnosis or a congenital abnormality. Thus, misdiagnosis is unlikely to substantially contribute to the risk of disability despite TH.
Epidemiology of hypoxic-ischemic encephalopathy
In high-income countries, studies using a consistent definition of HIE over time reported a fall in the incidence of moderate to severe HIE from 1–5/1000 live births in the early 2000s to 0.5–1.9/1000 livebirths in 2008–2010, which was attributed to improved antenatal and perinatal care.13 More recently, a population-based cross-sectional study in the US of hospital live births of infants born ≥ 35 weeks’ gestation age, found a stable incidence of perinatal HIE of 1.7 per 1000 live births between 2012 and 2019.14 By contrast, in low- and middle-income countries (LMICs) the incidence of HIE remains high, ranging between 1.5 to 20.3/1000 live births, with more severe encephalopathy and a higher case fatality rate.15
Patterns of HIE on modern imaging
Magnetic resonance imaging (MRI) is the recommended neuro-imaging technique for infants with neonatal encephalopathy (NE), including infants treated with hypothermia.16 MRI is recommended for infants with probable HIE, particularly in the presence of seizures. In more than 90% of neonates admitted with NE and seizures, MRI showed abnormalities suggestive of a diagnosis.17 For all infants presenting with NE the use of T1- and T2-weighted sequences, diffusion-weighted imaging (DWI), and susceptibility weighted imaging (SWI) is recommended. Slice thickness should be 2 mm or less. When arterial ischemic stroke or sinovenous thrombosis is suspected, MR angiography and MR venography should be added to the protocol. 1H-MR spectroscopy will provide very useful information and should be performed when available.17 Advanced imaging techniques, such as DTI, TBSS, arterial spin labeling (ASL), and the use of machine learning significantly improve prediction, at least in a research setting.18 The best time to perform the MRI in an infant treated with TH using diffusion weighted imaging (DWI) is shortly after rewarming (day 4–6). This will allow more reliable identification of acute injury especially in the central grey nuclei.19 Performing the MRI during TH will interrupt cooling and may not show the full extent of the injury but may be helpful when redirection of care is considered.20 Pseudo-normalization of DWI occurs by the end of the first week.21 When the MRI is performed during the second week of life, signal intensity changes will have become clear on T1- and T2 weighted sequences. Meta-analysis suggests that the diagnostic odds ratio (DOR = (true positives/false positives)/(false negatives/true negatives)) is greater when the MRI was performed during the first rather than the second week of life.22
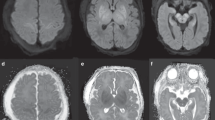
The use of a scoring system is recommended to support systematic assessment of the presence and extent of the main patterns of injury. 1) predominant injury in the basal ganglia and thalami (BGT), mainly the ventrolateral thalami and posterior putamina, and the perirolandic cortex following acute profound asphyxia; 2) injury in the parasagittal cortex and the subcortical white matter following partial prolonged asphyxia (watershed/white matter injury) and 3) diffuse injury in both the BGT and white matter (near total pattern) following very severe and prolonged asphyxia (Fig. 1). Several scoring systems have been introduced since the Barkovich score.23 The Rutherford score, Trivedi score, Weeke score, and NICHD NRN score are reported to have similar area under the curve (AUC > 0.66) for adverse outcome24; of these the Weeke and Trivedi scores had the highest inter-rater reliability.
By contrast, in a retrospective cohort study of 162 infants with probable HIE who were treated with TH, the AUC for ROC analysis for composite adverse outcome of death or moderate or severely delayed development at 2 years of age was significantly better for the Weeke score, 0.81 (95% CI 0.74–0.88), than the Barkovich score, 0.73 (95% CI 0.66–0.81).25 Further, the Weeke score was reported to provide better prediction of language score at two years than the Barkovich or NICHD systems (adjusted R2 = 0.166, P = 0.005).26 The Trivedi and Weeke scoring systems include DWI plus abnormalities in the cerebellum and brainstem. The Weeke score also includes intraventricular, extra-axial hemorrhages, sinovenous thrombosis, and 1H-MRS. Because it includes more detailed information the Weeke score may be especially useful for infants with mild HIE27; further studies are needed to validate this suggestion.
While moderate to severe injury to the central grey nuclei is associated with poor motor outcome, watershed injury is more likely to be associated with adverse cognitive outcomes, which may not be apparent until the children are seen at school age and seem to be more clearly defined in early adolescence.28 Another brain structure that may be a potential biomarker of cognitive outcome is the mammillary body. These are part of the limbic system, are small, and can only be assessed on 2 mm slices. An increase in signal intensity with swelling is best seen on the T2 sequence or with restricted diffusion. Mammillary body involvement can be an isolated finding, with or without associated hippocampal injury, or other brain injury. Within 4–6 weeks, the MRI will show atrophy of these structures, which is best seen on the sagittal T1 or T2 sequence.29
Quantitative MRI-based apparent diffusion coefficient (ADC) metrics for the whole brain and within the thalami and white matter, were recently reported in a secondary analysis of 416 of 500 infants recruited to the HEAL trial, based on automatically measuring areas with ADC values less than 800 × 10−6 mm2/s.30 A total volume of reduced ADC > 1 ml was associated with an increased odds ratio (OR 13.9; CI 5.93–32.45) for death and neurodevelopmental impairment at 2 years of age than the presence of any acute neural injury (OR 4.5; CI 2.6–7.8). Other advanced MRI techniques, such as arterial spin labeling (ASL) and diffusion data analyzed using frameworks such as tract based statistical analysis (TBSS) may also be helpful in predicting outcomes.31,32
When available, proton magnetic resonance spectroscopy (1H-MRS) can provide additional prognostic insight. MRS measures the signal from protons in different chemical metabolites, the main ones being choline (Cho), Creatine (Cr), N-acetylaspartate (NAA). With HI injury, NAA declines over days to weeks and then remains low. In contrast, lactate peaks during the first few days after acute brain injury and then declines toward “normal” by the end of the first week of life.33,34 Comparing the predictive value of clinical assessment, aEEG, MRI including DTI and MRS, Lac-NAA ratio (AUC 0.94 (95% CI 0.89–0.97) and NAA concentration (AUC 0.99, 95% CI 0.94—1.00) had the highest AUC.34 In another study MRS was performed at 24–96 h and day 7–14 of life, total NAA concentration in the central grey nuclei had the highest prognostic value at both time points (AUC at 24–97 h 0.97 and at 7–14 days 1.00). ADC values (AUC 0.90; 95% CI 0.64–0.98) and lactate-NAA ratios (0.97; 95% CI 0.90–0.99) also had high prognostic values on the early scan.35
Neuromonitoring (aEEG, EEG, NIRS)
While neuro-imaging techniques will provide important information, they are not informative within the first 24 h of life, as the echogenicity that can be seen on cranial ultrasound will take 24–48 h to develop, and although DWI -MRI will already show some abnormalities, retrospective studies show that they do not reach their full extent at that time.36 Neurophysiology, including aEEG and continuous video multichannel EEG (cEEG) are increasingly used immediately after admission and may also be of value when deciding whether or not to use TH. In most centers, aEEG is immediately available. It can reliably assess background activity but is less reliable for identifying seizures. Having a multichannel EEG with display of 2 aEEG channels will allow beside monitoring of the aEEG with simultaneous access to the raw EEG, while the neurophysiologist will be able to assess the multichannel EEG at regular time-points and on request. Both aEEG and cEEG are reliable predictors of neurodevelopmental outcomes. The greatest prognostic value of aEEG/cEEG background in those receiving TH is later than for neonates who do not receive TH, with the greatest prognostic value of aEEG/EEG recording around 48–72 h after birth.37 Seizures are seen in about one-third of infants who receive TH, often within 12–24 h after birth. Although the presence of seizures per se does not predict outcomes, the magnitude of the seizure burden has been independently associated with adverse outcomes.38,39
Seizures can be seen during and after rewarming, more often in infants with a worse EEG background and seizures during hypothermia. aEEG/cEEG monitoring should therefore be performed during hypothermia and continued during rewarming.40,41 Interestingly, a small trial of continuous EEG monitoring in 35 infants randomized to treatment of electrographic seizures or clinical seizures only, showed that treatment of electrographic seizures reduced seizure burden,42 and although the treatment groups did not appear to have different outcomes, seizure burden in the combined group was associated with both MRI injury and neurodevelopment outcome. Large RCTs are needed to resolve whether seizure treatment improves long-term outcomes.
Near infrared spectroscopy (NIRS) is a non-invasive, bedside monitoring system that continuously measures regional cerebral tissue oxygenation (rScO2). Several studies have reported an increase in rScO2 between day 1 and 2 in infants with an abnormal neonatal MRI and an adverse outcome.43 This association of increased rScO2 with brain injury/poor outcome likely reflects impaired mitochondrial oxidative metabolism and luxury perfusion.
Management of HIE
Foundation studies established that the brain often shows partial or even complete recovery of energy metabolism after acute HI in a short “latent” phase, typically lasting ~6 h, only to decline hours to days later, during a “secondary” deterioration phase characterized by seizures, cytotoxic edema and mitochondrial failure (Fig. 2).44 Critically, translational studies then showed that by inducing mild hypothermia as soon as possible during the latent phase and continuing it for 72 h it was possible to dramatically improve histological and functional outcomes.
Therapeutic hypothermia
Meta-analysis of subsequent large randomized controlled trials (RCTs) in high income countries (HICs) confirmed that within strict parameters TH significantly improved survival without disability after moderate-to-severe HIE, with a number needed to treat for each survivor without disability of 8 (95% CI 5 to 14).45 Based on this evidence, TH is now part of standard care for HIE in high-income countries,46 and remains the only proven treatment for HIE.11
Subsequent studies have focused on trying to further improve outcomes and to assess groups who were not included in the original RCTs. In infants with moderate or severe HIE, delayed initiation of TH at 6–24 h after birth and continued for 96 h, demonstrated no significant effect on death or disability.47 There was a 76% posterior probability (adjusted posterior risk ratio, 0.86; 95% credible interval, 0.58–1.29) of reduced death or disability compared with non-cooled infants, using Bayesian analysis assuming a neutral prior.47 However, the probability of death or disability in cooled (0.244) and non-cooled group (0.278) was too close to be certain of benefit of initiating cooling 6 h after birth.48 In a large RCT (n = 364), prolonged (120 h) or deeper (32.0°C) hypothermia did not reduce the risk of death or moderate or severe disability compared with the standard regimen of TH to 33.5 °C for 72 h.49 Death or moderate or severe disability occurred in 29.3% in the 33.5 °C for 72 h group; 34.5% in the 32.0 °C for 72 h group; 34.4% in the 33.5 °C for 120 h group and 28.2% in the 32.0 °C for 120 h group. It is important to note that although there was no overall effect, the adjusted relative risk for death was higher in the group cooled to 33.5°C for 120 h compared with 33.5°C for 72 h (RR: 2.52; 95% CI, 1.06–5.95).
Infants with mild HIE were excluded from the initial RCTs of the TH. However, meta-analysis of 20 studies found adverse outcomes in 86/341 (25%) infants with mild HIE.50 It is still unknown whether infants with mild HIE would benefit from TH. A recent RCT of 101 infants found no significant effect on cerebral magnetic resonance indices of outcome. However, there was evidence of a structural bias, such that infants treated with TH had greater severity of illness, including greater need for invasive resuscitation at birth.51 Large, well-designed trials will be important to determine whether infants with mild HIE should receive TH. At present two trials are enrolling, the COMET RCT and the COOL-PRIME comparative effectiveness trial.52,53
Lack of benefit with adjunct therapies during therapeutic hypothermia
The endogenous growth factor erythropoietin is neuroprotective as monotherapy after HI in multiple species and paradigms, provided that it is started within the first few hours of the latent phase.54,55 However, there is conflicting evidence for whether it can augment TH.54,56 Meta-analysis of 3 small studies in LMICs suggested that erythropoietin monotherapy reduced death or disability from 49.7% to 27.6% in the erythropoietin group (risk ratio 0.56 (95% CI: 0.42–0.75)).57 This encouraging finding supports large, well-designed studies to evaluate this approach for infants in whom TH is not appropriate.
By contrast, phase II and III studies of adjunct therapies for TH suggest lack of further benefit. In the High-Dose Erythropoietin For Asphyxia And Encephalopathy (HEAL) trial of 500 infants receiving TH for moderate or severe HIE, 5 doses of 1000 u/Kg erythropoietin on day 1, 2, 3, 4 and 7 days of age did not reduce the risk of death or neurodevelopment impairment compared to placebo, RR:1.03; 95% CI, 0.86 to 1.24, and increased the risk of having a high externalizing behavior score, RR:4.86, 95% CI,1.46 to 16.17.12 Moreover, the erythropoietin plus TH group had slightly higher mean number of serious adverse events per child than the placebo group (RR: 1.26; 95% CI, .01 to 1.57), and of at least one serious adverse event (RR:1.21; 95% CI 1.00 to 1.45).
Further, in a phase 2 study (n = 92), inhalation of 30% xenon for 24 h commenced at 10 h of age in infants receiving TH compared to TH alone did not reduce the Lactate/NAA ratio in the thalamus (mean (SD) 0.68(1.12) vs 0.47(0.94)) or preserve fractional anisotropy in the posterior limb of the internal capsule (mean (SD) 0.40 (0.01) vs 0.41 (0.01) or death (26.08% vs 21.74%) or moderate or severe disability (25% vs 23%).58,59 Relatively delayed start time of treatment (within 26 h after birth) and intermittent dosing may have contributed to the lack of neuroprotection in these clinical trials. Many other potential add-on therapies are being evaluated, such as melatonin and stem cells, but so far there is only preliminary evidence from small studies, as previously reviewed.60
Outcomes after therapeutic hypothermia at school-age
Although children at school age (6–8 years), who underwent TH for neonatal HIE had significantly improved outcomes, many still have motor or cognitive problems. At school age, the relative risk of CP in children cooled for HIE compared with non-cooled children was between 0.59 to 0.6 with 95% CI ranging between 0.31 to 1.18.61 All children with CP had IQ < 70, and 9% of children without CP had an IQ < 70 and 31% had an IQ of 70 to 84.62 Compared to typically developing children, even children without CP had borderline to extremely low IQs, borderline motor impairment, poorer communication, attention, visuospatial skills and executive function, and behavioral problems63,64 and were reported to be less ready for school.65
On MRI, children cooled for HIE had significantly smaller white matter and grey matter volumes, hippocampi, thalami, and globus pallidi volumes than healthy controls (Fig. 3).66 Abnormal mammillary bodies were reported in 35% of children at 6–8 years cooled for HIE and 38% of children cooled and not cooled for HIE at 10 years of age.67 Children cooled for HIE who had abnormal mammillary bodies on imaging also had decreased fractional anisotropy in the left fornix and reduced hippocampi volumes (Fig. 4).68 Both thalamic and hippocampi volumes were associated with full scale IQ and total motor scores after adjusting for sex, age, and total brain volume in children cooled for HIE but not in controls.66
Grey matter volume (a); white matter volume (b) and subcortical grey matter volumes (c). Children cooled for HIE who did not develop CP had significantly lower grey matter (a) and white matter (b) volumes than healthy controls. Although the thalami, hippocampi, and pallidi volumes (c) (asterisk) were lower in children cooled for HIE than controls, they were not independent of total brain volume. Adapted from,66 Creative Commons Attribution License.
Normal (a), equivocal (b), and abnormal (c). Left and right Mammillothalamic tract (MTT) (d) and fornix (e) shown. Right MTT had increased radial diffusivity in children cooled for HIE (cases) with abnormal mammillary bodies than cases with normal or equivocal mammillary bodies and higher than controls. Left fornix had lower fractional anisotropy in cases with abnormal mammillary bodies than controls. Adapted from ref. 68. Creative Commons Attribution License.
Further, children cooled for HIE compared to healthy controls have reduced corpus callosum area, volume, fractional anisotropy, and increased radial diffusivity,69 with reduced regional fractional anisotropy (Fig. 5),70 and reduced cognitive scores compared with healthy controls, with significant correlation between the magnitude of changes and IQ.66 Changes in the white matter subnetwork connecting parietal, limbic, temporal and occipital areas were significantly associated with IQ, and the white matter subnetwork connecting the nodes, right lingual gyrus left superior parietal gyrus and right cuneus cortex, were associated with processing speed.69,70 Fractional anisotropy in the midbody of corpus callosum, the cingulum and the superior longitudinal fasciculus were correlated with general communication composite scores in children cooled for HIE.64 Similarly, brain regions involved in attention and visuospatial processing including the precuneus, thalamus, left superior parietal gyrus and left inferior temporal gyrus showed lower connectivity in children cooled for HIE than controls.70
Decreased Fractional anisotropy in regions of corpus callosum in children cooled for HIE (cases) compared to controls (a), areas of increased RD in case children (b) and areas in which males exhibit larger case-control differences in RD than females (c). Results of voxel-wise comparison of FA on the white matter skeleton (green) showing widespread reduction in FA in children cooled for HIE and did not have CP compared with control children after adjusting for age and sex (d). The color bar shows significant differences (p < 0.05, Threshold free cluster enhancement corrected. These are overlaid on the MNI standard template. Labels indicate some major white matter tracts and regions. Abbreviations are as follows: anterior thalamic radiation (ATR), corpus callosum (CC), external capsule (EC), internal capsule (IC), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), uncinate fasciculus (UF). Differences are most notable in the IC, CC, fornix, and cingulum. Adapted from.69,70 Creative Commons Attribution License.
Lack of benefit of therapeutic hypothermia in low- and middle-income countries (LMICs)
By contrast with the clear benefit of TH in high-income countries, the Hypothermia for moderate or severe neonatal encephalopathy in low and middle-income countries (HELIX) trial found that TH had no overall effect on death or disability in infancy, and was associated with an apparent increase in the risk of death.71 It is possible that this is partly because only 8% of the babies in the hypothermia group and 13% in the normothermia group in the HELIX trial had a sentinel event, compared with 25% or more in studies from HICs.72,73 The presence of clinical seizures before initiation of hypothermia and the predominance of white matter injury compared to basal ganglia and thalamic injury on MRI suggested that these infants were likely exposed to subacute HI starting well before birth.71
Infants born after sentinel events may have better outcomes after TH, suggesting that those infants exposed to an acute hypoxic-ischemic insult may benefit more than the infants exposed to subacute hypoxic-ischemia. For example, in a retrospective single-center study of 182 neonates with HIE treated with hypothermia, those who had acute sentinel events had less severe injury on MRI and better motor and language outcomes at 18–36 months of age.74 Thus, it is plausible that, in part, the low rate of sentinel events in the HELIX trial may have contributed to the lack of benefit after hypothermia.
Consistent with these findings, a recent case-control study of the whole blood transcriptome of term and near-term infants with HIE from Italy and in the HELIX trial,75 found that infants with adverse outcomes despite TH for HIE in high-income settings showed downregulation of eukaryotic translation initiation factor 2, consistent with acute hypoxia. By contrast, in low- and middle-income settings, infants with adverse outcomes expressed aldosterone signaling, which has been associated with intermittent and chronic hypoxia. Although the HELIX trial was not powered to analyze the effect of sentinel events on outcomes after hypothermia, taken together, these data raise the speculative possibility that it might be possible to target treatment to the subset of babies who have had sentinel events in the LMIC.
Dysglycemia in hypoxic-ischemic encephalopathy
In studies of infants who underwent TH for HIE hypoglycemia, hyperglycemia, and glycemic lability were common. For example, in a secondary analysis of the CoolCap RCT of TH vs normothermia for HIE (n = 234 infants), hypoglycemia (OR: 6.2, 95% CI 1.4–27.3), and hyperglycemia (OR:2.7, 95% CI 1.5–4.9) were associated with death and/or severe neurodevelopmental disability at 18 months compared with normoglycemia, independent of TH and severity of HIE.76 Further, hypoglycemia, but not hyperglycemia, was associated with multiorgan impairment compared with normoglycemia; however, the comparison was not adjusted for confounders.77 More recently, in the HEAL trial (n = 491 infants), hypoglycemia was associated with increased risk of death (OR: 2.85, 95% CI 1.09 to7.43) and neurodevelopment impairment (OR:2.50, 95% CI 1.09–7.43), while hyperglycemia was associated with increased risk of death (OR:2.52, 95% CI 1.10 to 5.77) but not neurodevelopment impairment, independent of the severity of asphyxia, encephalopathy and illness and treatment with erythropoietin.78
Consistent with these findings, meta-analysis of studies of infants treated with TH (n = 446 infants) reported that hypoglycemia was associated with increased risk of abnormal neonatal brain imaging (OR: 1.863, 95% CI: 1.18 to 2.83), especially white matter injury, and death or neuro disability (n = 501 infants, OR: 2.17, 95% CI: 1.36 to 3.45).79 In two cohort studies (n = 428 infants), hyperglycemia increased the odds of abnormal brain imaging compared with normoglycemia (OR:2.41, 95% CI: 1.63 to 3.56), particularly the odds of basal ganglia, thalami, and posterior limb of the internal capsule injury (OR: 2.91 to 3.49) was higher than the white matter or cortical injury (OR: 2.0 to 2.07). Further, hyperglycemia was also associated with higher odds of death or neurodevelopmental impairment at 18 months of age in 5 cohort studies (n = 515 infants, OR: 2.74, 95% CI:1.49 to 5.04). Similarly, in the Long-Term Outcome of Neonatal Hypoxic Encephalopathy in the Era of Neuroprotective Treatment with Hypothermia (LyTONEPAL) cohort study of 545 patients with moderate-to-severe HIE, hyperglycemia was associated with greater risk of adverse outcomes compared to normoglycemia (aOR 1.81; 95% CI 1.06–3.11).80
It is important to consider that intermittent blood sampling could miss episodic glycemic lability. Supporting this speculation, in a prospective cohort of 108 neonates with NE, intermittent glucose testing missed 50% of hypo- and hyperglycemic events on the first day after birth compared with continuous glucose monitoring (CGM).79 This study also showed that a CGM value of > 10.1 mmol/L during the first 48 h after birth was associated with basal ganglia (53% positive predictive value and 91% negative predictive value) and watershed injury (47% positive predictive value and 96% negative predictive value). Importantly, although hyperglycemia > 10.1 mmol/L during the first 48 h after birth was associated with cerebral palsy and developmental scores at 18 months including neuromotor score and Child Behavior Checklist internalizing scores, these associations were not independent of the brain injury patterns on the neonatal MRI. Smaller cohort studies (n = 44 to 47 infants cooled for HIE), showed an association between duration of both hypo (OR 7.1, 95% CI 1.8–20.3) and hyperglycemia (OR 5.4, 95% CI 1.6–15.7) and death or moderate or severe disability at 18–24 months of age,81 and occurrence (OR 26.55, 95% CI 2.02–348.5), duration (OR 2.11, 95% CI 1.08–4.14) and greater area under the curve (OR 1.80, 95% CI 1.11–2.91) of hypoglycemia was associated with death and brain injury.82 Controlled studies of tight glycemic control are now essential to determine whether dysglycemia is the cause of brain injury or just an association.
Intracranial hemorrhage and HIE
In the original RCTs of TH versus normothermia for HIE, the incidence of intracranial hemorrhage was 39% and 33% in cooled and non-cooled infants at 8 days of age, predominantly subdural hemorrhage (30%), and 7% and 8% respectively, at 14–16 days of age.83,84 More recently, in a retrospective population-based study of infants with HIE (n = 7265), intraventricular hemorrhage (IVH) was reported in 0.9% of infants.85 In a prospective cohort study of infants cooled for HIE (n = 160), the incidence of IVH was 9% (95% CI 5.3–15.0%) and occurred frequently in infants with coagulopathy during TH.86 However, the magnitude of hemorrhage was usually small, and not significantly associated with neurodevelopmental outcomes at 18 to 26 months of age (OR:1.24, 95% CI 0.14 to 10.65).
Interactions between genetics and HIE
In the HEAL trial (n = 500 infants), 5% (95% CI 3% to 7%) had genetic or congenital anomalies. Both were associated with a higher risk of death or neurodevelopmental impairment (75% vs 50%, p = 0.02) and cerebral palsy (32% vs 13%, p = 0.02) compared with the rest of the cohort.87 The mechanism of this association is unknown but could reflect either greater risk of HI or vulnerability to HI injury. Supporting the latter possibility, in a cohort study of children with CP, 387 / 1578 (24.5%) had a genetic diagnosis; the subgroup with CP associated with HIE had a significantly greater proportion of genetic diagnoses compared with CP related to other causes, suggesting the possibility of a genetic vulnerability to HIE.88 Further, a small cohort of 55 infants with HI suggested that gene-gene interaction in inflammation pathways seemed to be associated with risk of epilepsy after HIE.89
Infection and neonatal encephalopathy
Acute perinatal and postnatal infection can lead to NE with abnormal tone, apnea, and risk of death or neurodevelopmental problems,90,91 and acute severe infection can present very similarly to HIE, with hypotonia, acidosis, and seizures. In the UK, prospectively collected data from an infection surveillance network of 30 intensive care units, found that from 2005 to 2014 there were 2171 cases,92 for an overall rate of bacterial infection of 6.1/1000 live births. The incidence of both early-onset sepsis and late-onset ( > 48 h) sepsis fell over this period. The majority of cases (76%) were late onset, and sepsis was more common in preterm infants (84%) and low birth weight infants ( < 2500 g, 81%). Although many different pathogens were seen, the most common in early-onset sepsis were group B streptococci and Escherichia coli, while Staphylococcus aureus and coagulase-negative staphylococci were most common in late onset sepsis.
There is growing evidence that neonatal infection remains highly associated with adverse outcomes. In a meta-analysis of 24 studies involving 121,645 infants (101,657 preterm and 19,988 term),93 sepsis was associated with increased risk of cognitive delay (adjusted OR, aOR 1.14 (95% CI: 1.01–1.28)), visual impairment (aOR 2.57 (95%CI: 1.14– 5.82)), hearing impairment (aOR 1.70 (95% CI: 1.02–2.81)) and cerebral palsy (aOR 2.48 (95% CI: 1.03–5.99)). Interestingly, a registry-based study of children with cerebral palsy found that 192 full-term infants with neonatal infection were more likely to have spastic triplegia or quadriplegia (OR 2.4),90 whereas infection in preterm infants was not associated with a specific phenotype.
Pathophysiology of NE related to infection
Multiple experimental studies have confirmed that acute exposure to the Gram negative cell wall component lipopolysaccharide (LPS) leads to a robust inflammatory response and adverse neurological outcomes.94,95 In turn, early initiation of anti-inflammatory therapies such as Interleukin-1 blockade improves EEG activity and markedly reduces microgliosis and oligodendrocyte loss.95,96 The key knowledge gap is the striking lack of information on whether treatment started after a clinically realistic delay would improve outcomes.97
Interactions of infection with HIE
It is important to appreciate that there is a higher incidence of both early neonatal infection and chorioamnionitis and fetal vasculitis in term infants with HIE.98 For example, in the Children’s Hospital Neonatal Database, of 1534 infants with HIE treated with TH, 2.3% had confirmed and 16.6% had suspected infection, far more than the population estimate of 0.5–1.0/1000 term or near-term live births.99 Further, in a subset of the HEAL trial, nearly 40% (124/321) had evidence of histological chorioamnionitis.100 This is consistent with a previous cohort study in term infants with HIE that found that diffuse chronic villitis was associated with abnormal neurodevelopmental outcomes after TH (OR, 9.29; 95% confidence interval, 1.11–77.73).101
Efficacy of therapeutic hypothermia for inflammation-sensitized hypoxia-ischemia
Overall, preclinical studies support concerns that early onset gram-negative inflammation can worsen outcomes after TH. In P7 rats exposed to systemic LPS injection 4 h before HI, LPS increased brain area loss, apoptosis, microgliosis, and astrogliosis compared with vehicle-HI controls, and treatment with hypothermia did not ameliorate these effects.102 Similarly, in LPS-sensitized HI in newborn piglets (human term-equivalent) TH for 14 or 24 h did not improve acute EEG recovery, MRS parameters, or cell survival.103,104 In contrast, in P7 rats, gram-positive bacterial mimetics given to induce inflammatory sensitization 8 h before HI, hypothermia was still neuroprotective,105 suggesting that hypothermia may still be effective after exposure to gram-positive bacterial infections. Note that these studies used short, sub-optimal durations of TH (14–24 h). Longer, clinical protocols of TH might be more efficacious.
Clinically, it remains unclear whether infants with NE exposed to proven infection or risk factors for early-onset neonatal infection in high-income countries do have worse outcomes. For example, group B streptococcal infection in infants with NE was associated with higher mortality than those with NE alone.106 However, in a population-based study from England and Wales (n = 5206), in infants treated with TH, the presence of risk factors for early neonatal sepsis such as prolonged rupture of membranes, maternal group B streptococcus, pyrexia or intrapartum antibiotics, and NE, did not increase the risk of death or short-term adverse outcome.93 Interestingly, exposure to chorioamnionitis in infants with NE was associated with reduced risk of moderate-severe brain injury and adverse cognitive outcomes,107 whereas exposure to chronic villitis was associated with impaired neurodevelopment, suggesting that the precise timing and nature of exposure critically modulates its effects.
Neonatal stroke
Perinatal arterial ischemic stroke (PAIS) occurs in 1 per 2000–3000 livebirths,108, and rarely may be confounded with HIE.109 In the HEAL RCT (n = 500), PAIS was seen in just 21/470 (4%) of infants who had high-quality MRI scans.110 Most infants with PAIS do not meet the criteria for HIE. Maternal fever, prolonged rupture of membranes, prolonged second stage, tight nuchal cord, and failed ventouse delivery were more common in PAIS, while thick meconium, sentinel events, and shoulder dystocia were more common in infants with HIE.111 Importantly, infants with PAIS develop seizures significantly later ( ≥ 12 h of age, OR 39.7.4; 95% CI 7.3, 217.0) and seizures are more likely to be unilateral clonic motor jerks (OR 13,4; 95% CI 2.1, 87.9) than in infants with HIE,112 with corresponding asymmetry in background activity and sleep-wake cycling.
Cerebral sinus venous thrombosis (CSVT) can mimic HIE but again tends to have much later onset of symptoms at a median age of 3 days (IQR 1–7).113 Infants with CSVT may present with seizures, coagulopathy, or persistent thrombocytopenia. Risk factors for CSVT include sepsis/infection and dehydration. Cranial ultrasound including color Doppler may suggest the diagnosis when an intraventricular hemorrhage (IVH) with thalamic extension is seen with lack of flow across one or more of the sinuses.114 MRI including MR venography is required to confirm the diagnosis and anticoagulation therapy can be considered as it may reduce the risk of thrombus propagation.115 Detailed discussion of the management of PAIS and CSVT and seizure detection can be found in the AHA/ASA Scientific statement on management of stroke in neonates and children.116
CNS malformations
Infants with severe malformations of the CNS seldom present with NE.117 More often they present with micro- or macrocephaly, hypertonia, hypotonia, and respiratory problems. A thorough neurological examination is mandatory and may show dysmorphisms and neurocutaneous lesions suggestive of a diagnosis. Severe CNS malformations, like alobar prosencephaly (abnormal separation of the cerebral hemispheres or lobes) and lissencephaly (smooth brain), are most often recognized on early fetal ultrasound and termination of the pregnancy will be considered. Other malformations, like cortical migrational disorder (CMD), will be harder or even impossible to detect on fetal ultrasound or MRI that are usually acquired around 20 weeks’ gestation, as migration~ and cortical folding are not complete. In infants with respiratory problems malformations of the brain stem (brainstem disconnection), kinking of the brainstem in the dystroglycanopathy / Walker-Warburg syndrome or elongated superior cerebellar peduncles, “molar tooth sign” in Joubert syndrome (ciliopathy) can be recognized on both pre- and postnatal MRI.118,119
Once abnormalities are recognized on fetal ultrasound, and preferably neurosonography (transvaginal rather than transabdominal ultrasound), additional neuro-imaging with fetal MRI is recommended. This provides more detailed information and in one study additional information was provided affecting prognosis and/or counseling in 18.1% of cases compared to ultrasound,120 although again CMD might still be difficult to diagnose with certainty early in the second trimester. Interestingly, MRI found more abnormalities in bilateral than unilateral ventriculomegaly (OR: 4.37, 95% CI 1.21–15.76; p = 0.04). MRI, especially when performed postnatally, identified associated CMD in about 10% of the cases with agenesis of the corpus callosum.121 Finally, genetic testing using whole exome sequencing (WES) or whole genome sequencing also plays an important role in fetal ventriculomegaly.122
Neurometabolic disorders
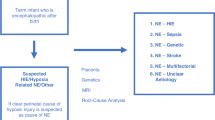
Some inborn errors of metabolism can present soon after birth with NE including seizures, such as urea cycle disorders (ammonia, plasma amino acids, and urine organic acids), non-ketotic hyperglycinemia (cerebrospinal fluid (CSF): plasma glycine), Molybdenum co-factor deficiency (low plasma uric acid & elevated urine s-sulphocysteine),123 sulfite oxidase deficiency (elevated urine s-sulphocysteine)124 and biotinidase deficiency (reduced biotinidase activity).125 A systematic approach in evaluating newborn infants with encephalopathy and suspected inborn errors of metabolism includes clinical examination for dysmorphic features, head size, liver involvement, and cardiac and eye abnormalities,126 plus assessment of key biochemical investigations such as metabolic acidosis, lactate, glucose, organic acids, serum ammonia, plasma free fatty acids, and urinary ketones (Fig. 6).
Diagnostic algorithm for NE proposed by Martinello et al.5. Creative Commons Attribution (CC BY 4.0) license.
Genetic and epileptic encephalopathies
Genetic encephalopathy
One of the major underdiagnosed types of NE is genetic encephalopathy. A retrospective cohort study of 193 cases of NE that was not due to HIE reported a diagnostic yield of 28/193 patients,127 many without dysmorphic features or associated congenital anomalies. Diagnosis was best made by exome sequencing. Prader - Willi was the single most common of the non-HIE causes of NE presenting with hypotonia. Similarly, in a retrospective cohort study of 28 infants cooled for HIE who had no known risk factors for perinatal asphyxia, genetic testing including microarrays, whole-exome sequencing, and comprehensive epilepsy panel identified ten patients with channelopathies (KCNQ2 and SCN9A mutation), inborn errors of metabolism (e.g., pyruvate dehydrogenase deficiency, MTFMT gene) and other potential candidate genes (e.g., CDKL5, ISY1, KIF1A).128
A large single center cohort study from China of 366 neonates with NE as part of the Chinese Newborn Genome Project between 2015–17 found on whole exome sequencing that 43 patients (11.7%) had pathogenic or likely pathogenic variants and 10 patients (2.7%) had variants of unknown significance. Neonates with genetic findings had a significantly higher incidence of seizures (96.2%, P = 0.0009), but a lower frequency of abnormal MRI (P < 0.0001).129 Epileptic encephalopathy related genes accounted for nearly half (46.4%) of all genetic defects of NE with seizures.
Other etiologies that can include a genetic basis are vascular conditions like stroke (Col4a1 and Col4a2), neuromuscular disorders like myotonic dystrophy, malformations like tubulinopathies, lissencephaly, holoprosencephaly, dystroglycanopathies and Dandy - Walker and Joubert syndromes. Metabolic syndromes encompass a broad array of disorders presenting with NE and can be diagnosed with appropriate laboratory studies like amino acids and organic acids and acylcarnitine profiling.7 NE can be multifactorial with overlap among the different causes. Therefore, all neonates with NE should be evaluated for infection, toxin exposure, seizures, and other forms of acute brain injury like trauma and stroke with appropriate laboratory studies, video EEG, and MRI.117
Genetic epileptic encephalopathies
As discussed above, nearly half of genetic etiologies of NE occur in patients who have seizures without HI. The epileptic encephalopathies include Early Infantile Epileptic Encephalopathy (EIEE), Early Myoclonic Epileptic Encephalopathy, KCNQ2 Encephalopathy, DEND syndrome (developmental delay, epilepsy, neonatal diabetes) and Epilepsy of infancy with Migrating Focal Seizures. Many of these conditions have distinctive EEG patterns, but metabolic studies, neonatal epilepsy panels, and whole exome sequencing are often needed.130,131
NE and risk factors in pregnancy and labor
In unmatched case-control studies, multiple pregnancy and intrapartum factors were associated with NE, after adjusting for confounders, including hypertension in pregnancy (OR: 3.77, 95% CI 1.49 to 9.55), augmentation of labor (OR: 2.23, 95% CI 1.17 to 4.23), no fetal monitoring (OR 2.75, 95% CI 1.71 to 4.22), acute intrapartum events (OR 8.74, 95% CI 1.70 to 45.02) and obstructed labor (OR 3.8, 95% CI 1.96 to 7.36).106 Pyrexia in labor may also be associated with risk of NE (OR 6.3, 95% CI 2.7 to 14.8), independent of parity, induction of labor, sex, or receiving epidural anesthesia.132 Infants exposed to drugs consumed by their mothers, which were either prescribed or illicit may present with abnormal neurological signs and encephalopathy. Exposure to maternal intake of drugs such as tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRI), antipsychotic medications, and mood stabilizers such as lithium may lead to abnormal tone, poor feeding, somnolence, and decreased deep tendon reflexes.7 Exposure to recreational drugs such as amphetamines, opiates, benzodiazepines, cocaine, and barbiturates can be associated with neonatal abstinence syndromes, with high-pitched cry, hypertonia, impaired feeding, and sleep. Infants with less convincing history of perinatal asphyxia and hypertonia, hyperreflexia persisting beyond 48 h after birth should be screened for exposure to possible toxins. Speculatively, exposure to perinatal asphyxia and toxins could contribute to neonatal encephalopathy in combination.
Diagnostic algorithm for NE
The reader may wish to consult the approach to evaluating the infant with NE from Martinello et al.5 (Fig. 6).
Can we reduce the overall risk of neonatal encephalopathy?
Low- and middle-income countries have a higher burden of NE secondary to birth asphyxia and antenatal factors such as socio-economic deprivation, undernutrition, and sub-optimal antenatal and intrapartum care, and chorioamnionitis increase the risk of NE.133 Institutional deliveries, better intrapartum care and support to pregnant women during labor may decrease the incidence of HI. Mathematical modeling data from 184 countries between 1990 and 2010 suggested that the incidence of NE decreased from 11.7 to 8.5 per 1000 live births, secondary to increased institutionalized deliveries and better intrapartum care.13 It will be challenging to further reduce the incidence of NE. A meta-analysis of continuous intrapartum cardiotocography with computer analysis compared with visual analysis of cardiotocography (n = 54,492 participants) did not reduce the risk of fetal metabolic acidosis.134 Nevertheless, there is evidence that artificial intelligence may be able to better utilize the fetal heart rate pattern to identify fetuses who are at high risk of developing acidosis or encephalopathy.135
Conclusions
In sum, recent evidence supports that thorough investigation of the protean causes of NE can lead to more accurate diagnosis and so potentially support our goal of providing the right treatment for the right condition. As discussed in this review, it is now well established that brain injury after both HI and infection/inflammation, and likely other types of NE evolve over time and that interventions need to be matched both specific etiologies and to the precise timing after the insult. Positively, it is now clear that current criteria for HIE are reliable, such that only a small minority have alternative or additional diagnoses. By contrast, infection-related brain injury evolves relatively slowly compared to HIE but a definitive diagnosis of sepsis is often delayed, and it is unknown how late is too late for the proposed interventions. Thus, we now need to find ways to establish the etiology and the timing of injury rapidly after birth. It is likely that a combination of history, examination, neurophysiology and rapid biochemical biomarkers, and genetic analysis, backed by modern imaging, will ultimately let us design novel therapeutics to target the specific diagnosis and timing. The use of these new therapeutics will more accurately be applied individually with the refinements of artificial intelligence, allowing true precision medicine for NE.
References
Gunn, A. J. & Thoresen, M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb. Clin. Neurol. 162, 217–237 (2019).
Vesoulis, Z. A. et al. Re-examining the arterial cord blood gas pH screening criteria in neonatal encephalopathy. Arch. Dis. Child. Fetal Neonatal. Ed. 103, F377–F382 (2018).
Yan, E. S. et al. Association between multi-organ dysfunction and adverse outcome in infants with hypoxic ischemic encephalopathy. J. Perinatol. 42, 907–913 (2022).
Penn, A. A. et al. Placental contribution to neonatal encephalopathy. Semin. Fetal Neonatal Med. 26, 101276 (2021).
Martinello, K., Hart, A. R., Yap, S., Mitra, S. & Robertson, N. J. Management and investigation of neonatal encephalopathy: 2017 update. Arch. Dis. Child. Fetal Neonatal. Ed. 102, F346–F358 (2017).
Fortin, O. et al. Risk factors and outcomes for cerebral palsy with hypoxic-ischemic brain injury patterns without documented neonatal encephalopathy. Neurology 102, e208111 (2024).
Sandoval Karamian, A. G. et al. Neonatal encephalopathy: etiologies other than hypoxic-ischemic encephalopathy. Semin. Fetal Neonatal. Med. 26, 101272 (2021).
Malin, G. L., Morris, R. K. & Khan, K. S. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ 340, c1471 (2010).
Branagan, A. et al. Consensus definition and diagnostic criteria for neonatal encephalopathy-study protocol for a real-time modified Delphi study. Pediatr. Res. 97, 430-436 (2024).
Andersson, C. B. et al. Umbilical cord pH levels and neonatal morbidity and mortality. JAMA Netw. Open 7, e2427604 (2024).
Volpe, J. J. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann. Neurol. 72, 156–166 (2012).
Wu, Y. W. et al. Trial of erythropoietin for hypoxic-ischemic encephalopathy in newborns. N. Engl. J. Med. 387, 148–159 (2022).
Lee, A. C. et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 74, 50–72 (2013).
Cornet, M. C. et al. Perinatal hypoxic-ischemic encephalopathy: incidence over time within a modern US birth cohort. Pediatr. Neurol. 149, 145–150 (2023).
Kukka, A. J. et al. Incidence and outcomes of intrapartum-related neonatal encephalopathy in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Glob. Health 7, e010294 (2022).
Wisnowski, J. L. et al. Integrating neuroimaging biomarkers into the multicentre, high-dose erythropoietin for asphyxia and encephalopathy (HEAL) trial: rationale, protocol and harmonisation. BMJ Open 11, e043852 (2021).
Weeke, L. C. et al. The aetiology of neonatal seizures and the diagnostic contribution of neonatal cerebral magnetic resonance imaging. Dev. Med. Child Neurol. 57, 248–256 (2015).
Lewis, J. D. et al. Automated neuroprognostication via machine learning in neonates with hypoxic-ischemic encephalopathy. Ann. Neurol. 97, 791-802 (2024).
Chakkarapani, E. et al. Reliability of early magnetic resonance imaging (MRI) and necessity of repeating MRI in noncooled and cooled Infants with neonatal encephalopathy. J. Child Neurol. 31, 553–559 (2016).
Barkovich, A. J. et al. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: preliminary report. AJNR Am. J. Neuroradiol. 22, 1786–1794 (2001).
Bednarek, N. et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 78, 1420–1427 (2012).
Ouwehand, S. et al. Predictors of outcomes in hypoxic-ischemic encephalopathy following hypothermia: a meta-analysis. Neonatology 117, 411–427 (2020).
Barkovich, A. J. et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am. J. Neuroradiol. 19, 143–149 (1998).
Langeslag, J. F. et al. Outcome prediction and inter-rater comparison of four brain magnetic resonance imaging scoring systems of infants with perinatal asphyxia and therapeutic hypothermia. Neonatology 119, 311–319 (2022).
Andorka, C. et al. The predictive value of MRI scores for neurodevelopmental outcome in infants with neonatal encephalopathy. Pediatr. Res. 97, 253-260 (2024).
Ní Bhroin, M. et al. Relationship between MRI scoring systems and neurodevelopmental outcome at two years in infants with neonatal encephalopathy. Pediatr. Neurol. 126, 35–42 (2022).
Machie, M. et al. MRI score ability to detect abnormalities in mild hypoxic-ischemic encephalopathy. Pediatr. Neurol. 116, 32–38 (2021).
Grossmann, K. R., Eriksson Westblad, M., Blennow, M. & Lindström, K. Outcome at early school age and adolescence after hypothermia-treated hypoxic-ischaemic encephalopathy: an observational, population-based study. Arch. Dis. Child. Fetal Neonatal. Ed. 108, 295–301 (2023).
Lequin, M. H. et al. Mammillary body injury in neonatal encephalopathy: a multicentre, retrospective study. Pediatr. Res. 92, 174–179 (2022).
Calabrese, E. et al. Correlating quantitative MRI-based apparent diffusion coefficient metrics with 24-month neurodevelopmental outcomes in neonates from the HEAL Trial. Radiology 308, e223262 (2023).
Tuura, R. O. et al. Elevated cerebral perfusion in neonatal encephalopathy is associated with neurodevelopmental impairments. Pediatr. Res. (2024).
Proisy, M. et al. Changes in brain perfusion in successive arterial spin labeling MRI scans in neonates with hypoxic-ischemic encephalopathy. Neuroimage Clin. 24, 101939 (2019).
Mitra, S. et al. Proton magnetic resonance spectroscopy lactate/N-acetylaspartate within 2 weeks of birth accurately predicts 2-year motor, cognitive and language outcomes in neonatal encephalopathy after therapeutic hypothermia. Arch. Dis. Child. Fetal Neonatal. Ed. 104, F424–F432 (2019).
Lally, P. J. et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol. 18, 35–45 (2019).
Shibasaki, J. et al. Comparison of predictive values of magnetic resonance biomarkers based on scan timing in neonatal encephalopathy following therapeutic hypothermia. J. Pediatr. 239, 101–109 e104 (2021).
Cizmeci, M. N. et al. Neonatal hypoxic-ischemic encephalopathy spectrum: severity-stratified analysis of neuroimaging modalities and association with neurodevelopmental outcomes. J. Pediatr. 266, 113866 (2024).
Meder, U. et al. Longitudinal analysis of amplitude-integrated electroencephalography for outcome prediction in hypoxic-ischemic encephalopathy. J. Pediatr. 246, 19–25.e15 (2022).
Alharbi, H. M. et al. Seizure burden and neurologic outcomes after neonatal encephalopathy. Neurology 100, e1976–e1984 (2023).
Kharoshankaya, L. et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev. Med. Child Neurol. 58, 1242–1248 (2016).
Chalak, L. F. et al. Association between increased seizures during rewarming after hypothermia for neonatal hypoxic ischemic encephalopathy and abnormal neurodevelopmental outcomes at 2-year follow-up: a nested multisite cohort study. JAMA Neurol. 78, 1484–1493 (2021).
Pavel, A. M. et al. Temporal evolution of electrographic seizures in newborn infants with hypoxic-ischaemic encephalopathy requiring therapeutic hypothermia: a secondary analysis of the ANSeR studies. Lancet Child Adolesc. Health 8, 214–224 (2024).
Srinivasakumar, P. et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics 136, e1302–e1309 (2015).
Agudelo-Pérez, S. et al. Cerebral rScO2 measured by near-infrared spectroscopy (NIRS) during therapeutic hypothermia in neonates with hypoxic-ischemic encephalopathy: a systematic review. J. Mother Child 28, 33–44 (2024).
Gunn, A. J. et al. Therapeutic hypothermia translates from ancient history in to practice. Pediatr. Res. 81, 202–209 (2017).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 1, CD003311 (2013).
Perlman, J. M. et al. Part 11: Neonatal Resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 122, S516–S538 (2010).
Laptook, A. R. et al. Effect of therapeutic hypothermia initiated after 6 h of age on death or disability among newborns with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 318, 1550–1560 (2017).
Walløe, L., Hjort, N. L. & Thoresen, M. Why results from Bayesian statistical analyses of clinical trials with a strong prior and small sample sizes may be misleading The case of the NICHD Neonatal Research Network Late Hypothermia Trial. Acta Paediatr. 108, 1190–1191 (2019).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 318, 57–67 (2017).
Conway, J. M., Walsh, B. H., Boylan, G. B. & Murray, D. M. Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome—a systematic review. Early Hum. Dev. 120, 80–87 (2018).
Montaldo, P. et al. Whole-body hypothermia vs targeted normothermia for neonates with mild encephalopathy: a multicenter pilot randomized clinical trial. JAMA Netw. Open 7, e249119 (2024).
Garegrat, R. et al. Whole-body hypothermia in mild neonatal encephalopathy: protocol for a multicentre phase III randomised controlled trial. BMC Pediatr. 24, 460 (2024).
Chalak, L. F., Slaughter, J. L., King, W. C., Sepulveda, P. & Wisniewski, S. R. A new horizon for understanding the comparative effectiveness for cooling prospectively infants with mild encephalopathy. Clin. Perinatol. 51, 605–616 (2024).
Wassink, G. et al. Partial white and grey matter protection with prolonged infusion of recombinant human erythropoietin after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow. Metab. 37, 1080–1094 (2017).
Wassink, G. et al. Non-additive effects of adjunct erythropoietin therapy with therapeutic hypothermia after global cerebral ischaemia in near-term fetal sheep. J. Physiol. 598, 999–1015 (2020).
Pang, R. et al. Melatonin and/or erythropoietin combined with hypothermia in a piglet model of perinatal asphyxia. Brain Commun. 3, fcaa211 (2021).
Ivain, P. et al. Erythropoietin monotherapy for neuroprotection after neonatal encephalopathy in low-to-middle income countries: a systematic review and meta-analysis. J. Perinatol. 41, 2134–2140 (2021).
Azzopardi, D. et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 15, 145–153 (2016).
Azzopardi, D. et al. Prospective qualification of early cerebral biomarkers in a randomised trial of treatment with xenon combined with moderate hypothermia after birth asphyxia. EBioMedicine 47, 484–491 (2019).
Molloy, E. J. et al. Neuroprotective therapies in the NICU in term infants: present and future. Pediatr. Res. 93, 1819–1827 (2023).
Azzopardi, D. et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 371, 140–149 (2014).
Pappas, A. et al. Cognitive outcomes after neonatal encephalopathy. Pediatrics 135, e624–e634 (2015).
Lee-Kelland, R. et al. School-age outcomes of children without cerebral palsy cooled for neonatal hypoxic-ischaemic encephalopathy in 2008-2010. Arch. Dis. Child. Fetal Neonatal. Ed. 105, 8–13 (2020).
Robb, T. J. et al. Communication skills in children aged 6-8 years, without cerebral palsy cooled for neonatal hypoxic-ischemic encephalopathy. Sci. Rep. 12, 17757 (2022).
Edmonds, C. J., Cianfaglione, R., Cornforth, C. & Vollmer, B. Children with neonatal Hypoxic Ischaemic Encephalopathy (HIE) treated with therapeutic hypothermia are not as school ready as their peers. Acta Paediatr. 110, 2756–2765 (2021).
Spencer, A. P. C. et al. Brain volumes and functional outcomes in children without cerebral palsy after therapeutic hypothermia for neonatal hypoxic-ischaemic encephalopathy. Dev. Med. Child Neurol. 65, 367–375 (2023).
Annink, K. V. et al. Mammillary body atrophy and other MRI correlates of school-age outcome following neonatal hypoxic-ischemic encephalopathy. Sci. Rep. 11, 5017 (2021).
Spencer, A. P. C. et al. Mammillary body abnormalities and cognitive outcomes in children cooled for neonatal encephalopathy. Dev. Med. Child Neurol. 65, 792–802 (2023).
Byrne, H. et al. Development of the corpus callosum and cognition after neonatal encephalopathy. Ann. Clin. Transl. Neurol. 10, 32–47 (2023).
Spencer, A. P. C. et al. Disrupted brain connectivity in children treated with therapeutic hypothermia for neonatal encephalopathy. Neuroimage Clin. 30, 102582 (2021).
Thayyil, S. et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob. Health 9, e1273–e1285 (2021).
Wyatt, J. S. et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 119, 912–921 (2007).
Grass, B. et al. Short-term neurological improvement in neonates with hypoxic-ischemic encephalopathy predicts neurodevelopmental outcome at 18-24 months. J. Perinat. Med. 48, 296–303 (2020).
Grass, B. et al. Therapeutic hypothermia for hypoxic-ischemic encephalopathy after perinatal sentinel events: less brain injury on MRI and improved neurodevelopmental outcome at 18-36 months. J. Perinatol. 40, 633–639 (2020).
Montaldo, P. et al. Whole-blood gene expression profile after hypoxic-ischemic encephalopathy. JAMA Netw. Open 7, e2354433 (2024).
Basu, S. K. et al. Hypoglycaemia and hyperglycaemia are associated with unfavourable outcome in infants with hypoxic ischaemic encephalopathy: a post hoc analysis of the CoolCap Study. Arch. Dis. Child. Fetal Neonatal Ed. 101, F149–F155 (2016).
Basu, S. K., Salemi, J. L., Gunn, A. J. & Kaiser, J. R. Hyperglycaemia in infants with hypoxic-ischaemic encephalopathy is associated with improved outcomes after therapeutic hypothermia: a post hoc analysis of the CoolCap Study. Arch. Dis. Child. Fetal Neonatal. Ed. 102, F299–F306 (2017).
Mietzsch, U. et al. Early glycemic state and outcomes of neonates with hypoxic-ischemic encephalopathy. Pediatrics 152, e2022060965 (2023).
Puzone, S. et al. Hypoglycaemia and hyperglycaemia in neonatal encephalopathy: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal. Ed. 109, 18–25 (2023).
Guellec, I. et al. Glycemia and neonatal encephalopathy: outcomes in the LyTONEPAL (Long-Term Outcome of Neonatal Hypoxic EncePhALopathy in the Era of Neuroprotective Treatment With Hypothermia) Cohort. J. Pediatr. 257, 113350 (2023).
Montaldo, P. et al. Continuous glucose monitoring profile during therapeutic hypothermia in encephalopathic infants with unfavorable outcome. Pediatr. Res. 88, 218–224 (2020).
Wang, J. et al. Association between continuous glucose profile during therapeutic hypothermia and unfavorable outcome in neonates with hypoxic-ischemic encephalopathy209 23-32. Early Hum. Dev. 187, 105878 (2023).
Rutherford, M. et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 9, 39–45 (2010).
Shankaran, S. et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal. Ed. 97, F398–F404 (2012).
Odd, D., Sabir, H., Jones, S. A., Gale, C. & Chakkarapani, E. Risk factors for infection and outcomes in infants with neonatal encephalopathy: a cohort study. Pediatr. Res. 96, 785–791 (2024).
Lakatos, A. et al. Neurodevelopmental effect of intracranial hemorrhage observed in hypoxic ischemic brain injury in hypothermia-treated asphyxiated neonates—an MRI study. BMC Pediatr. 19, 430 (2019).
Morell, A. S. et al. Genetic and congenital anomalies in infants with hypoxic-ischemic encephalopathy. Pediatr. Neurol. 154, 44–50 (2024).
Wang, Y. et al. Exome sequencing reveals genetic heterogeneity and clinically actionable findings in children with cerebral palsy. Nat. Med. 30, 1395–1405 (2024).
Esih, K., Goričar, K., Soltirovska-Šalamon, A., Dolžan, V. & Rener-Primec, Z. Genetic polymorphisms, gene-gene interactions and neurologic sequelae at two years follow-up in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Antioxidants 10, 1495 (2021).
Smilga, A. S. et al. Neonatal infection in children with cerebral palsy: a registry-based cohort study. Pediatr. Neurol. 80, 77–83 (2018).
Ortgies, T., Rullmann, M., Ziegelhöfer, D., Bläser, A. & Thome, U. H. The role of early-onset-sepsis in the neurodevelopment of very low birth weight infants. BMC Pediatr. 21, 289 (2021).
Cailes, B. et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch. Dis. Child. Fetal Neonatal. Ed. 103, F547–F553 (2018).
Ong, W. J. et al. Impact of neonatal sepsis on neurocognitive outcomes: a systematic review and meta-analysis. BMC Pediatr. 24, 505 (2024).
Kelly, S. B. et al. Progressive inflammation reduces high-frequency EEG activity and cortical dendritic arborisation in late gestation fetal sheep. J. Neuroinflamm. 20, 124 (2023).
Kelly, S. B. et al. Interleukin-1 blockade attenuates white matter inflammation and oligodendrocyte loss after progressive systemic lipopolysaccharide exposure in near-term fetal sheep. J. Neuroinflamm. 18, 189 (2021).
Galinsky, R. et al. Tumor necrosis factor inhibition attenuates white matter gliosis after systemic inflammation in preterm fetal sheep. J. Neuroinflamm. 17, 92 (2020).
Kelly, S. B. et al. A systematic review of immune-based interventions for perinatal neuroprotection: closing the gap between animal studies and human trials. J. Neuroinflamm. 20, 241 (2023).
Fox, A., Doyle, E., Geary, M. & Hayes, B. Placental pathology and neonatal encephalopathy. Int. J. Gynaecol. Obstet. 160, 22–27 (2023).
Rao, R. et al. Antimicrobial therapy utilization in neonates with hypoxic-ischemic encephalopathy (HIE): a report from the Children’s Hospital Neonatal Database (CHND). J. Perinatol. 40, 70–78 (2020).
Chalak, L. et al. Acute and chronic placental abnormalities in a multicenter cohort of newborn infants with hypoxic-ischemic encephalopathy. J. Pediatr. 237, 190–196 (2021).
Mir, I. N. et al. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am. J. Obstet. Gynecol. 213, 849.e841–847 (2015).
Osredkar, D. et al. Hypothermia does not reverse cellular responses caused by lipopolysaccharide in neonatal hypoxic-ischaemic brain injury. Dev. Neurosci. 37, 390–397 (2015).
Martinello, K. A. et al. Hypothermia is not therapeutic in a neonatal piglet model of inflammation-sensitized hypoxia-ischemia. Pediatr. Res. 91, 1416–1427 (2022).
Andersen, M. et al. No neuroprotective effect of therapeutic hypothermia following lipopolysaccharide-sensitized hypoxia-ischemia: a newborn piglet study. Front. Pediatr. 11, 1268237 (2023).
Falck, M. et al. Hypothermic neuronal rescue from infection-sensitised hypoxic-ischaemic brain injury is pathogen dependent. Dev. Neurosci. 39, 238–247 (2017).
Tann, C. J. et al. Perinatal risk factors for neonatal encephalopathy: an unmatched case-control study. Arch. Dis. Child. Fetal Neonatal. Ed. 103, F250–F256 (2018).
Tann, C. J. et al. Neonatal encephalopathy with group b streptococcal disease worldwide: systematic review, investigator group datasets, and meta-analysis. Clin. Infect. Dis. 65, S173–S189 (2017).
Kirton, A. et al. Perinatal stroke: mapping and modulating developmental plasticity. Nat. Rev. Neurol. 17, 415–432 (2021).
Harbert, M. J. et al. Hypothermia is correlated with seizure absence in perinatal stroke. J. Child Neurol. 26, 1126–1130 (2011).
Gonzalez, F. F. et al. Perinatal arterial ischemic stroke diagnosed in infants receiving therapeutic hypothermia for hypoxic-ischemic encephalopathy. Pediatr. Res. (2024).
Martinez-Biarge, M. et al. Risk factors for neonatal arterial ischemic stroke: the importance of the intrapartum period. J. Pediatr. 173, 62–68 e61 (2016).
Rafay, M. F. et al. Predictive value of clinical and EEG features in the diagnosis of stroke and hypoxic ischemic encephalopathy in neonates with seizures. Stroke 40, 2402–2407 (2009).
Sorg, A. L. et al. Incidence estimates of perinatal arterial ischemic stroke in preterm- and term-born infants: a national capture-recapture calculation corrected surveillance study. Neonatology 118, 727–733 (2021).
Kersbergen, K. J. et al. Anticoagulation therapy and imaging in neonates with a unilateral thalamic hemorrhage due to cerebral sinovenous thrombosis. Stroke 40, 2754–2760 (2009).
Rossor, T., Arichi, T., Bhate, S., Hart, A. R. & Raman Singh, R. Anticoagulation in the management of neonatal cerebral sinovenous thrombosis: a systematic review and meta-analysis. Dev. Med. Child Neurol. 60, 884–891 (2018).
Ferriero, D. M. et al. Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke 50, e51–e96 (2019).
Russ, J. B., Simmons, R. & Glass, H. C. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neoreviews 22, e148–e162 (2021).
Srivastava, R. & Morneau-Jacob, F. D. Teaching neuroImages: the curious case of the brainstem kink. Neurology 92, e1933–e1934 (2019).
Gana, S., Serpieri, V. & Valente, E. M. Genotype-phenotype correlates in Joubert syndrome: a review. Am. J. Med Genet C. Semin Med. Genet. 190, 72–88 (2022).
Di Mascio, D. et al. Role of prenatal magnetic resonance imaging in fetuses with isolated severe ventriculomegaly at neurosonography: a multicenter study. Eur. J. Obstet. Gynecol. Reprod. Biol. 267, 105–110 (2021).
ENSO Working Group. Role of prenatal magnetic resonance imaging in fetuses with isolated anomalies of corpus callosum: multinational study. Ultrasound Obstet. Gynecol. 58, 26–33 (2021).
Etchegaray, A., Juarez-Peñalva, S., Petracchi, F. & Igarzabal, L. Prenatal genetic considerations in congenital ventriculomegaly and hydrocephalus. Childs Nerv. Syst. 36, 1645–1660 (2020).
Bjoraker, K. J. et al. Neurodevelopmental outcome and treatment efficacy of benzoate and dextromethorphan in siblings with attenuated nonketotic hyperglycinemia. J. Pediatr. 170, 234–239 (2016).
Schwahn, B. C. et al. Consensus guidelines for the diagnosis and management of isolated sulfite oxidase deficiency and molybdenum cofactor deficiencies. J. Inherit. Metab. Dis. 47, 598–623 (2024).
Forny, P., Wicht, A., Rüfenacht, V., Cremonesi, A. & Häberle, J. Recovery of enzyme activity in biotinidase deficient individuals during early childhood. J. Inherit. Metab. Dis. 45, 605–620 (2022).
Judy, R. L., Reynolds, J. L. & Jnah, A. J. Identifying metabolic diseases that precipitate neonatal seizures. Neonatal Netw. 43, 139–147 (2024).
Lenahan, A. et al. Characteristics, genetic testing, and diagnoses of infants with neonatal encephalopathy not due to hypoxic ischemic encephalopathy: a cohort study. J. Pediatr. 260, 113533 (2023).
Woodward, K. E., Murthy, P., Mineyko, A., Mohammad, K. & Esser, M. J. Identifying genetic susceptibility in neonates with hypoxic-ischemic encephalopathy: a retrospective case series. J. Child Neurol. 38, 16–24 (2023).
Yang, L. et al. Clinical features and underlying genetic causes in neonatal encephalopathy: a large cohort study. Clin. Genet. 98, 365–373 (2020).
Ahmad, S. F., Ahmad, K. A. & Ng, Y. T. Neonatal epileptic encephalopathies. Semin. Pediatr. Neurol. 37, 100880 (2021).
Specchio, N. & Curatolo, P. Developmental and epileptic encephalopathies: what we do and do not know. Brain 144, 32–43 (2021).
Impey, L. W. et al. The relationship between intrapartum maternal fever and neonatal acidosis as risk factors for neonatal encephalopathy. Am. J. Obstet. Gynecol. 198, 49.e41–46 (2008).
Krishnan, V. et al. Need for more evidence in the prevention and management of perinatal asphyxia and neonatal encephalopathy in low and middle-income countries: a call for action. Semin. Fetal Neonatal. Med. 26, 101271 (2021).
Campanile, M. et al. Intrapartum cardiotocography with and without computer analysis: a systematic review and meta-analysis of randomized controlled trials. J. Matern. Fetal Neonatal. Med. 33, 2284–2290 (2020).
O’Sullivan, M. E. et al. Challenges of developing robust AI for intrapartum fetal heart rate monitoring. Front Artif. Intell. 4, 765210 (2021).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670 (2005).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 (2009).
Jacobs, S. E. et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch. Pediatr. Adolesc. Med. 165, 692–700 (2011).
Simbruner, G., Mittal, R. A., Rohlmann, F. & Muche, R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 126, e771–e778 (2010).
Akisu, M., Huseyinov, A., Yalaz, M., Cetin, H. & Kultursay, N. Selective head cooling with hypothermia suppresses the generation of platelet-activating factor in cerebrospinal fluid of newborn infants with perinatal asphyxia. Prostaglandins Leukot. Essent. Fat. Acids 69, 45–50 (2003).
Lin, Z. L. et al. Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia: an experience from a single neonatal intensive care unit. J. Perinatol. 26, 180–184 (2006).
Zhou, W. H. et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: A multicenter randomized controlled trial in China. J. Pediatr. 157, 367–372.e361-363 (2010).
Robertson, N. J. et al. Pilot randomized trial of therapeutic hypothermia with serial cranial ultrasound and 18-22 month follow-up for neonatal encephalopathy in a low resource hospital setting in Uganda: study protocol. Trials 12, 138 (2011).
Weeke, L. C. et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J. Pediatr. 192, 33–40.e32 (2018).
Thoresen, M. et al. MRI combined with early clinical variables are excellent outcome predictors for newborn infants undergoing therapeutic hypothermia after perinatal asphyxia. EClinicalMedicine 36, 100885 (2021).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
E.C.—wrote the first draft and edited the manuscript and provided figures. Ld.V.—wrote and edited the manuscript and provided figures. D.F.—wrote and edited the manuscript. A.G.—wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
E.C.—research supported by the NIHR, Wellcome studentship funding, and Action Medical Research for children. Support from Chiesi Pharmaceuticals for conference organization. No other conflicts. Ld.V.—Declares no conflict of interest. Received honorarium as a speaker of the “cranial ultrasound course” held at Imperial College London, UK every year and Royalties being a co-author of “an atlas of aEEG in the newborn”; “An atlas of Neonatal Brain Sonography” and “Neurology of the Newborn”. No other conflicts. D.F.—grants from the NIH and honorarium from the LeDucq foundation and Pediatric Research. No other conflicts. A.G.—Declares no conflict of interest. A.G. has received public good grants from the Health Research Council of New Zealand (current, HRC grant 22/559).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chakkarapani, E., de Vries, L.S., Ferriero, D.M. et al. Neonatal encephalopathy and hypoxic-ischemic encephalopathy: the state of the art. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-03986-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-03986-2
This article is cited by
-
Whole-body hypothermia in late preterm and early term infants: a retrospective analysis from a neurocritical care unit
Pediatric Research (2026)
-
Long-term disability after neonatal encephalopathy in low-resource settings
Pediatric Research (2026)
-
From differential to definitive: diagnosing and treatment of neonatal encephalopathy
Pediatric Research (2025)
-
Brain imaging as a predictor of neurodevelopmental outcomes in neonatal encephalopathy
Pediatric Research (2025)
-
Comparison of perinatal outcomes in small-for-gestational-age newborns in breech presentation near term by delivery mode: a multicenter retrospective study
Scientific Reports (2025)