Abstract
Background
International guidelines recommend germline genetic testing for men with metastatic prostate cancer. If offered to all patients by genetic healthcare professionals, there will be insufficient capacity to cope with the high patient numbers. In a mainstreaming pathway, non-genetic healthcare professionals (ngHCPs) discuss and order germline genetic testing instead of referring patients to genetic healthcare professionals. We aimed to evaluate the experience of ngHCPs with pre-test genetic counselling and to explore the feasibility from the ngHCPs’ perspective.
Methods
We carried out a prospective cohort study in 15 hospitals in the Netherlands. All participating ngHCPs (i.e. urologists, medical oncologists, specialist nurses and nurse practitioners) completed an online training module of 45 min. The ngHCPs completed a questionnaire both before the training and at three and nine months after it. Paired analyses were used to compare the first with the last questionnaires on attitude, confidence in the ability to discuss and order germline genetic testing, and perceived and actual knowledge of genetics and genetic testing.
Results
167 ngHCPs were invited to participate of whom 69 completed the first questionnaire and started or completed the last one. They had a positive attitude towards offering genetic testing themselves. After nine months of providing pre-test genetic counselling, significantly more ngHCPs considered mainstreaming helpful (94% after versus 81% before, p = 0.01). Both perceived and actual knowledge increased significantly. Pre-test genetic counselling took less than 10 minutes for 82% of ngHCPs and the majority (88%) were in favour of continuing the mainstream pathway. Only six participating ngHCPs considered mainstreaming possible without completing a training module beforehand.
Conclusions
After completing a short online training module, ngHCPs feel well-prepared to discuss germline genetic testing with metastatic prostate cancer patients.
Similar content being viewed by others
Introduction
International guidelines recommend germline genetic testing for men with metastatic prostate cancer (mPCa) [1,2,3]. Pathogenic germline variants found in high-risk cancer predisposition genes may have important consequences for a patient and his relatives, and germline genetic testing should therefore be accessible to all eligible patients. Patients with metastatic castration-resistant prostate cancer and a pathogenic germline or somatic variant in one of the BRCA genes might benefit from treatment with PARP inhibitors [4,5,6]. Patients with a pathogenic germline variant in BRCA2 also have an increased risk of developing breast cancer [7]. In addition, relatives of men with a pathogenic germline variant in one of the genes that are related to breast cancer in women (BRCA1, BRCA2, ATM, CHEK2, PALB2) might benefit from genetic testing of the index patient. They may carry the same variant and are therefore at increased risk of developing cancer, e.g. breast, ovarian, prostate and pancreatic cancer, depending on the gene involved [8,9,10].
In the traditional genetic testing pathway, patients are referred to a genetic healthcare professional who discusses and requests genetic testing. If germline genetic testing were to be carried out in all patients with mPCa, the traditional genetic counselling and testing pathway is not prepared for such large numbers. An alternative approach would be a mainstream genetic testing pathway [11] in which pre-test genetic counselling is provided by a non-genetic healthcare professional (ngHCP) instead of a genetic healthcare professional. Only patients with a pathogenic germline variant or a relevant family history (e.g. breast or ovarian cancer) undergo extensive post-test genetic counselling with a genetic healthcare professional.
The most extensive experience with mainstream genetic testing has been gained in ovarian and breast cancer care, almost always after concise training for the ngHCPs [12,13,14,15,16,17,18,19,20]. So far, there are only three studies that implemented mainstream genetic testing in patients with prostate cancer [21,22,23]. These studies assessed patients’ experience, but only one also assessed the experience in a small group of medical oncologists and fellows [21]. As urologists and nurses (specialists and practitioners) also have key roles in prostate cancer care, it is important that they also endorse this alternative pathway. This has not yet been evaluated.
We therefore implemented a mainstreaming pathway for mPCa patients and aimed 1) to assess the attitudes of urologists, medical oncologists and nurses (specialists and practitioners) towards this pathway; 2) to assess ngHCPs’ knowledge of genetics in prostate cancer and self-efficacy towards discussing and ordering genetic testing; and 3) to determine the feasibility of incorporating the mainstreaming pathway in daily practice from the ngHCPs’ perspective.
Patients and methods
Study procedure
In this prospective cohort study, ngHCPs discussed and ordered germline genetic testing for patients with mPCa. The study was conducted in 15 hospitals in the Netherlands and the study procedures were described in detail in our study protocol [24]. The University Medical Center Utrecht’s Institutional Review Board determined that the Dutch Medical Research Involving Human Subjects Act does not apply to this questionnaire study and official approval from an ethics committee is therefore not required.
To educate ngHCPs about the basics of genetic counselling and genetic testing, they were invited to complete an online training module of 45 min, which included among others a simulated conversation with a patient. An overview of topics in the training module are published priorly in the study protocol [24]. After completion, they had a meeting with the study researcher (MV) to go over the mainstreaming manual. Three questionnaires were sent to ngHCPs; a first questionnaire (T0) before accessing the online training, a second questionnaire to detect practical issues three months after completing the training (T1) and a third questionnaire six months later (T2). Healthcare professionals who did not complete the training within 3 months received two reminders and if they did not respond, an invitation was sent to fill in a short questionnaire (Questionnaire B), adapted from Bokkers et al., to assess their reasons for not attending or not completing the module [12].
Participants
Healthcare professionals (urologists, medical oncologists, specialist nurses and nurse practitioners) were invited if they were involved in prostate cancer care at one of the 15 participating hospitals and did not have a background in clinical genetics.
Outcomes
The study variables for each questionnaire and the origins of the questions or statements used are summarized in Supplementary Table S1. The questionnaires consisted of statements about attitudes towards and self-efficacy of mainstream genetic testing, partially based on previous questionnaires and partially self-developed [12, 16]. In addition, questions about perceived knowledge of genetics and genetic testing, questions about actual knowledge of genetics and genetic testing and questions about the feasibility of mainstream genetic testing (e.g. time investment of mainstreaming and evaluation of online training module) were also partially based on previous questionnaires and partially self-developed [12, 25]. The statements about attitudes, self-efficacy and perceived knowledge were rated using a 5-point Likert scale (strongly disagree to strongly agree). The results of Questionnaire T1 will not be discussed in detail in this paper because it was only used for detecting practical issues.
Statistics
Descriptive statistics were used to describe the ngHCPs’ characteristics and evaluation of feasibility questions. In the statements where a 5-point Likert scale was used and for knowledge questions we performed paired analysis between the T0 and T2 questionnaires with the Wilcoxon signed-rank test. Only ngHCPs who completed both questionnaires (T0 and T2) were included in these analyses. In the 5-point Likert scale, ‘agree’ and ‘strongly agree’ were recoded as positive and the other answers (‘not agree nor disagree’, ‘disagree’ and ‘strongly disagree’) were recoded as negative. To determine whether the ngHCPs included in the paired analysis were a good representation of the entire group, characteristics of ngHCPs, and answers to the T0 questionnaire, were compared between the ngHCPs who did and who did not complete the T2 questionnaire.
Results
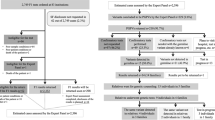
Of the 167 ngHCPs invited, 93 (55%) completed the training module (Fig. 1). Of those, 61 completed the T2 questionnaire in full and 8 ngHCPs started T2 without completing it. Characteristics of participating ngHCPs who completed questionnaire T0 and started or completed T2 are summarized in Table 1. The vast majority (60/69, 87%) of the ngHCPs indicated that they had discussed or requested genetic testing with their patients. Characteristics of non-participants (n = 74) are shown in Supplementary Table S2 and were comparable to those of the participants, except for the significantly higher percentage of disciplines categorized as ‘other’. Twenty-one non-participants filled out Questionnaire B. The 24 ngHCPs who dropped out from the study did not differ significantly in baseline characteristics and answers to the T0 questionnaire from those who filled out the T2 questionnaire (data not shown).
Attitude, self-efficacy, perceived knowledge
Participating ngHCPs had a positive attitude towards offering germline genetic testing themselves, both before implementing the mainstreaming pathway and nine months afterwards, with 88% agreeing or strongly agreeing before and 86% afterwards (Table 2). Seven ngHCPs switched from being positive at T0 to negative at T2 and stated that they did not have sufficient time (n = 2), that it was not one of their tasks (n = 2) because genetic testing yielded too little (n = 1) or for unspecified reasons (n = 2); five switched from being negative to being positive. After implementing the mainstreaming pathway, a significantly larger proportion of ngHCPs deemed it helpful that they could offer genetic testing themselves; 81% agreed or strongly agreed before implementation and 94% afterwards (p = 0.01). Only half of the ngHCPs felt mainstreaming necessary (48% before and 57% after implementing mainstreaming) and the large majority (78% before and 83% afterwards) stated that mainstreaming improved healthcare (p = 0.44). At T2, only 6 out of 61 ngHCPs (10%) considered mainstreaming possible without completing an online training module beforehand; 50 (82%) ngHCPs considered it possible after following an online training module only.
Perceived knowledge significantly increased after nine months of mainstreaming, as well as confidence in discussing the advantages and disadvantages of genetic testing (p = 0.01). Confidence in discussing genetic testing with a prostate cancer patient was high both before (82%) and after (85%) implementing the pathway.
Knowledge
Participating ngHCPs showed a significant increase in knowledge (p < 0.001), with a mean total number of correct answers in the T0 of 10 out of 14 (SD 2.9) and 11 in the T2 (SD 2.0), (Supplementary Table S3). Questions with the largest increase in correct answers had to do with criteria for referring prostate cancer patients to the genetics department in routine healthcare.
Feasibility of mainstream genetic testing
Providing pre-test genetic counselling took less than 10 minutes for the majority (82%) of ngHCPs, which was ‘better or much better than expected’ for 66% of the ngHCPs (Table 3). When taking discussing and ordering genetic testing together, 84% determined it feasible. After nine months’ experience with mainstream genetic testing, 88% (n = 53/60) of ngHCPs were in favour of continuing with the mainstreaming pathway.
Evaluation of supporting materials and reasons for not completing the online training module
Nearly all (n = 60/61, 98%) ngHCPs found it helpful to receive information about genetic testing before providing pre-test genetic counselling. They received information through an online training module of 45 min, which was deemed clear or very clear by a large majority (n = 90/93, 97%) and highly useful (n = 91/93, 98%), (Supplementary Table S4). Additionally, an FAQ form was given, which 34/61 ngHCPs (56%) stated was useful. 54/61 ngHCPs (89%) found it helpful to give written information to their patients after discussing genetic testing.
Of the 21 ngHCPs who did not complete the online training module and filled out Questionnaire B, 13 (62%) did not try to log in, mainly because of time constraints. Three ngHCPs experienced technical problems that prevented them from completing the online training module.
Discussion
To the best of our knowledge, this is the largest study that has examined the experiences of ngHCPs (i.e. urologists, medical oncologists, specialist nurses and nurse practitioners) with mainstream genetic testing in mPCa. We have shown that the majority of these ngHCPs are positive about offering germline genetic testing themselves to patients with mPCa and support continuing the mainstreaming pathway, with an online training module seen as a prerequisite for implementing the mainstreaming pathway. Moreover, ngHCPs are well prepared, have high perceived knowledge and self-efficacy for discussing genetic testing and they consider mainstreaming feasible. This may improve access to germline genetic testing.
Our mainstreaming pathway and online training module are based on prior studies in breast and ovarian cancer [12, 13]. In general, surgical oncologists, gynaecological oncologists and specialist nurses—the main ngHCPs in the studies previously mentioned—have more experience with genetic testing than urologists, the latter being the largest contributors to our study. Patients with mPCa have been eligible for genetic testing in the USA since 2018 [26] and in Europe since 2021 [27], whereas it has been common for breast and ovarian cancer for decades [28]. Despite the limited experience with genetic testing in mPCa, the large majority of ngHCPs are positive about providing pre-test genetic counselling. This is in line with previous studies among ngHCPs in breast and ovarian cancer care [14, 16, 18].
Until now, only three studies have shown data about mainstream genetic testing in prostate cancer [21,22,23] and only one assessed the ngHCPs’ experiences [21]. In two studies, ngHCPs were also trained with a short training course: one training item about germline genetic testing, pre-test counselling and study-specific information consisted of a face-to-face meeting of one hour [21]. The other—about genetic counselling, the importance of genetic testing and the current guidelines—consisted of a presentation of 30–60 min by study team members [23]. Our online training of 45 min consisted of basic genetic knowledge, indications for genetic testing, risks and benefits for patients and relatives and pre-test counselling information; this training was accredited. Our training added a simulated conversation and could be watched again, which quarter of the participating ngHCPs did. It is described in more detail in our study protocol [24]. An online training module was described as a prerequisite for implementing a mainstreaming pathway by nearly all of our participating ngHCPs. A positive influence on the level of knowledge of the online training module was observed, as more questions were answered correctly over time. This could be caused solely by the online training module or also because ngHCPs were more aware of genetics when offering genetic testing themselves. Based on existing scientific literature at the start of the study, we chose to only include patients with mPCa due to the highest prevalence of pathogenic germline variants. However, patients with mPCa, but patients with non-metastatic prostate cancer and relevant family history are also eligible for genetic testing [2]. In our training module, we explained the eligibility criteria for genetic testing for these patients and advised ngHCPs to refer these patients to the genetics department. This could raise awareness about genetics in prostate cancer and non-metastatic prostate cancer patients might therefore benefit as well. However, in the future, these patients may also be included in a mainstreaming pathway.
An interview study on barriers and facilitators of genetic counselling and germline testing in prostate cancer showed that many urologists felt a barrier towards conducting genetic testing due to time constraints [29]. However, in our study, 84% considered the time for discussing and ordering genetic testing feasible. The majority of ngHCPs (82%) were able to discuss genetic testing in less than 10 min and for 66% this was faster than expected. In previous ovarian, prostate and breast cancer studies, discussing genetic testing by ngHCPs was deemed feasible as well, mostly within the timeframe of a standard consultation [11, 14, 16, 18, 21]. The time that ngHCPs need to discuss genetic testing is less than genetic healthcare professionals [30], but this is acceptable as long as patients are given the key information to make an informed choice about whether or not to undergo genetic testing. A previous study comparing breast cancer patients’ experiences with pre-test counselling by ngHCPs or genetic healthcare professionals showed no significant differences [31]. Key topics in pre-test counselling are an explanation of the genes being tested and corresponding cancer risks, as well as the possible implications of genetic testing for a patient and his relatives [11]. To reduce the time required, not all trained ngHCPs need to perform both counselling and requesting genetic testing. We showed that physicians and nurses can divide the tasks (i.e., physicians counsel and a nurse requests or vice versa). In addition to or instead of mainstream genetic testing, other pre-test counselling options are being published or assessed that could reduce the burden on the Genetics department. These include video-based, web-based of telephone-based pre-test counselling [32,33,34].
Limitations
Of the 167 ngHCPs who received an invitation, 93 (56%) completed the first questionnaire and online training module, which may have resulted in response bias. A large proportion (n = 21, 28%) of ngHCPs who did not participate were from ‘other’ disciplines, e.g. residents. They are only at the participating departments for a short period and therefore did not opt for the training. Moreover, not every ngHCP at a participating department needs to conduct mainstream genetic testing, as long as there are a few dedicated ngHCPs per department. It is known from previous studies that 3–5 per centre is sufficient for maintaining the mainstreaming pathway [12, 19,20,21]. In our study, an average of 5 ngHCPs per hospital discussed genetic testing with their patients.
Of the 93 who completed the first questionnaire, 69 ngHCPs (75%) started the last questionnaire and 61 (66%) completed it in full. We compared the answers from the dropouts against the other answers and did not find any significant differences in baseline characteristics, attitude, actual or perceived knowledge, or self-efficacy. It might be possible that response bias affected the results of our study. However, the ngHCPs who dropped out mainly consisted of ngHCPs who included none or only one patient (17 out of 24). Therefore, the result represents the findings among ngHCPs who had the largest experience with mainstream genetic testing.
This article does not cover the experiences of mPCa patients who received pre-test counselling from ngHCPs. However, this will be addressed in a subsequent article.
Conclusion
Non-genetic healthcare professionals were positive towards mainstream genetic testing in patients with metastatic prostate cancer and found that the mainstreaming pathway was feasible. They supported the pathway, but only after online training.
Data availability
The data sets generated during the present study are available from the corresponding author upon reasonable request.
References
Cornford P, Tilki D, van den Bergh R, Briers E, Advocate EP, Eberli D, et al. EAU - EANM - ESTRO - ESUR - ISUP - SIOG: Guidelines on Prostate Cancer (2024).
National Comprehensive Cancer Network. Prostate cancer (Version 4.2024).
Lowrance W, Dreicer R, Jarrard DF, Scarpato KR, Kim SK, Kirkby E, et al. Updates to advanced prostate cancer: AUA/SUO Guideline (2023). J Urol. 2023;209:1082–90.
De Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102.
Fizazi K, Piulats JM, Reaume MN, Ostler P, McDermott R, Gingerich JR, et al. Rucaparib or physician’s choice in metastatic prostate cancer. N Engl J Med. 2023;388:719–32.
Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383:2345–57.
Tai YC, Domchek S, Parmigiani G, Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811–4.
Li S, Silvestri V, Leslie G, Rebbeck TR, Neuhausen SL, Hopper JL, et al. Cancer risks associated with BRCA1 and BRCA2 pathogenic variants. J Clin Oncol. 2022;40:1529–42.
Hall MJ, Bernhisel R, Hughes E, Larson K, Rosenthal ET, Singh NA, et al. Germline pathogenic variants in the ataxia telangiectasia mutated (ATM) gene are associated with high and moderate risks for multiple cancers. Cancer Prev Res. 2021;14:433–40.
Stolarova L, Kleiblova P, Janatova M, Soukupova J, Zemankova P, Macurek L, Kleibl Z. CHEK2 germline variants in cancer predisposition: stalemate rather than checkmate. Cells. 2020;9:2675.
Bokkers K, Vlaming M, Engelhardt EG, Zweemer RP, van Oort IM, Kiemeney LA, et al. The feasibility of implementing mainstream germline genetic testing in routine cancer care—a systematic review. Cancers. 2022;14:1059.
Bokkers K, Bleiker E, Aalfs C, van Dalen T, Velthuizen M, Duijveman P, et al. Surgical oncologists and nurses in breast cancer care are ready to provide pre-test genetic counseling. Ann Surgical Oncol. 2023;30:3248–58.
Bokkers K, Zweemer RP, Koudijs MJ, Stehouwer S, Velthuizen ME, Bleiker E, Ausems MG. Positive experiences of healthcare professionals with a mainstreaming approach of germline genetic testing for women with ovarian cancer. Fam Cancer. 2021;21:1–10.
Colombo N, Huang G, Scambia G, Chalas E, Pignata S, Fiorica J, et al. Evaluation of a streamlined oncologist-led brca mutation testing and counseling model for patients with ovarian cancer. J Clin Oncol. 2018;36:1300–7.
Flaum N, Morgan RD, Burghel GJ, Bulman M, Clamp AR, Hasan J, et al. Mainstreaming germline BRCA1/2 testing in non-mucinous epithelial ovarian cancer in the North West of England. Eur J Hum Genet. 2020;28:1541–7.
George A, Riddell D, Seal S, Talukdar S, Mahamdallie S, Ruark E, et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci Rep. 2016;6:1–8.
Gleeson M, Kentwell M, Meiser B, Do J, Nevin S, Taylor N, et al. The development and evaluation of a nationwide training program for oncology health professionals in the provision of genetic testing for ovarian cancer patients. Gynecol Oncol. 2020;158:431–9.
Kemp Z, Turnbull A, Yost S, Seal S, Mahamdallie S, Poyastro-Pearson E, et al. Evaluation of cancer-based criteria for use in mainstream BRCA1 and BRCA2 genetic testing in patients with breast cancer. JAMA Netw Open. 2019;2:e194428.
Powell CB, Laurent C, Ciaravino G, Garcia C, Han L, Hoodfar E, et al. Streamlining genetic testing for women with ovarian cancer in a Northern California health care system. Gynecol Oncol. 2020;159:221–8.
Yoon S-Y, Wong SW, Lim J, Ahmad S, Mariapun S, Padmanabhan H, et al. Oncologist-led BRCA counselling improves access to cancer genetic testing in middle-income Asian country, with no significant impact on psychosocial outcomes. J Med Genet. 2022;59:220–9.
Scheinberg T, Goodwin A, Ip E, Linton A, Mak B, Smith DP, et al. Evaluation of a mainstream model of genetic testing for men with prostate cancer. JCO Oncol Pract. 2021;17:e204–e216.
Hamilton JG, Symecko H, Spielman K, Breen K, Mueller R, Catchings A. et al. Uptake and acceptability of a mainstreaming model of hereditary cancer multigene panel testing among patients with ovarian, pancreatic, and prostate cancer. Genetics Med. 2021;23:1–9.
Abusamra SM, Solorzano MA, Luke M, Quarles J, Jacobs MF, Das S, et al. Satisfaction with clinician-led germline genetic counseling in patients with prostate cancer. J Urol. 2022;208:1007–17.
Vlaming M, Bleiker EMA, van Oort IM, Kiemeney LALM, Ausems MGEM. Mainstream germline genetic testing in men with metastatic prostate cancer: design and protocol for a multicenter observational study. BMC Cancer. 2022;22:1365.
Claes E, Evers‐Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E. Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet Part A. 2003;116:11–19.
National Comprehensive Cancer Network. Prostate cancer (Version 1.2018).
Mottet N, Cornford P, van den Bergh R, Briers E, De Santis M, Gillessen S. et al. EAU - EANM - ESTRO - ESUR - ISUP - SIOG: Guidelines on Prostate Cancer (2021).
American Society of Clinical Oncology. Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility. J Clin Oncol. 1996;14:1730–6.
Loeb S, Li R, Sanchez Nolasco T, Byrne N, Cheng HH, Becker D, et al. Barriers and facilitators of germline genetic evaluation for prostate cancer. Prostate. 2021;81:754–64.
Eijzenga W, Aaronson NK, Hahn DE, Sidharta GN, van der Kolk LE, Velthuizen ME, et al. Effect of routine assessment of specific psychosocial problems on personalized communication, counselors’ awareness, and distress levels in cancer genetic counseling practice: a randomized controlled trial. J Clin Oncol. 2014;32:2998–3004.
Bokkers K, Bleiker E, Velthuizen M, Koelemij R, Burgmans J, Klinkenbijl J, et al. Patients’ experiences with pre-test genetic counseling provided by breast cancer healthcare professionals: results from a large prospective multicenter study. Breast. 2023;69:349–57.
Russo J, McDougall C, Bowler N, Shimada A, Gross L, Hyatt C, et al. Pretest genetic education video versus genetic counseling for men considering prostate cancer germline testing: a patient-choice study to address urgent practice needs. JCO Precis Oncol. 2021;5:1377–86.
Loeb S, Keith SW, Cheng HH, Leader AE, Gross L, Sanchez Nolasco T, et al. TARGET: a randomized, noninferiority trial of a pretest, patient-driven genetic education webtool versus genetic counseling for prostate cancer germline testing. JCO Precis Oncol. 2024;8:e2300552.
Kwon DH, Gordon KM, Tong B, Borno HT, Beigh M, Fattah D, et al. Implementation of a telehealth genetic testing station to deliver germline testing for men with prostate cancer. JCO Oncol Pract. 2023;19:e773–e783.
Funding
This study was funded by the Dutch Cancer Society (KWF), grant number 12601.
Author information
Authors and Affiliations
Contributions
Concept and design: MV, MA, LK, GS, EB, IvO. Acquisition of data: MV, MA, GS, HvM, MN, DS, HvdP, CW, BW, RH, JvM, BvB, RM, MB, HvdB, DR, BD, IvO. Analysis and interpretation of data: MV, MA, LK, GS, EB, IvO. Drafting of the Manuscript: MV, MA, IvO. Critical revision of the Manuscript for important intellectual content: MA, GS, HvM, MN, DS, HvdP, CW, BW, RH, JvM, BvB, RM, MB, HvdB, DR, BD, IvO. Statistical analysis: MV, MA, IvO. Obtaining funding: MA, LK, EB, IvO. Administrative, technical, or material support: GS. Supervision: MA, IvO.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki. The Medical Ethics Committee of UMC Utrecht ruled that the Dutch Medical Research Involving Human Subjects Act did not apply to this study. Participation in the initial questionnaire prior to the online training module, completion of the online training module, and completion of the final questionnaire were considered as implied consent; therefore, no signature was required for participation in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vlaming, M., Ausems, M.G.E.M., Kiemeney, L.A.L.M. et al. Experience of urologists, oncologists and nurse practitioners with mainstream genetic testing in metastatic prostate cancer. Prostate Cancer Prostatic Dis 28, 789–794 (2025). https://doi.org/10.1038/s41391-024-00925-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-024-00925-w