Abstract
Background
For de-novo oligometastatic prostate cancer (omHSPC) treated with standard of care androgen suppression and prostate radiotherapy, the patterns of progression vis-a-vis index metastatic sites are not well understood.
Methods
This single-centre study included patients with de-novo omHSPC (CHAARTED criteria) staged with a PSMA-PET/CT scan at diagnosis, treated with systemic therapy and prostate radiotherapy, and re-staged with PSMA-PET/CT at biochemical progression. Disease status at index oligometastases was noted at progression.
Results
From 2015 to 2024, 79 patients with omHSPC were found eligible (M1a = 22, M1b = 57). Over a median follow-up of 39 months (IQR 28–69), 15 patients (19%) had disease progression. Restaging PSMA-PET/CT revealed progression of the index oligometastases for 11/15 patients (73%), with additional metastases in 7 of these.
Conclusion
The high proportion of progression at the index oligometastases supports the potential benefit of metastasis-directed therapy for local ablation.
Similar content being viewed by others
Introduction
De novo oligometastatic hormone-sensitive prostate cancer (omHSPC) is typically treated with a combination of lifelong androgen deprivation therapy (ADT), androgen receptor pathway inhibitors (ARPI), and radiotherapy to the prostate. The role of metastasis-directed therapy (MDT) is not established yet. The Advanced Prostate Cancer Consensus Conference (APCCC) 2024 expert panel did not reach consensus on utilisation of MDT for de-novo omHSPC, with only 17% experts favouring MDT based on conventional imaging and 57% voting for additional next-generation imaging before deciding about MDT [1]. Prostate-specific membrane antigen (PSMA) based imaging (such as PSMA-PET/CT) is now preferred for more accurate disease staging and localisation of smaller metastases [1, 2]. Currently, MDT is not recommended by most global guidelines, and its utilisation is discretionary [1].
Key trials in omHSPC, such as STAMPEDE and PEACE-1 have reported on the benefit of prostate radiotherapy in omHSPC, staged with conventional imaging [3, 4]. Details of the patterns of progression are not known, which would help understand the potential role of MDT for the index oligometastases. Therefore, we explored the institutional cohort of PSMA-staged de novo omHSPC treated with ADT and prostate radiotherapy, for sites of disease progression in consideration of the index metastatic lesions.
Materials and Methods
This IRB-approved study queried a prospectively maintained database at a large tertiary cancer care centre. We included patients diagnosed with de novo omHSPC, staged with PSMA-PET/CT at diagnosis, and fulfilling the CHAARTED criteria - disease in non-regional lymph nodes or <5 bone metastases, in the absence of visceral metastases [5]. All patients were treated with standard of care lifelong systemic therapy and radiotherapy to the prostate to a dose of 36.25 Gy/5Fr, without MDT. Follow up was based on PSA, and all patients with biochemical or clinical signs of progression underwent a restaging PSMA-PET/CT. Biochemical recurrence was defined as per the Phoenix definition of a rise of >2 ng/mL over the nadir PSA. Patients with high volume disease, those with castration resistant disease prior to radiotherapy, or those with diagnostic or restaging scans unavailable, were excluded. PET/CT acquisition was done on Philips Medical Systems, GEMINI TOF 64. Tracer was administered intravenously 60 min before the study and at a dose of 67–111 MBq. CT was acquired in caudocranial direction with parameters of slice thickness‑2 mm, pitch‑0.83, voltage 120 kV, FOV 600 mm, rotation time‑0.5 s, automated mA, and image matrix‑512 × 512. Eighty milliliters of low‑osmolar nonionic intravenous contrast was administered in all eligible patients at a rate of 1.8 ml/s, and scan delay was 50 s. All PET scans were acquired in 3D‑mode with an acquisition time of 2 min per bed position. Images were reconstructed iteratively using RAMLA algorithm. Images were viewed on display system having extended brilliance workspace software version 4.5.3.40140 (Philips Healthcare, Cleveland, Ohio, USA). The individual sites of disease recurrence in the restaging scans were mapped with respect to the index site of metastases in the index scan by nuclear medicine physicians with over 5 years of experience in reporting PSMA-PET/CTs at a high-volume tertiary care cancer centre. The PSMA PET Progression (PPP) [6] criteria were utilised to determine if a patient had disease progression. Known prognostic factors - ISUP grade group (4–5 vs 1–3), nodal involvement (N1 vs N0), bone metastases (M1b vs M1a), systemic intensification (intensified ADT vs ADT alone), 6-month nadir PSA ( ≤ 0.1 ng/mL vs higher) - were evaluated for risk of progression of the index metastasis by calculating odds ratio, using binary logistic regression. A p-value of < 0.05 was considered statistically significant. Data was analysed using IBM SPSS v21.0.
Results
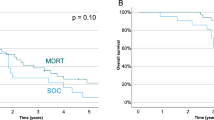
A total of 79 eligible patients treated from January 2015 to January 2024 were included (Table 1). Majority (82%) had ISUP grade group 4 or 5 disease. Bony metastases were present in 72% of patients. About 75% received ADT intensified with either ARPI or docetaxel. At a median follow-up of 39 months (IQR 28–69), 19% patients (n = 15) had either biochemical or clinical progression to castration-resistant prostate cancer (CRPC). Median time to progression was 28 months (IQR 24–37) from the start of ADT. Median PSA at progression was 3.16 ng/ml (IQR 1.71 – 6.0). Restaging PSMA-PET/CT showed visible progression at the index metastases in 11/15 (73.3%) patients. Four patients (26.7%) had progression limited to the index metastatic site only, while 7 (46.6%) showed progression in the index site with new metastatic lesions [Fig. 1]. PSMA uptake was seen at the prostate and N1 regional nodal areas for 5 patients each in the restaging PSMA PETCT. All the patients who had PSMA uptake at the prostate had concurrent disease progression seen at an extra-prostatic site as well. None of the prognostic factors showed statistically significant association with progression of index metastases [Table 2].
Discussion
To our knowledge, this is the first study to report patterns of recurrence for PSMA-staged omHSPC treated with ADT and prostate radiotherapy without MDT. At progression, restaging PSMA-PET/CT showed progression of index metastases in 75% of the patients, with about a quarter of the patients having progression only at the index site. This suggests a potentially important role of MDT for local control at the site of index oligometastases.
Although without robust evidence, the argument in favour of adding MDT to prostate radiotherapy in omHSPC can be threefold. First, very high control rates exceeding 90% have been demonstrated with ablative radiotherapy for metastases, with minimal added toxicities [7,8,9,10]. Next line systemic therapy was reported to be safely deferred by using MDT in these non-randomised studies, though PSMA-based imaging was not used. Secondly, there might be a clinical advantage of treating the metastases in the de novo omHSPC setting rather than the metachronous setting, supporting total ablation of visible de novo oligometastases [8]. Finally, ablation of index lesions in addition to prostate radiotherapy may disrupt polyclonal seeding of further metastases [11]. Overall, the potential benefit of MDT would likely depend on reliably mapping the oligometastases with PSMA-based imaging, as well as effective ablation of putative hormone-resistant disease in these lesions.
Acknowledging the inherent limitations of a small cohort from a single centre, our results are strengthened by PSMA-based staging at diagnosis and recurrence, formal review of all scans by experienced nuclear medicine physicians, and a prospectively maintained database. While the value of MDT is under evaluation by the phase III STAMPEDE-2 randomised trial, this study provides an interesting insight into the progression of de novo omHSPC after systemic therapy and prostate-only radiotherapy. The high proportion of progression at the index site of metastases (75%), without additional metastases in a third of these, suggests a potential role of ablation of oligometastases to optimize overall disease control.
Data availability
The datasets generated and analysed during the current study are not publicly available due to institutional policy regarding patient privacy, but are available from the corresponding author on reasonable request.
References
Gillessen S, Turco F, Davis ID, Efstathiou JA, Fizazi K, James ND, et al. Management of Patients with Advanced Prostate Cancer. Report from the 2024 Advanced Prostate Cancer Consensus Conference (APCCC). Eur Urol. 2025;87:157–216. https://doi.org/10.1016/j.eururo.2024.09.017.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16. https://doi.org/10.1016/S0140-6736(20)30314-7.
Parker CC, James ND, Brawley CD, Clarke NW, Ali A, Amos CL, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Med. 2022;19:e1003998 https://doi.org/10.1371/journal.pmed.1003998.
Bossi A, Foulon S, Maldonado X, Sargos P, MacDermott R, Kelly P, et al. Efficacy and safety of prostate radiotherapy in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2024;404:2065–76. https://doi.org/10.1016/S0140-6736(24)01865-8.
Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36:1080–7. https://doi.org/10.1200/JCO.2017.75.3657.
Fanti S, Hadaschik B, Herrmann K. Proposal for Systemic-Therapy Response-Assessment Criteria at the Time of PSMA PET/CT Imaging: The PSMA PET Progression Criteria. J Nucl Med. 2020;61:678–82. https://doi.org/10.2967/jnumed.119.233817.
Deodato F, Pezzulla D, Cilla S, Ferro M, Romano C, Bonome P, et al. Stereotactic radiosurgery for bone metastases in oligometastatic prostate cancer patients: DESTROY-2 clinical trial subanalysis. Clin Transl Oncol. 2022;24:1177–83. https://doi.org/10.1007/s12094-021-02764-w.
Qi X, Li HZ, Gao XS. Radiotherapy of the Primary Tumor and All Metastatic Lesions in Oligometastatic Prostate Cancer: 5-Year Results of Prolong Study. Int J Radiat Oncol. 2024;120:S147 https://doi.org/10.1016/j.ijrobp.2024.07.2156.
Nickols NG, Tsai S, Kane N, Tran S, Ghayouri L, Diaz-Perez S, et al. Systemic and Tumor-directed Therapy for Oligometastatic Prostate Cancer: The SOLAR Phase 2 Trial in De Novo Oligometastatic Prostate Cancer. Eur Urol. 2024;86:190–3. https://doi.org/10.1016/j.eururo.2024.02.008.
Reverberi C, Massaro M, Osti MF, Anzellini D, Marinelli L, Montalto A, et al. Local and metastatic curative radiotherapy in patients with de novo oligometastatic prostate cancer. Sci Rep. 2020;10:17471. https://doi.org/10.1038/s41598-020-74562-3.
Warner EW, Van Der Eecken K, Murtha AJ, Kwan EM, Herberts C, Sipola J, et al. Multiregion sampling of de novo metastatic prostate cancer reveals complex polyclonality and augments clinical genotyping. Nat Cancer. 2024;5:114–30. https://doi.org/10.1038/s43018-023-00692-y.
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Contributions
VM conceptualized the study. VM, PM, PV, GSK, and NM were involved in patient accrual, data acquisition, and study procedures. SC, SG, and AA provided technical and material support. PV and PM did a statistical analysis. The manuscript was drafted by PV, VM, and PM. All authors approved the final manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
We confirm that all methods were performed in accordance with the relevant guidelines and regulations. Ethical approval was taken from the Institutional Ethical Committee of Tata Memorial Hospital, Mumbai. No informed consent was taken as the Ethics Committee granted wavier.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Verma, P., Maitre, P., Krishnasamy, G.S. et al. Progression of index metastases in oligometastatic hormone-sensitive prostate cancer: implications for metastases directed therapy?. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-025-01056-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41391-025-01056-6