Abstract
Objective
To evaluate the therapeutic efficacy and clinical applicability of the novel P100 extracorporeal shock wave therapy (ESWT) device in the treatment of type IIIB chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).
Methods
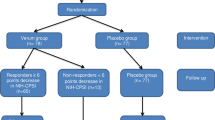
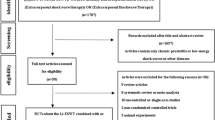
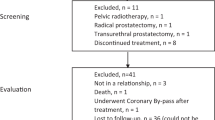
In this randomized, single-blind, sham-controlled trial, 83 patients with type IIIB CP/CPPS were enrolled and randomly assigned to either the P100 treatment group (n = 51) or the control group (n = 32). Patients in the treatment group received four weekly low-intensity ESWT sessions (0.2 mJ/mm²), while the control group received identical procedures with shock transmission blocked. The primary endpoint was the clinical response rate (≥6-point reduction in NIH-CPSI score) at week 4; week 8 outcomes were further analyzed to assess sustained efficacy. Secondary endpoints included IPSS, IIEF-5, and VAS scores.
Results
At week 4, the clinical response rate was 78.4% in the P100 group compared with 25% in the control group (P < 0.001). Median NIH-CPSI scores decreased from 35 at baseline to 13 at week 4 and 12 at week 8, indicating sustained improvement. Significant reductions in PDS, IPSS, and VAS scores were observed as early as week 2 (P < 0.05), and symptom relief remained stable through week 8 without rebound. Exploratory analyses suggest that lower baseline estradiol levels and lower E2/T ratios may be associated with more sustained improvements in erectile function. No treatment-related adverse events were reported.
Conclusion
The P100 ESWT device provided rapid, significant, and sustained symptom relief for type IIIB CP/CPPS, particularly in pain and urinary domains. Hormonal balance (E2/T) may influence the long-term maintenance of erectile function after ESWT. These findings support P100 as a safe and effective non-invasive therapeutic option for CP/CPPS, warranting further validation in larger studies with longer-term follow-up.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All raw data and informed consent for this study are available upon request from corresponding authors.
References
Auersperg V, Trieb K. Extracorporeal shock wave therapy: an update. EFORT Open Rev. 2020;5:584–92. https://doi.org/10.1302/2058-5241.5.190067.
Liu Y, Chen X, Guo A, Liu S, Hu G. Quantitative assessments of mechanical responses upon radial extracorporeal shock wave therapy. Adv Sci. 2018;5:1700797. https://doi.org/10.1002/advs.201700797.
Wess O, Mayer J. The interaction of shock waves with biological tissue—momentum transfer, the key for tissue stimulation and fragmentation. Int J Surg. 2025;111:2810–8. https://doi.org/10.1097/js9.0000000000002261.
Sun H, Chen H, Mu G, Fu H, Yue L. Comparison of different treatment regimens of extracorporeal shockwave therapy in chronic low-back pain: a randomized controlled trial. Pain physician. 2022;25:E1211–e1218.
Porst H. Review of the current status of low intensity extracorporeal shockwave therapy (Li-ESWT) in Erectile Dysfunction (ED), Peyronie’s Disease (PD), and sexual rehabilitation after radical prostatectomy with special focus on technical aspects of the different marketed ESWT devices including personal experiences in 350 patients. Sex Med Rev. 2021;9:93–122. https://doi.org/10.1016/j.sxmr.2020.01.006.
Capogrosso P, Frey A, Jensen CFS, Rastrelli G, Russo GI, Torremade J, et al. Low-intensity shock wave therapy in sexual medicine-clinical recommendations from the european society of sexual medicine (ESSM). J Sex Med. 2019;16:1490–505. https://doi.org/10.1016/j.jsxm.2019.07.016.
Vardi Y, Appel B, Jacob G, Massarwi O, Gruenwald I. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58:243–8. https://doi.org/10.1016/j.eururo.2010.04.004.
Kitrey ND, Gruenwald I, Appel B, Shechter A, Massarwa O, Vardi Y. Penile low intensity shock wave treatment is able to shift PDE5i nonresponders to responders: a double-blind, sham controlled study. J Urol. 2016;195:1550–5. https://doi.org/10.1016/j.juro.2015.12.049.
Kennady EH, Bryk DJ, Ali MM, Ratcliffe SJ, Mallawaarachchi IV, Ostad BJ, et al. Low-intensity shockwave therapy improves baseline erectile function: a randomized sham-controlled crossover trial. Sex Med. 2023;11:qfad053. https://doi.org/10.1093/sexmed/qfad053.
Mykoniatis I, Pyrgidis N, Sokolakis I, Ouranidis A, Sountoulides P, Haidich AB, et al. Assessment of combination therapies vs monotherapy for erectile dysfunction: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:e2036337 https://doi.org/10.1001/jamanetworkopen.2020.36337.
Kalyvianakis D, Mykoniatis I, Pyrgidis N, Kapoteli P, Zilotis F, Fournaraki A, et al. The Effect of low-intensity shock wave therapy on moderate erectile dysfunction: a double-blind, randomized, sham-controlled clinical trial. J Urol. 2022;208:388–95. https://doi.org/10.1097/ju.0000000000002684.
Lu Z, Lin G, Reed-Maldonado A, Wang C, Lee YC, Lue TF. Low-intensity extracorporeal shock wave treatment improves erectile function: a systematic review and meta-analysis. Eur Urol. 2017;71:223–33. https://doi.org/10.1016/j.eururo.2016.05.050.
Karakose A, Yitgin Y. A new alternative approach to management of acute phase Peyronie’s disease: low intensity extracorporeal shockwave therapy and platelet-rich plasma. Minerva Urol Nephrol. 2024;76:367–72. https://doi.org/10.23736/s2724-6051.23.05458-7.
Sokolakis I, Pyrgidis N, Lahme S, Hatzichristodoulou G. Low-intensity shockwave therapy in Peyronie’s disease: long-term results from a prospective, randomized, sham-controlled trial. Int J Impot Res. 2022;34:487–94. https://doi.org/10.1038/s41443-021-00447-2.
Krieger JR, Rizk PJ, Kohn TP, Pastuszak A. Shockwave therapy in the treatment of Peyronie’s disease. Sex Med Rev. 2019;7:499–507. https://doi.org/10.1016/j.sxmr.2019.02.001.
Yu X, Gao Q. Multidisciplinary diagnosis and treatment guideline for chronic prostatitis with integrated Chinese and Western medicine. Chin J Men’s Sci. 2020;26:369–76. https://doi.org/10.13263/j.cnki.nja.2020.04.015.
Khan FU, Ihsan AU, Khan HU, Jana R, Wazir J, Khongorzul P, et al. Comprehensive overview of prostatitis. Biomed Pharmacother. 2017;94:1064–76. https://doi.org/10.1016/j.biopha.2017.08.016.
Liang ZZ, Xia SJ, Deng CH. Chinese expert consensus on prostatic pelvic syndrome. J Mod Urol. 2024;29:756–61.
Krieger JN, Nyberg L, Nickel JC. NIH consensus definition and classification of prostatitis [5]. JAMA. 1999;282:236–7. https://doi.org/10.1001/jama.282.3.236.
Schaeffer AJ. Chronic prostatitis and the chronic pelvic pain syndrome. N Engl J Med. 2006;355, https://doi.org/10.1056/NEJMcp060423.
Litwin MS, McNaughton-Collins M, Fowler FJ Jr., Nickel JC, Calhoun EA, Pontari MA, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collab Res Netw J Urol. 1999;162:369–75. https://doi.org/10.1016/s0022-5347(05)68562-x.
Cheah PY, Liong ML, Yuen KH, Teh CL, Khor T, Yang JR, et al. Terazosin therapy for chronic prostatitis/chronic pelvic pain syndrome: a randomized, placebo controlled trial. J Urol. 2003;169:592–6. https://doi.org/10.1097/01.ju.0000042927.45683.6c.
Qin Z, Zhang C, Guo J, Kwong JSW, Li X, Pang R, et al. Oral pharmacological treatments for chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis of randomised controlled trials. EClinicalMedicine. 2022;48:101457. https://doi.org/10.1016/j.eclinm.2022.101457.
Zhang K, Zhang Y, Hong S, Cao Y, Liu C. Comparative analysis of efficacy of different combination therapies of α-receptor blockers and traditional Chinese medicine external therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: Bayesian network meta-analysis. PLoS ONE. 2023;18:e0280821 https://doi.org/10.1371/journal.pone.0280821.
Sakr AM, Fawzi AM, Kamel M, Ali MM. Outcomes and clinical predictors of extracorporeal shock wave therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: a prospective randomized double-blind placebo-controlled clinical trial. Prostate Cancer Prostatic Dis. 2022;25:93–9. https://doi.org/10.1038/s41391-021-00464-8.
Mykoniatis I, Pyrgidis N, Sokolakis I, Sountoulides P, Hatzichristodoulou G, Apostolidis A, et al. Low-intensity shockwave therapy for the management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and meta-analysis. BJU Int. 2021;128:144–52. https://doi.org/10.1111/bju.15335.
Li G, Man L. Low-intensity extracorporeal shock wave therapy for male chronic pelvic pain syndrome: a systematic review and meta-analysis. Transl Androl Urol. 2021;10:1202–11. https://doi.org/10.21037/tau-20-1423.
Wagenlehner FM, van Till JW, Magri V, Perletti G, Houbiers JG, Weidner W, et al. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. Eur Urol. 2013;63:953–9. https://doi.org/10.1016/j.eururo.2012.10.042.
Propert KJ, Litwin MS, Wang Y, Alexander RB, Calhoun E, Nickel JC, et al. Responsiveness of the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI). Qual Life Res. 2006;15:299–305. https://doi.org/10.1007/s11136-005-1317-1.
Karakus S, Musicki B, Burnett AL. Mirabegron improves erectile function in men with overactive bladder and erectile dysfunction: a 12-week pilot study. Int J Impot Res. 2022;34:588–92. https://doi.org/10.1038/s41443-021-00455-2.
Fojecki GL, Tiessen S, Osther PJS. Effect of low-energy linear shockwave therapy on erectile dysfunction—a double-blinded, sham-controlled, randomized clinical trial. J Sex Med. 2017;14:106–12. https://doi.org/10.1016/j.jsxm.2016.11.307.
Tang P, Wen T, Lu W, Jin H, Pan L, Li H, et al. The efficacy of extracorporeal shock wave therapy for knee osteoarthritis: an umbrella review. Int J Surg (Lond, Engl). 2024;110:2389–95. https://doi.org/10.1097/js9.0000000000001116.
Guu SJ, Liu CC, Juan YS, Li CC, Tsai CC. The 12-month follow-up of the low-intensity extracorporeal shockwave therapy in the treatment of patients with chronic pelvic pain syndrome refractory to 3-As medications. Aging Male. 2020;23:793–800. https://doi.org/10.1080/13685538.2019.1597341.
Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, Wang Y, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102:1161–73. https://doi.org/10.1210/jc.2016-2935.
Punjani N, Bernie H, Salter C, Flores J, Benfante N, Mulhall JP. The utilization and impact of aromatase inhibitor therapy in men with elevated estradiol levels on testosterone therapy. Sex Med. 2021;9:100378 https://doi.org/10.1016/j.esxm.2021.100378.
Gravas S, De Nunzio C, Campos Pinheiro L, Ponce de León J, Skriapas K, Milad Z, et al. Development and validation of a clinical nomogram to predict prostatic inflammation in men with lower urinary tract symptoms. Prostate Cancer Prostatic Dis. 2025;28:405–10. https://doi.org/10.1038/s41391-024-00857-5.
Wang HJ, Cheng JH, Chuang YC. Potential applications of low-energy shock waves in functional urology. Int J Urol. 2017;24:573–81. https://doi.org/10.1111/iju.13403.
Fischer S, Mueller W, Schulte M, Kiefer J, Hirche C, Heimer S, et al. Multiple extracorporeal shock wave therapy degrades capsular fibrosis after insertion of silicone implants. Ultrasound Med Biol. 2015;41:781–9. https://doi.org/10.1016/j.ultrasmedbio.2014.10.018.
Fode M, Russo GI, Verze P. Therapeutic areas of Li-ESWT in sexual medicine other than erectile dysfunction. Int J Impot Res. 2019;31:223–30. https://doi.org/10.1038/s41443-019-0114-2.
Cai T, Tamanini I, Odorizzi K, Gallelli L, Lanzafame M, Mazzoli S, et al. The diagnostic yield of the Meares & Stamey test can be significantly improved by symptom-based patient selection and the experience of the test performer. Prostate Cancer Prostatic Dis. 2024;27:300–4. https://doi.org/10.1038/s41391-024-00824-0.
Ma Y, Zhu T, Yang P, Gao R, Shen L, Gao P, et al. The neurological decline and psychological factors caused by coronavirus disease 2019 may be predictors of erectile dysfunction. Andrology. 2024;12:1851–6. https://doi.org/10.1111/andr.13613.
Xu J, Chen Y, Gu L, Liu X, Yang J, Li M, et al. Hypothalamic-pituitary-adrenal axis activity and its relationship to the autonomic nervous system in patients with psychogenic erectile dysfunction. Front Endocrinol. 2023;14:1103621. https://doi.org/10.3389/fendo.2023.1103621.
Liang X, Wang Z, Wang S, Ruan F, Zhang Y, Shao D, et al. Magnetic mesoporous silica nanoparticles loaded with peptides for the targeted repair of cavernous nerve injury underlying erectile dysfunction. Biomaterials. 2025;314:122811. https://doi.org/10.1016/j.biomaterials.2024.122811.
Huang WL, Tung SY, Tseng CS, Wang TD, Lee WJ, Chen JH, et al. The flow index provides a comprehensive assessment of erectile dysfunction by combining blood flow velocity and vascular diameter. Sci Rep. 2022;12:16099. https://doi.org/10.1038/s41598-022-19364-5.
Fojecki GL, Tiessen S, Osther PJ. Extracorporeal shock wave therapy (ESWT) in urology: a systematic review of outcome in Peyronie’s disease, erectile dysfunction and chronic pelvic pain. World J Urol. 2017;35:1–9. https://doi.org/10.1007/s00345-016-1834-2.
Sokolakis I, Hatzichristodoulou G. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: a systematic review and meta-analysis of randomised controlled trials. Int J Impot Res. 2019;31:177–94. https://doi.org/10.1038/s41443-019-0117-z.
Yao H, Wang X, Liu H, Sun F, Tang G, Bao X, et al. Systematic review and meta-analysis of 16 randomized controlled trials of clinical outcomes of low-intensity extracorporeal shock wave therapy in treating erectile dysfunction. Am J Mens Health. 2022;16:15579883221087532. https://doi.org/10.1177/15579883221087532.
Sontag A, Ni X, Althof SE, Rosen RC. Relationship between erectile function and sexual self-confidence: a path analytic model in men being treated with tadalafil. Int J Impot Res. 2014;26:7–12. https://doi.org/10.1038/ijir.2013.31.
Pan D, Xu ZH, Gao Q, Li M, Guan Y, Zhao ST. Relationship between penile erection and the ratio of estradiol to testosterone: a retrospective study. Andrologia. 2020;52:e13701. https://doi.org/10.1111/and.13701.
Fukuma N, Tokiwa H, Numata G, Ueda K, Liu PY, Tajima M, et al. Endothelial oestrogen-myocardial cyclic guanosine monophosphate axis critically determines angiogenesis and cardiac performance during pressure overload. Cardiovasc Res. 2024;120:1884–97. https://doi.org/10.1093/cvr/cvae202.
Shi V, Morgan EF. Estrogen and estrogen receptors mediate the mechanobiology of bone disease and repair. Bone. 2024;188:117220. https://doi.org/10.1016/j.bone.2024.117220.
Sun Y, Leng P, Guo P, Gao H, Liu Y, Li C, et al. G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1. Mol Med. 2021;27:96 https://doi.org/10.1186/s10020-021-00360-w.
Wang N, Lu Y, Rothrauff BB, Zheng A, Lamb A, Yan Y, et al. Mechanotransduction pathways in articular chondrocytes and the emerging role of estrogen receptor-α. Bone Res. 2023;11:13 https://doi.org/10.1038/s41413-023-00248-x.
Acknowledgements
We would like to express our gratitude to Wikkon Precision Technologies for developing and providing the new device, as well as for the training and technical support during the pilot phase. At the same time, we are very grateful for the company’s support in allowing us to independently conduct the subsequent clinical trial, which ensured the reliability and fairness of this study. To ensure the independence and transparency of the study, all participants adhered to strict conflict of interest management procedures throughout the research process. The study design, data collection, analysis, and interpretation of results were conducted solely by the research team, with no intervention or influence from the sponsor. Furthermore, no direct or indirect financial support was received from the sponsor by any of the researchers during the study. All potential conflicts of interest and ethical considerations have been disclosed in the manuscript. The results and conclusions of this study are entirely based on data and scientific evidence, and the research team ensures that all findings are independent of the sponsor.
Funding
The study was funded by the Program for Research-oriented Physicians of Shanghai Tenth People’s Hospital (grant number 2023YJXYSA016).
Author information
Authors and Affiliations
Contributions
HZ, WS, and JN contributed to the study conception and design, and data acquisition. HoZ, ZJ, GY, and YZ performed the data analysis and interpretation. HZ and WS drafted the manuscript. KW, YC, and BP provided critical revision of the manuscript for important intellectual content and supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Peng Bo reports that the project was funded by the Shanghai Tenth People’s Hospital’s Research Physician Programme and technical support for the P100 Shockwave Therapy Instrument from Wikkon Precision Technologies. To ensure the independence and transparency of the study, all participants adhered to strict conflict of interest management procedures throughout the research process. The study design, data collection, analysis, and interpretation of results were conducted solely by the research team, with no intervention or influence from the sponsor. Furthermore, no direct or indirect financial support was received from the sponsor by any of the researchers during the study. All potential conflicts of interest and ethical considerations have been disclosed in the manuscript. The results and conclusions of this study are entirely based on data and scientific evidence, and the research team ensures that all findings are independent of the sponsor. There are no disputes of interest and we would like to express our gratitude.
Consent to participate and consent to publish
All authors of this article participated in the study and agreed to the article being submitted to the journal, and a signed statement from all authors can be uploaded if required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Song, W., Ni, J. et al. Efficacy of the new P100 extracorporeal shock wave therapy device in the treatment of type IIIB chronic prostatitis/chronic pelvic pain syndrome: a sham treatment controlled, prospective clinical trial. Prostate Cancer Prostatic Dis (2026). https://doi.org/10.1038/s41391-026-01072-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41391-026-01072-0