Abstract
Background/Objectives
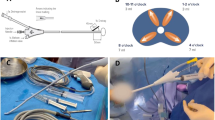
Endorsing the principles of minimal invasiveness in benign-prostatic hyperplasia (BPH) surgery, we conducted the first evaluation of transurethral intraprostatic anesthesia (TUIA) using Schelin catheter® (SC) prior to iTIND positioning.
Subjects/Methods
Of 23 patients enrolled, 11 (48%) received TUIA via SC whereas the remaining underwent standard anesthesia protocol. Pain was assessed using visual analogue scale (VAS).
Results
No differences between cohorts were observed for pain during the device implantation and removal. Conversely, significantly lower median VAS scores were reported at 24- (1.0 vs. 3.0) and 48- (1.0 vs. 2.5) hour follow-up favoring TUIA.
Conclusions
SC TUIA offers effective pain control during iTIND procedures, supporting its use in outpatient settings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
References
Nguyen DD, Li T, Ferreira R, Baker Berjaoui M, Nguyen ALV, Chughtai B, et al. Ablative minimally invasive surgical therapies for benign prostatic hyperplasia: A review of Aquablation, Rezum, and transperineal laser prostate ablation. Prostate Cancer Prostatic Dis. 2023;27:22–8.
Manfredi C, Arcaniolo D, Spatafora P, Crocerossa F, Fusco F, Verze P, et al. Emerging minimally invasive transurethral treatments for benign prostatic hyperplasia: a systematic review with meta-analysis of functional outcomes and description of complications. Minerva Urol Nephrol. 2022;74:389–99.
Amparore D, Fiori C, Valerio M, Schulman C, Giannakis I, De Cillis S, et al. 3-Year results following treatment with the second generation of the temporary implantable nitinol device in men with LUTS secondary to benign prostatic obstruction. Prostate Cancer Prostatic Dis. 2020;24:349–57.
Amparore D, De Cillis S, Schulman C, Kadner G, Fiori C, Porpiglia F. Temporary implantable nitinol device for benign prostatic hyperplasia-related lower urinary tract symptoms: over 48-month results. Minerva Urol Nephrol. 2023;75:743–51.
Sibona M, Destefanis P, Vercelli E, Secco S, Gontero P, Cindolo L. Ejaculation physiology and dysfunction after BPH surgery: the role of the new MISTs. Prostate Cancer Prostatic Dis. 2023;26:475–82.
Gemma L, Pecoraro A, Sebastianelli A, Spatafora P, Sessa F, Nicoletti R, et al. Impact of minimally invasive surgical procedures for Male Lower Urinary Tract Symptoms due to benign prostatic hyperplasia on ejaculatory function: a systematic review. Prostate Cancer Prostatic Dis. 2024;27:404–21.
Tzeng M, Basourakos SP, Lewicki PJ, Hu JC, Lee RK. New Endoscopic In-office Surgical Therapies for Benign Prostatic Hyperplasia: A Systematic Review. Eur Urol Focus. 2022;8:522–31.
Schelin S. Transurethral resection of the prostate after intraprostatic injections of mepivacain epinephrine: a preliminary communication. Scand J Urol Nephrol. 2009;43:63–67.
Hamouda A, Ibrahim A, Corsi N, Siena G, Elterman DS, Chughtai B et al. Use of the Schelin Catheter for transurethral intraprostatic anesthesia prior to Rezūm treatment. Can J Urol. 2024;31. www.canjurol.com.
Siena G, Sessa F, Cindolo L. Use of a Schelin Catheter for analgesia during Rezum treatment of the prostate. Prostate Cancer Prostatic Dis. 2024;27:147–9.
Gravas S, Malde S, Cornu JN, Gacci M, Gratzke C, Herrmann TRW, et al. From BPH to male LUTS: a 20-year journey of the EAU guidelines. Prostate Cancer Prostatic Dis. 2023;27:48–53.
Author information
Authors and Affiliations
Contributions
SS: project development, data collection, drafting of the manuscript; AO: project development, data collection; ML: data analysis, drafting of the manuscript; PD: data collection; ST: data collection, ED: critical revision of the manuscript; TS: critical revision of the manuscript; GS: critical revision of the manuscript; AMB; critical revision of the manuscript; AG: critical revision of the manuscript; LC: project development, critical revision of the manuscript, drafting of the manuscript.
Corresponding author
Ethics declarations
Competing interests
AO, ML, PD, ST, ED, TS, GS, AMB, AG, LC: no conflicts of interest to declare; SS: proctor iTIND (Medi-Tate LTd.®, Or Akiva, Israel).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Secco, S., Olivero, A., Longoni, M. et al. Use of a Schelin catheter for transurethral intraprostatic anesthesia (TUIA) prior to iTIND procedure. Prostate Cancer Prostatic Dis 28, 832–833 (2025). https://doi.org/10.1038/s41391-024-00892-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-024-00892-2
This article is cited by
-
Best of 2024 in Prostate Cancer and Prostatic diseases
Prostate Cancer and Prostatic Diseases (2025)