Abstract
Background
There are limited data on the proposed association of early gut microbiota composition and the risk of respiratory tract infections (RTI) in infants from prospective studies.
Methods
We investigated the maternal and infant gut microbiota in infants prospectively followed up for their RTIs in the HELMi cohort from Helsinki, Finland. The 16S rRNA gene amplicon data was assessed at weeks 3 and 6 from 461 infants, of whom 178 developed RTIs within 3 and 6 months of life. Fecal samples collected near the due date were available from 261 mothers.
Results
There was no difference in the maternal or early infant gut microbiota in the overall microbiota composition in alpha or beta diversity between infants with or without RTIs within the first 3 and 6 months of life. The relative abundances of adult-type butyrate producers and some Enterobacteriaceae were significantly more higher at 3 and to some extent also at 6 weeks of age in the infection group compared to controls, while their mothers’ microbiota was significantly enriched with Enterococcus, Citrobacter, and Enterobacter spp., and Clostridium being less abundant.
Conclusion
The maternal and early-life infant gut microbiota may play a role in predisposition to RTIs in infants.
Impact
-
The maternal and early-life infant gut microbiota profile was associated with infants’ respiratory tract infections within the first 6 months of life.
-
In infants, the higher abundance of adult-type butyrate producers and some Enterobacteriaceae were associated with respiratory tract infections, while mothers’ microbiota was significantly enriched with Enterococcus, Citrobacter, and Enterobacter spp. in the group of infants with infections.
-
The results indicate that maternal and infant gut microbiota may play a role in predisposing an infant to infections during early life. Further studies are warranted on how this link is mediated.
Similar content being viewed by others
Introduction
Healthy, full-term infants experience four to ten respiratory tract infection (RTI) episodes in the first year of life in a high-income country.1,2,3 The full burden of RTIs is seen not only in the reduced quality of life in infants but also in the socioeconomic effects including parental worry and absenteeism from work.4,5,6,7,8,9,10
Early gut microbiota composition has been suggested to be associated with RTIs in children.11,12 There is evidence from animal studies that early-life gut microbiota composition might influence respiratory immunity and, therefore, increase the susceptibility to RTIs.13,14 However, results from human studies are inconsistent. There is evidence that low alpha diversity and low relative abundance of particular gut-commensal bacterial genera (Bifidobacterium, Faecalibacterium, Ruminococcus, and Roseburia) are associated with childhood respiratory diseases, especially wheezing, and asthma,15,16,17,18,19,20 whereas evidence between gut microbiota and RTIs in infancy is less studied due to a lack of longitudinal large studies with fecal sampling during the first months of life, standard follow-up times, and definitions of RTIs.20,21,22,23,24,25
Most birth cohort studies15,16,17,18,26 and case-control studies19,20,21 investigating the relationship between the gut microbiota and RTIs have explored wheezing or asthma. Only a few studies have reported RTIs as an outcome.22,23,24,25 Furthermore, there are limited data on the association between the gut microbiota and RTIs in infants using a careful follow-up of respiratory symptoms in prospective cohort studies.22,24,25 Finally, maternal microbiota has rarely been investigated in previous studies. Considering the suggested role of gut microbiota composition in the risk of childhood RTIs, we hypothesized that the early gut microbiota is associated with the occurrence of RTIs in the first 6 months of life.
In this prospective study cohort, using a systematic follow-up of respiratory symptoms, we set out to investigate the proposed association between early gut microbiota composition and the occurrence of RTIs in infants.
Methods
Study design
This was a nested case-control study retrieved from a prospective HELMI cohort study (see below the details of the cohort). We compared the maternal and infant gut microbiota between infants with an RTI episode within 3 and 6 months of life and infants with no such episodes serving as controls. Infants with an RTI episode were defined as infants who presented with a lower RTI (LRTI) or an upper RTI (URTI) episode with fever or otitis media in the first 6 months of life. In the HELMI cohort study, the families used a prospective online diary weekly for the first 4 months of life, then biweekly until 7 months, including infection symptoms and doctoral visits, which was to detect infants who developed RTIs in the first 6 months of life and their controls without such infections.1
The study population of mothers and infants originated from the prospective HELMi (Health and Early Life Microbiota) birth cohort recruited in the Helsinki region of Finland, from February 2016 to March 2018. A detailed description of the HELMi cohort has previously been published.27 In brief, the inclusion criteria were singleton, term newborn infants born healthy, with birth weight exceeding 2.5 kg. To study the gut microbiota composition, we used fecal samples from mothers collected close to the due date (±2 weeks) and infant samples collected at weeks 3 and 6. Parents collected the fecal samples at home, froze them immediately at −20 °C, and transported them in a frozen form to the laboratory, which kept them at −80 °C until DNA extraction. Parental background data were recorded at the enrollment.27,28
Study groups
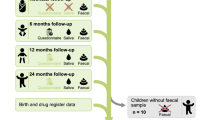
From the original HELMi cohort of 1052 infants, 189 (18%) infants developed an RTI episode in the first 6 months of life (Fig. 1). Of 189 infants, there were good quality microbiota data available from 178 infants and 136 mothers (Table 1). A random sample of infants with no RTI episodes during 6 months of life (n = 143) and their mothers (n = 125) served as controls. Baseline characteristics were comparable between the groups except there were more first-born infants in the control group (Table 2). In total, we included samples from 461 infants and 261 mothers for microbiota analyses. In the initial analyses, we compared the 3- and 6-week microbiota between infants with RTI episodes in the first 6 months of life and the control infants. For the sensitivity analysis, after excluding infants with any RTIs before the first fecal sample was taken, we had samples of 109 infants who developed an RTI episode in the first 6 months of life and 182 controls (Fig. 1) matched for sex, year of birth, season of birth, mode of delivery, exposure to intrapartum antibiotics and the number of older siblings (Supplemental Table S1).
Sample size
In the previous birth cohort studies investigating the relationship between gut microbiota and the occurrence of RTIs, the sample size has been around 120 children22,24 and in case-control studies, the included number of children in the microbiota analyses have ranged from 49 to 155 altogether.21,23 These studies have found statistically significant differences in alpha- and beta-diversity of gut microbiota.21,22,23,24 Thus, the sample size of the study was deemed sufficient.
Ethics
The study protocol was reviewed by the ethical committee of The Hospital District of Helsinki and Uusimaa (263/13/03/03 2015), Finland. The study was conducted following the principles of the Helsinki Declaration. All families gave their written informed consent for the HELMi study.
Microbiota and statistical analyses
The data on infections and background variables were presented as a median and interquartile range (IQR) unless otherwise stated. Fecal DNA extraction and preparation of the samples for V3-V4 16S ribosomal ribonucleic acid (rRNA) gene amplicon sequencing has been previously described.28 Samples with <2000 reads were excluded from the analyses. Statistical analyses were performed using the R package mare.29 Alpha diversity was calculated as the inverse Simpson diversity index and richness as the number of OTUs (operational taxonomic unit). For beta-diversity analyses, unsupervised Principal-coordinate Analyses (PcoA) were calculated with the capscale function and the Bray-Curtis dissimilarities with the function vegdist of the R package vegan, complemented with permutational ANOVA using adonis function of the same package.30
GroupTest function of the mare package was used for comparison of the relative abundances of bacterial genera and families between the groups. The function selects the most optimal model for each taxon based on its distribution, using either the glm.nb function from the MASS package,31 lm function from base R with log-transformation if necessary, or the gls function from the nlme package.32 The GroupTest function calculates a model that is appropriate for each taxon separately and attempts to find a suitable model for the taxon. Each model is checked for fulfilling the assumptions (residual heteroscedasticity and normality). If these are not met, the model is corrected to fulfill the criteria (e.g., by including a residual variance parameter in the model). This level of scrutiny is normally not done, but rather all taxa are tested using the same model, which is unlikely to fit all taxa equally due to differences in their distributions. Thus, the presented p values are more robust than what is typical in the field. When no suitable model is found, and the model assumptions are not met, no P value is reported for the taxa. The total read counts per sample were used as the offset in the models. Standard Benjamini-Hochberg corrections for false discovery rate (FDR) were applied. FDR-corrected P values of <0.1 were considered significant. This research paper is an exploratory analysis in early-stage research, which justifies FDR < 0.1 in this study.
After this, we conducted a sensitivity analysis, by excluding infants with any infections before the first fecal sample was taken. Gut microbiota compositions were compared between infants who developed an RTI episode and control infants matched for sex, year of birth, season of birth, mode of delivery, exposure to intrapartum antibiotics, and the number of older siblings (see below and Supplemental Table S1).
The main analysis was based on the assumption that gut microbiota composition may reflect the association of environmental and perinatal factors on the risk of RTIs. The rationale for the analyses using matched controls was that the selected covariates may act as confounding covariates as they influence both the gut microbiota composition and the risk of RTIs.
Results
From the HELMI cohort, we included all 178 infants who developed an RTI episode within the first 6 months of life and had an available fecal sample at weeks 3 and 6 as well as 143 infants without such infections serving as controls, and their mothers (n = 261) (Table 2, Supplemental Table S1).
RTI episodes
In the 178 infants with an RTI episode within the first 6 months of life, the median duration of RTI symptoms was 11 days (IQR 7–15) (Table 3). Most of these RTIs were URTIs, including fever 49%, followed by otitis media 47%, and LRTIs 4%. Of the 178 infants with an RTI episode, 30% (n = 53) developed at least one episode during the first 3 months of life and a median duration of 10 days (IQR 6.5-15). Of these early RTIs, otitis media was the most common (53%), followed by URTI with fever 41% and LRTI 6%. During the first 6 months of life, 61% (n = 108/178) of infants with an RTI had a visit to a doctor, and 13.5% (n = 24/178) visited the hospital emergency department. Among the control group, 21% (n = 30/143) reported a doctor’s visit (not listed as an RTI episode), and 2% (n = 3/143) visited the hospital emergency department without a specific diagnosis. None of the infants died.
Maternal gut microbiota composition and RTI episodes in infants
Based on the beta diversity of microbiota, we found no statistically significant differences in the gut microbiota composition between mothers of infants with and without an RTI episode during the first 6 months of life (Fig. 2). Beta diversity analysis, representing the dissimilarities between microbial communities using PCoA, showed no differences between the gut microbiota of mothers of infants with and without an RTI episode at family level (p = 0.39) (Fig. 2a) or at genus level (p = 0.30) (Supplemental Fig. S1a). The microbial richness and diversity defined by the number of OTUs did not differ between mothers of infants developing an RTI episode and mothers of controls (Supplemental Table S2).
Comparison between mothers of infants who developed a respiratory tract infection episode (RTI) in the first 6 months of life and mothers of randomly selected infants with no such infection episode. PCoA plots based on Bray-Curtis dissimilarities of the samples, showing the richness of the microbiota as background (a). Clusters are shown by circles, which were drawn based on the standard deviations of the data points in each category of the samples (a). The comparisons are between mothers of infants who developed an RTI in the first 6 months of life and mothers of infants remaining healthy (p = 0.39). Clustered stacked column graphs demonstrate microbiota differences at the family level (b). The comparisons are between mothers of infants who developed an RTI in the first 6 months of life (YES) and mothers of infants remaining healthy (NO).
In the taxonomic distribution, the most predominant bacteria of the maternal microbiota on family level were Ruminococcaceae (38% in the RTI group and 38% in the control group) and Lachnospiraceae (31% and 30%, respectively) (Fig. 2b). The relative abundance analysis at family level revealed that mothers of infants with RTI had a higher abundance of Enterococcaceae and Enterobacteriaceae together with Family XIII Incertae Sedis and Pasteurellacceae, while Clostridiaceae was less abundant compared to mothers of infants with no infections (Table 4). At genus level mothers of infants with RTI had a higher abundance of Enterococcus, Enterobacter, and Citrobacter and a lower abundance of Clostridium (Table 4). All microbiota results in the relative abundance analysis between mothers on family and genus levels in the microbiota samples are listed in Supplemental Tables S3 and S4.
Early gut microbiota composition and RTI episodes
Based on beta diversity, there was no difference in the overall composition of microbiota at 3 or 6 weeks of age between infants who developed an RTI episode and the control group (Figs. 3–4). At 3 weeks of age, we observed no significant difference in PCoA between infants who developed an RTI episode within the first three (p = 0.87) or 6 months (p = 0.43) of life when compared to infants in the control group at microbiota family level (Fig. 3a, b) nor at genus level within the first three (p = 0.68) or 6 (p = 0.34) months of life (Supplemental Fig. S2a, b). We did not find significant differences at 6 weeks of age in PCoA between infants developing RTI episodes within the first 3 (p = 0.39) or 6 (p = 0.95) months of life when compared to infants in the control group at family level (Fig. 3c, d) nor at genus level within the first 3 (p = 0.46) or 6 (p = 0.93) months of life (Supplemental Fig. S2c, d). At weeks 3 and 6 the overall microbial richness and diversity defined by the number of OTUs showed no differences between infants developing an RTI episode during the first 3 or 6 months of life and the controls (Supplemental Table S2).
PCoA plots are based on Bray-Curtis dissimilarities of the samples, and show richness as the background at time points of 3 (a, b) and 6 weeks of age (c, d). The comparisons are between infants who developed an RTI episode in the first 3 (a, p = 0.87 and c, p = 0.39) and 6 (b, p = 0.43 and d, p = 0.95, respectively) months of life and randomly selected infants remaining healthy in the first 6 months of life.
Clustered stacked column graphs demonstrate microbiota differences at the family level at time points of 3 and 6 weeks of age. At week three and six, the comparisons are between infants who developed an RTI episode (YES) in the first 3 (a, c) and 6 (b, d) months of life and randomly selected infants remaining healthy (NO) in the first 6 months of life.
In the taxonomic distribution, the two most predominant bacteria of the microbiota at family level were Bifidobacteriaceae and Enterobacteriaceae at the age of 3 and 6 weeks in all infant groups (Fig. 4). The most significant difference in the microbiota composition at family level in the fecal samples at 3 weeks of age was that infants who developed an RTI within the first 3 months of life had a higher abundance of Rikenellaceae and Verrucomicrobiaceae, followed by increased Prevotellacaeae, Actinomycetaceae, Coriobacteriaceae and Micrococcaceae compared to that in control group (Table 5). The higher abundance of Prevotellacaeae was also seen in infants developing RTI within the first six months of life. Only this higher abundance of Prevotellacaeae persisted in the samples taken at 6 weeks of age but only in infants developing an RTI within the first 3 months of life (Table 6). At genus level, in the samples taken at 3 weeks of age, altogether 11 genera (Alistipes and Akkermansia were the most elevated followed by Peptoniphilus, Faecalibacterium, and Serratia) were increased in infants developing an RTI within the first 3 months of life compared to that in the control group (Table 5). The higher relative abundance of Faecalibacterium, a major butyrate producer, and Peptoniphilus was also observed in infants developing RTIs within the first 6 months of life, while Anaerostipes was less abundant (Table 5). The relative abundance analysis from the samples taken at 6 weeks of age revealed a concomitant strong decrease of Anaerostipes in infants developing an RTI within the first three and 6 months of life (Table 6). We also found a higher abundance of six genera (Prevotella, Macellibacteroides, Catenibacterium, Subdoligranulum, Faecalibacterium, and Atopobium) in infants developing infection within the first 3 months of life (Table 6). All other microbiota results in the relative abundance analysis on family and genus levels are listed in Supplemental Tables S5–8. The taxonomic distribution at genus level at the ages of 3 and 6 weeks is shown in Supplemental Fig. S3.
Early gut microbiota composition and RTI episodes in cases and controls matched for environmental and perinatal factors (sensitivity analysis)
After excluding infants with any infection symptoms before obtaining the early fecal samples, we compared the gut microbiota composition in infants later developing RTIs to that in controls matched for environmental and perinatal covariates. We observed no statistically significant differences in the overall composition of microbiota at 3 weeks of age based on beta diversity between the groups (Fig. 5).
The comparisons are between infants who developed an RTI episode during the first 3 (a) and 6 months of life (b). Clustered stacked column graphs demonstrate microbiota differences at the family level. The comparisons are between infants who developed an RTI episode (YES) in the first 3 (c) and 6 (d) months of life and carefully matched infants remaining healthy (NO) for the first 6 months of life. Infants with any infections before the 3-week stool sample were excluded.
The gut microbiota composition was highly similar at 3 weeks of age between infants who developed an RTI episode within the first 3 (p = 0.55) or 6 months (p = 0.30) of life and matched controls as visualized by PCoA at family level (Fig. 5a, b) and at genus level within the first 3 (p = 0.66) or 6 months of life (p = 0.25) (Supplemental Fig. S4a, b). The bacterial richness and diversity defined by the number of OTUs did not differ at 3 weeks of age between infants developing an RTI episode within the first 3 or 6 months of life and matched controls (Supplemental Table S2).
The taxonomic distribution of the dominant taxa was comparable to the unmatched analysis and the two most predominant bacteria of the microbiota at family level were Bifidobacteriaceae and Enterobacteriaceae at the age of 3 weeks (Fig. 5c and d). In the relative abundance analysis, the significant findings at family level were that in infants developing an RTI during the first 6 months of life had a higher abundance of Acidaminococcaceae and a lower abundance of Veillonellaceae compared to infants in the control group (Table 7). No such observation was made between infants developing infections in the first 3 months of life and matched controls. At genus level in the infant group developing RTIs within the first 3 months of life altogether 8 genera (Dialister, Faecalibacterium, Roseburia, Proteus, Ruminococcaceae uncultured, Serratia, Pseudobutyrivibrio, and Rothia) were increased, and 2 genera (Akkermansia and Salmonella) were less abundant (Table 7). Whereas in the infant group developing RTIs in the first 6 months of life altogether 6 genera (Faecalibacterium, Proteus, Peptoniphilus, Pseudobutyrivibrio, Roseburia, and Anaerospora) were increased, and 2 genera (Anaerostipes and Veillonella) were less abundant (Table 7). The most significant findings at genus level were that a higher abundance of butyrate-producing genera Roseburia, Pseudobutyrivibrio, and Faecalibacterium, and also genus Proteus was found both in infants who developed an RTI episode within the first 3 and 6 months of life (Table 7). All other results of the relative abundances of the sensitivity analysis on family and genus levels are listed in Supplemental Tables S9–10. The taxonomic distribution at genus level is shown in Supplemental Fig. S5.
When comparing the results to the unmatched analysis, we found similarities in the relative abundances at genus level that persisted when the groups were matched. Infants developing RTIs in the first 6 months of life had a lower abundance of a butyrate producer genus Anaerostipes in the samples taken at 3 and 6 weeks of age (Tables 5–7). We also observed that in the samples taken at 3 and 6 weeks of age, the relative abundance of another major butyrate producer genus Faecalibacterium was higher in infants developing RTIs during the first 3 and 6 months of life in both unmatched and matched analysis (Tables 5–7). The analysis also revealed that in the samples taken at 3 weeks of age, the genera Serratia and Rothia were more abundant in infants developing RTIs within the first 3 months of life and Peptoniphilus and Proteus were more abundant in infants developing RTIs within the first 6 months of life (Tables 5–7).
Discussion
This prospective study demonstrated that alterations in the relative abundance of certain taxa of the maternal and early-life gut microbiota are associated with the occurrence of RTIs within the first 3 and 6 months of life. However, no association between maternal or early-life overall gut microbiota composition, measured with beta diversity, and the occurrence of RTIs in infants was found. We did observe an association in higher abundances of Prevotellaceae and adult-type butyrate producers, including Faecalibacterium spp., and some Enterobacteriaceae at three and to some extent also at 6 weeks of age in infants with RTIs compared to controls.
The gut microbiota of mothers of infants with infections was enriched in relative abundance of Enterobacter and Citrobacter, members of phylum Proteobacteria, and Enterococcus, which are all considered opportunistic pathogens. The Enterobacteriaceae family, a part of the Proteobacteria phylum found in the healthy human gut but with low abundance,33 comprises both commensal and opportunistic disease-causing pathogens. It has been suggested that the enrichment of Enterobacteriaceae in the gut is a marker for an unstable microbial composition, which is reportedly associated with various diseases such as inflammatory bowel disease, colorectal cancer, or metabolic syndrome.34 It has been proposed that perturbations to the maternal or neonatal microbiota might increase and prolong the window of risk for bronchiolitis in infancy.35 In previous studies, there has been limited data on the associations between maternal microbiota and infections in infants. In one prospective study, the composition of the maternal gut microbiota with higher counts of enterococci was shown to be associated with the increased risk of infant wheezing in the first 6 months of life.36 In line with this, we found here that the mothers of infants with RTIs showed an increased relative abundance of Enterococcus spp. in their gut microbiota around delivery.
The evidence from prior case-control studies evaluating bacterial profiles in infants and RTIs show inconsistent results.20,21,23 Hasegawa et al. found in 40 infants hospitalized with bronchiolitis that at 3 months of age, the likelihood of bronchiolitis was associated with a microbiota profile dominated by Bacteroides,21 whereas Li et al., based on analysis of 26 children, mostly aged from 2 to 6 years, found an association between recurrent RTIs and a lower alfa-diversity, and also a lower relative abundance of genera Faecalibacterium, Bifidobacterium, and Eubacterium, and a higher abundance of Enterococcus.23 Our results do not support these observed changes in the composition of the bacterial profile. Instead, we found genus Faecalibacterium to be more abundant in infants developing RTIs compared to healthy controls. A retrospective case-control study design cannot exclude the possibility that the infections per se result in a deviation in the gut microbiota. In our prospective cohort, we were able to avoid bias due to reverse causation by excluding all infants with any infections before the first gut microbiota samples were obtained at 3 weeks of age. In addition, we were able to match the controls for clinically important covariates such as mode of delivery and perinatal antibiotics, which are known to influence the early gut microbial composition.37,38 We did observe that infants developing RTIs in the first 6 months of life had a lower abundance of Anaerostipes spp. in the samples taken at 3 and 6 weeks of age (Tables 5–7). Anaerostipes spp. are known to produce butyrate from sugars but notably also from lactate and acetate, that are abundant short-chain fatty acids in early life produced by Bifidobacteria from breast milk.39,40 Hence the depletion of Anaerostipes spp. in the the gut of RTI infants may point towards an increased level of lactate and reduced levels of butyrate while there are also indications that this is related to a deviating immune response.41
The results from birth cohorts focusing on the relation between gut microbiota and RTIs have been variable. A Danish birth cohort assessed wheezing at 3 years of age and found no difference in alfa- or beta-diversity or the relative abundance of the gut microbiota at 9 months of age.24 In a prospective birth cohort from the USA, Moroishi et al. observed that higher gut microbiome diversity at 6 weeks of age increased the odds for RTIs by the age of 1 year.25 In a well-executed prospective birth cohort from the Netherlands, Reyman et al. found that enrichment of Bifidobacterium spp. and reduction of Enterococcus and Klebsiella spp. at 1 week of life was associated with the number of RTIs in the first year of life.22 Similarly, a recent study in the UK described a correlation with the microbiota in the first week of life and hospital admissions for viral LRTIs, suggesting a protective role for Bifidobacterium spp.42 We did not observe this in our HELMi cohort, where we monitored mild RTIs treated at home and where virtually all infants were breast-fed and contained Bifidobacterium spp. In contrast, we observed here that the gut microbiota of infants with infections contained an increased relative abundance of fecal butyrate-producing bacteria, including Faecalibacterium, Ruminococcus, and Roseburia spp. In healthy infants, these butyrate producers are linked to the conversion of solid foods as these are found to develop from around 4 to 6 months of age when weaning off breastmilk.43 We consider their increased relative abundance in infants with infections at 3 weeks and, to some extent, also at 6 weeks of age as reflecting a premature gut microbiota maturation.
The strength of our study lies in the large prospective longitudinal birth cohort of full-term infants born healthy. The follow-up starting at birth provided us with a large scale of weekly information on the occurrence of common RTIs mainly treated at home. The information on symptoms provided by parents gave a more comprehensive understanding of the real rate of common RTIs, whilst making our study hard to be comparable to other studies with a more severe outcome or an outcome defined by a healthcare professional. The fecal samples available enabled us the opportunity to study not only the microbiota of infants but also the maternal gut microbiota.
The main limitation of this work is that the cohort represented highly educated families in a high-income country. Although the development of infant microbiota appears universal, the pattern is affected by local culture and feeding practices.44 Almost all the mothers breastfed their infants, for instance, and very few reported smoking. Thus, the results may not be generalizable to child cohorts with different background characteristics. Moreover, our HELMi cohort included only infants who were born healthy, term, and singleton with birth weight exceeding 2.5 kg. The current methodology did not allow assessment of the functional properties of gut microbiota warranting further studies on the metabolic pathways of the microbiota and their possible associations with susceptibility to RTIs in early life.
Conclusion
This study reported the association between maternal and infant gut microbiota and early-life RTIs in the offspring using a prospective online follow-up for respiratory symptoms.
This prospective study demonstrated that alterations in the relative abundance of taxa of the maternal and early-life gut microbiota are associated with infants’ early RTIs in full-term infants. Ideally, data on gut microbiota from observational cohort studies could be used in planning intervention studies in the future.
Data availability
Sequencing data are accessible in ENA along with limited metadata (Study ID: PRJEB55243). Additional data can be obtained from the corresponding author upon reasonable request.
References
Hyvonen, S. et al. Perinatal and other risk factors for common infections in infancy: a prospective cohort study. Pediatr. Infect. Dis. J. 42, e447–e453 (2023).
Toivonen, L. et al. Rhinovirus infections in the first 2 years of life. Pediatrics 138, e20161309 (2016).
von Linstow, M. L. et al. Acute respiratory symptoms and general illness during the first year of life: a population-based birth cohort study. Pediatr. Pulmonol. 43, 584–593 (2008).
Enserink, R. et al. Gastrointestinal and respiratory illness in children that do and do not attend child day care centers: a cost-of-illness study. PLoS ONE 9, e104940 (2014).
Heikkinen, T., Silvennoinen, H., Heinonen, S. & Vuorinen, T. Clinical and socioeconomic impact of moderate-to-severe versus mild influenza in children. Eur. J. Clin. Microbiol. Infect. Dis. Publ. Eur. Soc. Clin. Microbiol. 35, 1107–1113 (2016).
Heikkinen, T., Ojala, E. & Waris, M. Clinical and socioeconomic burden of respiratory syncytial virus infection in children. J. Infect. Dis. 215, 17–23 (2017).
Koopman, L. P. et al. Respiratory infections in infants: interaction of parental allergy, child care, and siblings—the PIAMA study. Pediatrics 108, 943–948 (2001).
Paalanne, M. et al. Absence from day care or school and parental absence from work during children’s respiratory infections. Acta Paediatr.112, 486–492 (2023).
Toivonen, L. et al. Burden of recurrent respiratory tract infections in children: a prospective cohort study. Pediatr. Infect. Dis. J. 35, e362–e369 (2016).
Willis, G. A. et al. The impact of influenza infection on young children, their family and the health care system. WAIVE Study Team, ed. Influenza Other Respir. Viruses 13, 18–27 (2019).
Alcazar, C. G. M. et al. The association between early-life gut microbiota and childhood respiratory diseases: a systematic review. Lancet Microbe 3, e867–e880 (2022).
Zama, D. et al. The relationship between gut microbiota and respiratory tract infections in childhood: a narrative review. Nutrients. 14, 2992 (2022).
Renz, H. et al. The neonatal window of opportunity-early priming for life. J. Allergy Clin. Immunol. 141, 1212–1214 (2018).
Wang, W. et al. Bifidobacterium infantis relieves allergic asthma in mice by regulating Th1/Th2. Med Sci. Monit. 26, e920583 (2020).
Depner, M. et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 26, 1766–1775 (2020).
Fujimura, K. E. et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 22, 1187–1191 (2016).
Patrick, D. M. et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir. Med. 8, 1094–1105 (2020).
Stokholm, J. et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat. Commun. 9, 141 (2018).
Arrieta, M. C. et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 7, 307ra152 (2015).
Arrieta, M. C. et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J. Allergy Clin. Immunol. 142, 424–434.e10 (2018).
Hasegawa, K. et al. The fecal microbiota profile and bronchiolitis in infants. Pediatrics 138, 07 (2016).
Reyman, M. et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 10, 4997 (2019).
Li, L., Wang, F., Liu, Y. & Gu, F. Intestinal microbiota dysbiosis in children with recurrent respiratory tract infections. Microb. Pathog. 136, 103709 (2019).
Laursen, M. F. et al. Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol. 15, 154 (2015).
Moroishi, Y. et al. The relationship between the gut microbiome and the risk of respiratory infections among newborns. Commun. Med. 2, 87 (2022).
Galazzo, G. et al. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology 158, 1584–1596 (2020).
Korpela, K. et al. Cohort profile: Finnish Health and Early Life Microbiota (HELMi) longitudinal birth cohort. BMJ Open 9, e028500 (2019).
Jokela, R. et al. Sources of gut microbiota variation in a large longitudinal Finnish infant cohort. EBioMedicine 94, 104695 (2023).
Korpela, K. et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat. Commun. 7, 10410 (2016).
Oksanen, J. et al. Community Ecology Package. R package version 2 4–0 (R Foundation for Statistical computing). Published online (2016).
Venables, W., Ripley, B. Modern Applied Statistics with S 4th edn (Springer, 2002).
Pinhero, J., Bates, D., DebRoy, S., Sarkar, D., R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–128 (R Foundation for Statistical Computing). Published online (2016).
Huttenhower, C. et al. Structure, function and diversity of the healthy human microbiome. Human Microbiome Project Consortium. Nature 486, 207–214 (2012).
Moreira de Gouveia, M. I., Bernalier-Donadille, A. & Jubelin, G. Enterobacteriaceae in the human gut: dynamics and ecological roles in health and disease. Biology. 13, 142 (2024).
Lynch, J. P. et al. The influence of the microbiome on early-life severe viral lower respiratory infections and asthma-food for thought?. Front. Immunol. 8, 156 (2017).
Lange, N. E. et al. Maternal intestinal flora and wheeze in early childhood. Clin. Exp. Allergy 42, 901–908 (2012).
Li, W. et al. Vertical transmission of gut microbiome and antimicrobial resistance genes in infants exposed to antibiotics at birth. J. Infect. Dis. 224, 1236–1246 (2021).
Tapiainen, T. et al. Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci. Rep. 9, 10635 (2019).
Shetty, S. A. et al. Unravelling lactate-acetate and sugar conversion into butyrate by intestinal Anaerobutyricum and Anaerostipes species by comparative proteogenomics. Environ. Microbiol. 22, 4863–4875 (2020).
Yokota, H., Tanaka, Y. & Ohno, H. Coculture of Bifidobacterium bifidum G9-1 With Butyrate-Producing Bacteria Promotes Butyrate Production. Microbiol. Immunol. 69, 389–396 (2025).
Feehley, T. et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 25, 448–453 (2019).
Garcia-Maurino, C. et al. Investigation of associations between the neonatal gut microbiota and severe viral lower respiratory tract infections in the first 2 years of life: a birth cohort study with metagenomics. Lancet Microbe. 6, 101072 (2025).
Backhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015).
Korpela, K. & de Vos, W. M. Early life colonization of the human gut: microbes matter everywhere. Curr. Opin. Microbiol. 44, 70–78 (2018).
Acknowledgements
We thank the nurses who worked with these children, especially Jaana Valkeapää, along with Heli Suomalainen, Anna Mantere, Eevi Heitto and Janica Bergström. We also thank Dr. Alise Ponsero and Evgenia Dikareva, MSc, for their contributions to the curation of the questionnaire data. Finally, we thank the participating families for their efforts.
Funding
This work was supported by the Signe and Ane Gyllenberg Foundation, Finland [5044 to S.H.], the Foundation for Pediatric Research, Finland [200141 and 210168 to SH and grant not numbered to K.L.K.], the Finnish Medical Foundation, Finland [5851 to S.H.], Juho Vainio Foundation, Finland [grant nor numbered to S.H.] and by Tekes/Business Finland [329/31/2015 to W.M.d.V.] to establish the HELMi cohort. None of these sources had any role in the design or execution of the research. Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Author information
Authors and Affiliations
Contributions
Sanni Hyvönen carried out the initial statistical analyses, drafted the initial manuscript, and approved the final manuscript as submitted. Aki Saarikivi carried out the initial statistical analyses, drafted the initial manuscript, and approved the final manuscript as submitted. Jani Mälkönen assisted in the statistical analyses and approved the final manuscript as submitted. Terhi Solasaari critically revised the manuscript and approved the final manuscript as submitted. Katri Korpela, Anne Salonen, and Willem M de Vos conceptualized the HELMi cohort, critically revised the manuscript and approved the final manuscript as submitted. Terhi Ruuska-Loewald conceptualized and designed the study, supervised the data analysis, co-authored the manuscript and approved the final manuscript as submitted. Kaija-Leena Kolho conceptualized the HELMi cohort, conceptualized and designed the study, supervised the data analysis, co-authored the manuscript and approved the final manuscript as submitted. All the authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
All families gave written informed consent for the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hyvönen, S., Saarikivi, A., Mälkönen, J. et al. The association of maternal and infant early gut microbiota with respiratory infections in infants. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04326-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04326-0
This article is cited by
-
Initial insights into perinatal gut microbiome and early infantile respiratory tract infections
Pediatric Research (2025)