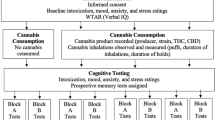
Abstract
Impaired decision-making is often seen in people with bipolar disorder (BD), even those undergoing treatment. Targeted therapeutics are therefore needed. People with BD report that cannabis use (CU) attenuates such cognitive and behavioral symptoms. We hypothesized that 1) people with BD who do not use cannabis would exhibit poor decision-making and functional capacity relative to healthy comparison (HC) participants and 2) CU in people with BD would be associated with decision-making and functional capacity comparable to that of HC participants who do not use cannabis. HC and BD participants that either reported regular (≥4x/weekly) CU or no-CU were recruited (n = 87). Participants were tested on decision-making and functional capacity using the Iowa Gambling Task and UCSD Performance-based skills assessment (UPSA-2), respectively. CU was associated with impaired decision-making in healthy participants while CU in participants with BD was associated with better decision-making than their non-using counterparts and equivalent to decision-making in non-CU HC participants. Additionally, CU in people with BD was associated with UPSA-2 scores comparable to non-CU HC participants. Studies are needed to determine whether cannabinoid-related treatments improve such decision-making and function in people with BD.
Similar content being viewed by others
Introduction
People with bipolar disorder (BD) often struggle with deficits in goal-directed behaviors such as decision-making and inhibitory control. These behavioral and cognitive deficits can often lead to disruptions in social, occupational, and family life, as well as poor health outcomes [1]. Identifying mechanisms underlying these deficits may enable the development of targeted treatments, thereby improving the lives of people with BD. Self-medication hypotheses of drug use in people with BD (i.e., the premise that these individuals use certain drugs to manage symptoms), reveal potential avenues of research. For example, cannabis use (CU) is exceptionally prevalent among people with BD, with over 70% reporting a lifetime history of regular CU versus 26% of the general population [2]. Prevalence of cannabis use continues to increase as its legalization and availability continues to grow. People with BD consistently report using cannabis to ameliorate cognitive and behavioral symptoms such as racing thoughts and hyperactivity [3,4,5]. Not surprisingly, cannabis is the most commonly used drug among people with BD [6].
Regular CU is often associated with cognitive deficits and risk-taking behaviors however [7, 8], calling into question its utility for self-medication and raising concerns for potential exacerbation of cognitive deficits in people with BD. This contrast in potential effects in people with BD was highlighted by a recent review by our group [9], which identified two studies reporting associations between CU and improved cognitive performance [10, 11], one study reporting an association with poorer cognitive performance [12], and three studies reporting no associations [13,14,15]. The markedly different criteria for CU and BD status, as well as the cognitive domains tested, may have contributed to these equivocal results. Nevertheless, considering the equivocal results of our review and continued patient reports of self-medication, more research is needed to better understand the relationship between CU and cognitive functions in people with BD. This need is additionally underscored by the increase in therapeutic CU concomitant with cannabis legalization across the United States [16]. An overall increase in CU [16], combined with its potential for improving cognition in BD, highlights the importance of research investigating cognitive domains in this population.
Decision-making is among the most critically affected cognitive domains in BD, with deficits being evident at all stages of the disorder [17]. Deficits in decision-making likely contribute to increased engagement in risky and impulsive behaviors characteristic of people with BD. While decision-making is a broad cognitive domain impacting everyday functioning, numerous tasks exist that can assess such functions in laboratory settings such as the Iowa Gambling Task (IGT), which has real-world significance [18]. While many clinical populations exhibit poor performance in the IGT, their performance is driven by different decision-making strategies, e.g., high-risk high-reward preference in people with BD versus elevated punish-sensitivity in people with depression [19]. A major advantage of the IGT is that it can be translated across species, thus enabling investigation of mechanisms underlying elevated risk preference in rodent models relevant to BD [20,21,22]. We previously demonstrated that mice with reduced expression of the dopamine transporter (DAT) exhibit deficient decision-making in the IGT as seen in people with BD, i.e., elevated overall risk preference driven by more frequent choice of risky options following receipt of small, high-probability rewards (elevated safe win-stay) [18]. Further, reduced DAT function in mice results in behavior consistent with people with BD including hyper-exploration [23, 24], inattention [25], and poor decision-making in the IGT [20, 26, 27]. Reduced DAT expression (from positron emission studies) was seen in unmedicated people with BD euthymia [28] and mania [29], and may arise from polymorphisms in the DAT gene [24] associated with BD [30, 31]. These mechanistic and behavioral links enable future translational and treatment studies using animals.
Reduced DAT expression drives hyperdopaminergia [32] which may play a role in BD [27,28,29, 33,34,35]. The primary constituent of cannabis, delta-9-tetrahydrocannabinol (THC), affects dopamine through indirect DAT interactions, particularly in brain regions that control cognitive functions affected in BD [36]. Acute THC administration activates the endocannabinoid (eCB) system to promote dopamine release [37]. On the other hand, chronic THC reduces dopamine transmission as revealed by changes in dopamine D2/3 receptor signaling in non-human primates [38] and rodents [39]. Dopaminergic signaling plays a critical role in cognitive function, including decision-making; thus, chronic CU may exert a unique effect on aberrant dopaminergic signaling in BD, potentially resulting in the observed downstream changes in cognitive function and behavior.
Here, we sought to determine whether chronic CU was differentially associated with decision-making in people with BD versus healthy comparison (HC) participants. First, we investigated the association between CU and cognitive function in BD by assessing 4 groups: HC, HC + CU, BD, and BD + CU on the IGT. Next, to better understand the effects of CU on real-world functional behavior, participants were also tested in the UCSD Performance-Based Skills Assessment (UPSA-2), a role-play test designed to evaluate a person’s functional capacity in selected areas. We hypothesized that people with BD who do not use cannabis would exhibit poorer decision-making and functional capacity compared to non-CU HC participants. In contrast, we hypothesized that CU in BD would be associated with decision-making and functional capacity comparable to non-CU HC participants.
Patients and methods
Participants
87 participants (18–50 years old) were recruited from the local area (San Diego, CA) via social media campaigns, online advertisements, and flyers posted in local coffee shops, libraries, bus stops, and community centers. 50 were healthy comparison (HC) participants who had never met DSM-5 criteria for any Axis I psychiatric disorder and the remaining 37 participants were previously diagnosed for any type of BD (e.g., BD I, BD II, cyclothymic, etc.). Diagnoses were confirmed through assessment by trained research staff using the SCID RV/NP (Version 1.0.0). The SCID RV was used to confirm BD diagnosis in our clinical cohort, whereas the NP version was used to assess the healthy comparison cohort to ensure the absence of any exclusionary conditions. Self-report data were collected on current medication use; however, data was limited on other forms of BD treatment (e.g., previous therapy and self-help group attendance, etc.). BD participants were excluded if they reported clinically severe mood symptoms at the time of testing (Young Mania Rating Score >20 [40] and Hamilton Depression Scale> 23 [41]). Participants were also excluded for: (1) current alcohol or substance use disorder (excluding mild, moderate or severe cannabis use disorder for CU groups); (2) a history of neurological conditions, head trauma, or seizures; (3) treatment with electroconvulsive therapy; (4) stroke or myocardial infarction; (5) a positive urine toxicology result for THC (non-CU groups only), other illicit drugs or non-prescribed medications (i.e., cocaine, amphetamine, methamphetamine, methadone, tricyclic antidepressants, opiates, phencyclidine, barbiturates, and benzodiazepines) assessed using a multi-drug screen urine dipstick test; (6) active suicidality (assessed by the SCID and symptom ratings). All participants provided written informed consent to the current protocol approved by the UCSD Institutional Review Board known as the Human Research Protections Program. Both BD and HC participants were recruited based on two groupings: no CU (less than 5x lifetime use and no use in the past 90 days) or + CU (4x/week or more for the past 90 days [42,43,44,45]). The four groups (HC, BD, HC + CU, BD + CU) were matched for gender, education, and ethnicity, but age differed significantly between no CU and + CU participants (Table 1). Presence of any current alcohol use and estimated drinks per week (mean = 1.8, SD = 2.5, range = 0–10) was also reported by all participants. Current BD-related medications were self-reported; BD and BD + CU participants were matched on relative proportions reporting medication use in each medication category.
Iowa gambling task
Participants were administered the Iowa Gambling Task (IGT 18]), a computerized decision-making task in which participants are instructed to select from 4 decks of cards (A, B, C, D) that yield hypothetical monetary rewards of various amounts at various levels of risk. After selecting a card, a participant either may win or lose a theoretical amount of money. Decks A and B (risky choices) contain both large amounts of monetary gains, but also large losses compared to Decks C and D (safe choices) which contain smaller amounts of monetary gains but also smaller losses, making decks C and D “lower risk” and more advantageous over time. The primary outcome measure was the Total Net Difference score, calculated by subtracting the total number of risky choices from the total number of safe choices. The secondary IGT outcomes were defined and calculated as follows:
Safe win-stay ratio (SWS): probability of choosing an advantageous option after being rewarded by an advantageous choice; {# safe choices after safe rewards/# safe rewards}
Safe lose-shift ratio (SLS): probability of choosing a disadvantageous option after being punished by an advantageous choice; {# risky choices after safe punishments/# safe punishments}
Risky win-stay ratio (RWS): probability of choosing a disadvantageous option after being rewarded by a disadvantageous choice; {# risky choices after risky rewards/# risky rewards}
Risky lose-shift ratio: (RLS): probability of choosing an advantageous option after being punished by a disadvantageous choice; {# safe choices after risky punishments/# risky punishments}.
UCSD performance-based skills assessment (UPSA-2)
Participants were administered the UPSA-2 which uses role-play situations in six different domains (comprehension/planning, finance, communication, transportation, household management, medication management) to evaluate functioning and neuropsychological deficits [46]. Performance is scored based on accuracy and completeness of participant responses within each situation. Trained research staff scored participant performance using a standardized scoring sheet. Each domain score ranges from 0–20 points with higher scores reflecting better performance. UPSA scoring was typically completed by research staff immediately after the experimental visit, as such they were not blinded to participant group status. UPSA scoring uses clearly defined scoring criteria (i.e., participants are marked as correct or incorrect) to minimize the potential for scoring bias.
Cannabis use survey
Participants were asked about their past-year CU. Survey questions included purposes of CU (i.e., recreational, medicinal, or both) and frequency of CU. These data were based on participant recollection, as such detailed data on cannabinoid content or potency (e.g., relative ratios of THC:CBD, or percent THC/CBD) are not available. Participants reported engaging in several modes of administration including inhalation (smoking, vaping) and consumption (edibles). Participants reported using products containing primarily THC or combinations of THC and CBD in addition to other constituents and no participants reported use of products containing primarily CBD. Cannabis use survey data is presented in Supplemental Data 2. For CU frequency, participants were asked to indicate number of CU times per day in an average week (e.g., 1 time 7 days/week, 1 time 3 days/week, etc.). Average weekly CU for each participant was calculated using these responses (# of times per day × days per week). Participants were stratified into the following CU frequency groups: no CU (0x/week), moderate CU (4–24x/week) and heavy CU (25x + /week) [46, 47] based on the distribution of CU frequency within this cohort (mean weekly CU frequency = 23.6, SEM = 2.8). Although our ranges are broad and higher than those used in other studies, they are representative of the higher prevalence of cannabis use at the study location and for this participant population. A similar classification has also been used in other published studies [47, 48].
Statistical analyses
Assumptions for equal variances (Levene’s or Box’s test of equality) and normality (Shapiro-Wilks test) were tested for demographic differences, IGT outcomes, UPSA scores; variance and normality were tested across the entire sample and within each group. Potential outliers were assessed using boxplots and Tukey’s method. Participants with missing cognitive data were excluded from analyses. Kruskal-Wallis tests with follow up pairwise Mann-Whitney U tests, or Chi-square tests were used to determine demographic or CU pattern differences between BD and/or CU groups. Chi-square tests were used to assess differences in current medications used was assessed between BD and BD + CU participants. Clinical and demographic covariates that differed between groups and/or were correlated with outcome variables (i.e., age and mania symptoms) were considered as covariates. Sensitivity analyses were conducted to determine the effects of these factors on outcome variables; the results remained consistent therefore we did not include covariates for these analyses.
IGT Net Difference score and IGT lose-shift ratios were normally distributed, as such these variables were analyzed using a 2 × 2 ANOVA with BD and CU status as between-subjects factors. Cohen’s d or η 2 effect sizes were calculated for main effects and interactions. Planned comparison tests t-tests were conducted between the HC group and the other groups, as well as within the BD group by CU status. Given our a priori hypotheses, the α level was set at 0.05.
IGT win-stay ratios and UPSA scores were not normally distributed, as such these variables were analyzed using non-parametric tests. IGT win-stay ratios were analyzed using Kruskal-Wallis tests and planned pairwise Mann-Whitney U tests to assess group differences between HC group and the other groups, as well as within the BD group by CU status. Spearman’s rho correlation coefficients (rs) were used to test for correlations between UPSA and IGT scores. UPSA scores were analyzed using the Kruskal-Wallis test and planned pairwise Mann-Whitney U tests, as described above.
Supplemental analyses were then carried out to explore the effect of CU frequency on IGT performance. 2 × 3 ANOVAs were conducted on IGT outcome variables using BD and CU frequency groups (no CU, moderate CU and heavy CU) as between-subjects factors (Supplemental Data). Secondary IGT outcome variables were analyzed using non-parametric testing described above due to non-normal distribution of the data. Pairwise Mann-Whitney U comparison tests were conducted between the HC group and all other 5 comparison groups (HC/BD × CU frequency comparison groups), as well as between CU frequency groups within the HC/BD cohorts. Significant (p < 0.05) interactions were reported, and the Bonferroni multiple comparisons correction was applied. All statistical analyses were performed using SPSS 28.0 (Chicago, IL, USA).
Results
No significant differences in CU patterns between healthy and BD participants
People with BD reported using cannabis to treat a greater number of symptoms (Supplemental Data 1; p < 0.05, d = 0.88). There was no significant difference in proportions of participants reporting recreational versus medicinal CU. There were no significant group differences in the reported weekly CU frequency.
CU was associated with better decision-making in people with BD
A significant BD × CU status interaction was observed on IGT Net Difference score (F(1,82) = 9.39; p < 0.01; \({\eta }^{2}\) = 0.103). IGT scores for the HC were higher than the HC + CU (t = −2.086, p = 0.042, d = −0.591) and BD (t = −2.50, p = 0.018, d = −0.883), but not the BD + CU group (Fig. 1; Supplementary Data 2a). The BD + CU group had higher IGT scores than the BD group (t = −2.328, p = 0.026, d = −0.823). Importantly, the BD + CU group exhibited comparable difference scores to HC group, indicating CU is not associated with worse decision-making in BD participants.
An overall interaction effect of BD × CU [F(1,82) = 9.39; p < 0.01; \({\eta }^{2}\) = 0.103] revealed that HC participants had significantly higher overall IGT scores compared to HC + CU and BD participants. BD + CU group had higher IGT scores than the BD group (p < 0.1) and, critically, did not differ from the HC group. *p < 0.05; Data presented as mean, SEM, and individual data points. Orange symbols represent males, yellow symbols represent females.
BD and CU are associated with different decision making strategies
Replicating our prior observations [27], we observed that decision-making strategies were significantly different between BD and HC participants and extend these findings to BD and HC participants who use cannabis. There was no significant BD × CU interaction on safe lose-shift (SLS; F(1,81) = 3.68; p = 0.058; \({\eta }^{2}\) = 0.43), though a significant main effect of CU (F(1,81) = 8.36; p = 0.005; \({\eta }^{2}\) = 0.094, Fig. 2a) was detected. SLS was significantly lower in the HC group compared to all other groups (ps < 0.05, Supplementary Data 2b), indicating that both BD and CU were associated with a higher likelihood of switching from safe to risky choices following a loss. There was no BD × CU interaction on risky lose-shift (RLS), but there was a main effect of BD (F(1,80) = 4.03; p = 0.048; \({\eta }^{2}\) = 0.048, Fig. 2b). Visual inspection of the group differences indicated a higher RLS in the BD + CU participants compared to HC participants (Fig. 2b); thus, BD + CU participants were more likely to switch from a risky deck to a safe deck following a loss. HC participants also had significantly higher safe win-stay ratios (SWS; Fig. 2c) compared to all other groups (ps<0.05; Supplementary Data 2c). This difference indicates that both BD and CU was associated with a lower likelihood of repeatedly selecting from a safe deck following a reward. Risky win-stay ratios (RWS; Fig. 2d) were significantly lower in BD + CU and HC + CU participants relative to HC participants (ps<0.05, Supplementary Data 2b), indicating that CU is associated with a lower likelihood of continuing to select from a risky deck following a reward.
a HC participants had significantly lower safe lose-shift ratios compared to all other groups (p < 0.01). b The BD + CU group had lower risky lose-shift ratios compared to HC participants. *p < 0.05; c HC participants had significantly lower safe win-stay ratios compared to all other groups (ps <0.05). d HC participants had significantly higher risky win-stay ratios compared to CU groups (ps<0.05). *p < 0.05; Lose-shift data presented as mean, SEM. Win-stay data presented as median, interquartile range. Orange symbols represent males, yellow symbols represent females.
Heavy, but not moderate, CU was associated with worse risk-based decision making
CU frequency has been associated with poor cognitive functioning in a dose-dependent manner in other populations [49, 50]. As such, we next sought to explore whether CU frequency was similarly associated with decision-making in people with BD. There was a significant BD status × CU frequency interaction detected on IGT net difference score (F(1,78) = 4.98; p = 0.009; effect size [\({\eta }^{2}\) = 0.113], Fig. 3a). After applying the Bonferroni correction significance threshold (p < 0.006), there were no significant differences between BD × CU frequency groups. However, there were nominally significant (p < 0.05) differences. Within HC group, heavy CU was associated with worse IGT performance compared to no-CU participants (t = 2.31, p = 0.03, d = 0.94; Fig. 3b). Within the BD group, moderate CU was associated with better IGT performance compared to no CU (t = −2.71, p = 0.012, d = −1.05). There was no difference in IGT performance between the moderate CU (BD or HC) groups and the HC group. IGT secondary outcome variables were also analyzed by BD × CU frequency groups (Supplementary Data 3). Again, although no group differences met the Bonferroni correction significance threshold, there were some nominally significant differences in safe but not risky decision-making strategies. Notably, SWS was higher in the HC group compared to all other groups, except the BD + moderate CU group (Supplementary Data 3a).
a A significant BD status × CU frequency interaction was detected on total IGT net difference score (F(1,78) = 4.978; p = 0.009; effect size [\({\eta }^{2}\) = 0.113]). Among the healthy comparison (HC) groups, both moderate and heavy CU was associated with worse IGT performance. The BD + moderate CU group, but not the BD + Heavy CU group, exhibited significantly higher IGT scores (safer decision-making) compared to the BD group. Data presented as median, interquartile range and individual data points. Orange symbols indicate males, yellow symbols indicate females. b Pairwise comparison test statistics for BD × CU frequency groups.
CU was associated with better functional medication management skills in BD
There was no significant correlation between IGT performance and total UPSA score (rs = 0.21, p = 0.054), however correlation analyses between UPSA sub-scores and IGT score (Fig. 4a) revealed that the UPSA Medication Management (MM) sub-score (rs = 0.27, p < 0.05, Fig. 4b) was significantly correlated with IGT score. During the Medication Management section of the UPSA, participants are asked participants are asked to engage in a role play scenario during which they plan out a medication routine using 4 drugs taken over the course of one day with various restrictions (i.e., with or without meals, number of doses, etc.). Participants with higher IGT scores had better functional capability in the MM section of the UPSA-2. Nonparametric testing of UPSA total (H = 6.52, p = 0.10) and UPSA MM scores (H = 9.90, p = 0.019) revealed that median scores differed significantly between groups (Fig. 4c). Planned pairwise Mann-Whitney U tests revealed that BD participants had significantly lower median scores on the UPSA MM compared to all other groups (ps < 0.05, Supplementary Table 4). Consistent with IGT scores, BD + CU participants did not have significantly different UPSA MM scores compared to HC participants.
a Correlations between UPSA sub-scores and IGT performance. b Net Iowa Gambling Task (IGT) score was positively correlated with UPSA MM score (rs=0.269, p < 0.05). c The BD participants had significantly lower UPSA MM scores compared to HC (p < 0.01) and BD + CU participants (p < 0.01). **p < 0.01; Data presented as median, interquartile range and individual data points. Orange symbols represent males, yellow symbols represent females.
Discussion
Here, we sought to determine whether chronic CU was associated with risk-based decision-making in people with BD. Consistent with our previous report, BD participants who did not use cannabis exhibited increased risk-preference in the IGT relative to HC participants [27]. Importantly, the current study demonstrated that decision-making by BD + CU participants was comparable to that of non-CU HC participants. Further, we also observed that CU was associated with reduced risk-preference (i.e., higher RLS) in BD but not HC participants. Similarly, only people with BD who do not use cannabis had significantly worse functional capacity compared to HC participants, specifically in the domain of medication management. Our secondary analyses suggest that the beneficial effects of CU on decision-making could be specific to moderate CU. Altogether, these data support the premise that people with BD may use cannabis because it confers cognitive benefits in this population.
A core neurochemical feature of BD mania is tonic hyperdopaminergia [51], likely mediated in-part by reduced DAT density [29] that may persist into euthymic and depressed states [28] (but see [52,53,54,55]). Studies of dopamine function in people with BD are few, although a heightened behavioral response to amphetamine has been reported relative to HC participants [56, 57], indicative of postsynaptic hyper-responsivity to dopamine [56]. PET imaging revealed that hyper-responsivity of the mesostriatal dopamine system (albeit presynaptic) was associated with risky decision-making in the IGT. Specifically, elevated amphetamine-induced increases in right ventrostriatal dopamine release were associated with poorer IGT performance in HC participants [58]. Additionally, in-task increases in striatal dopamine signaling predicted impaired IGT performance in individuals with gambling disorder, but better performance in HC participants [59]. The contribution of any postsynaptic dopamine hyper-responsivity to the IGT deficit of cannabis-abstinent people with BD remains to be directly evaluated, although its putative functional consequences could ostensibly be exacerbated by BD-associated DAT hypoexpression [28]. Indeed, prior studies observed elevated HVA levels in people with BD, and although these results were not stratified by cannabis use, they do support a hyperdopaminergic state present in people with BD.
While acute THC generally increases cortical and striatal dopamine release in humans [37, 60, 61] and animals [62,63,64], chronic exposure via regular CU leads to a long-term reduction in dopamine synthesis and transmission [65,66,67] (reviewed in [68, 69]:). Such a reduction could mitigate the BD-related elevations in dopamine signaling proposed above, possibly driving the improved IGT performance associated with moderate CU. This mechanism may also contribute to impaired performance of the HC + CU participants (both here and in previous reports [70,71,72,73]), given the association between IGT-related striatal dopamine release and better decision-making in healthy participants [59]. Interestingly, a positive association was previously also observed between plasma anandamide levels and IGT performance in healthy humans [74], strengthening the link between eCB signaling and decision-making. There is evidence to suggest a complex regulatory interaction between dopamine and anandamide [75,76,77]; thus, changes in anandamide levels in people with BD may occur in response to changes in dopamine transmission. Future studies should confirm the degree of involvement of pre- versus post-synaptic dopamine mechanisms on the effects of chronic CU in people with BD and their decision-making and increase sample sizes to evaluate the effects of chronic CU on anandamide.
IGT performance is predictive of clinical and functional outcome in BD. Emergence of hypomanic/manic symptoms has been predicted based on performance in a reward-based decision-making card task [78]. Previously, our group reported that poor cognitive task performance was correlated with worse functional capacity in people with BD patients, with manic/hypomanic patients performing significantly worse compared to depressed or euthymic BD patients [79]. Our current study supports previous findings that worse IGT performance was negatively correlated with higher levels of mania symptoms (Supplementary Data 5). Importantly, CU has previously been associated with worsening of mania and psychosis symptoms [80,81,82]. This association, combined with the high prevalence of CU in BD highlighted in the Introduction, has led to the hypothesis that CU may be a risk factor for the development of BD, rather than a form of self-medication. Although we do not have detailed data on whether CU was initiated prior to or after onset of BD symptoms, we did not observe a significant difference in mania symptoms between no-CU and CU groups (Supplementary Data 6). Though not statistically significant, BD + moderate CU participants reported lower mania symptoms compared to no CU and heavy CU. BD participants were clinically stable at the time of testing and sensitivity analyses conducted including Young Mania Rating Score and Hamilton Depression Rating Scale Score as a covariate did not alter the significance or directionality of the results. These data demonstrate that mania or depression severity may not be a primary driver of the differences in decision-making and are contrary to the hypothesis that CU contributes to worse BD mood symptoms. However, limiting our BD cohort to those without severe mood symptoms may also be a source of influence that should be explored in future studies. This research is particularly important, considering the altered neurochemical activity present during acute mood episodes in BD that may result in a differential response to cannabis.
In further support of the self-medication hypothesis of CU in BD is a recent study in which current CU in people with BD was associated with higher UPSA scores relative to BD participants who do not use cannabis [83]; our study replicates these findings by identifying an association between better functional capacity and chronic CU. With the caveat that we observed this finding with a single (albeit important and highly practical) domain of functional outcome (i.e., medication management; MM). Successful completion of the MM section of the UPSA requires grossly intact executive functioning and working memory; thus, it may be the case that CU in BD improves executive functions, such as decision-making, with beneficial effects on real-world functioning. Indeed, CU was associated with improved executive functioning in other populations likely required to manage extensive medication regimens [8, 48, 84], and the current data extends this premise to BD.
This study does have several limitations that require addressing however, particularly in that the cross-sectional design and static group comparisons limit any causal conclusions about causal CU effects on cognition. The IGT is also a laboratory-based measure which limits its reflection of decision-making across all contexts; nonetheless, the risk-based decision making assessed by the IGT was associated with real-world functional behaviors highly relevant to people with BD, such as substance use [85,86,87] and suicide risk [86, 88,89,90]. The CU frequency data make a strong case for direct effects of CU on cognition in people with BD; however, interpretation of the CU frequency findings are limited. For example, while the CU frequency as defined in the present study improves on prior literature in which weekly CU is loosely defined (e.g., 3–4/week may represent 3–4 days/week without considering # of times per day of use), these findings are not likely generalizable to other locations and participant populations where cannabis is not readily available and as such use frequency is inherently lower. Further, our dataset lacks critical data on potency and route of administration as most participants could not recall this information, which would have enhanced the interpretation of our results. Notably, participants who vape high cannabis concentrate 20 times a week may not be directly comparable to participants who smoke low-potency cannabis 4 times a week. Additionally, the primary reason for use (i.e., medicinal versus recreational) could also impact the THC potency individuals prefer to use; however, we did not observe a difference in cognitive performance between medicinal and recreational users. The differences in CU pattern variables highlights the need for future studies to consider: 1) standardized methods of reporting CU frequency; 2) collection of detailed CU variable data (e.g., route of administration and potency via certificate of analysis provided on cannabis products); and 3) use of multivariate or principal component analyses that include variables such as cumulative lifetime exposure (as suggested by Reis et. al [91]), potency, route of administration and CU frequency. Participants reported using products containing primarily THC or a combination of THC and CBD with additional constituents. Given the differential effects of CBD and THC on neurochemistry, any mechanistic interpretation of these findings is therefore limited. Nonetheless, the translational nature of the IGT enables future cross-species validation of this work, which could test the impact of different cannabinoids (e.g., THC or CBD), cannabis potency, and dosage/frequency of cannabinoid administration on IGT performance in the DAT knockdown mouse model of mania [18].
This study is also limited by a number of variables that may have independent effects on cognitive performance, such as age, mood symptoms, medication use, other substance or alcohol use and other sociodemographic variables. Sensitivity analyses suggest that neither age nor mood symptoms (mania and depression) have a significant effect on decision-making in this cohort. Reported medication use within the BD groups did not include detailed information such as dosage and frequency of medications, and as such could not be included as a covariate in our analyses. While we endeavored to collect dose and frequency information from the participants, many participants could not recall the exact prescribing details of their medications, not atypical for this population. We did not have access to their medical records. As shown in Table 1, there were no differences in the percentage of BD participants that reported medication use in any given category; however, future studies would benefit by including detailed medication use given the reported effects of some BD medications on cognitive functioning. Interestingly, a greater percentage of HC + CU participants reported current alcohol use compared to the other groups, though the number of drinks per week were below what might be considered problematic use (Table 1); future research should collect detailed other substance use and alcohol use to better understand how these factors may impact any potential cognitive effects of CU. Social determinants of health, such as income, housing, and social support, also differs in people with BD [92] or those with cannabis use disorder [93], relative to healthy adults, potentially influencing their decision-making processes and thereby contributing to the observed differences in risk-based decision making and functional capacity observed in our study. Finally, this study is limited by sample size, most notably in the non-CU BD group. Challenges in participant recruitment for this group were expected given the high prevalence of CU in people with BD.
In summary, people with BD who use cannabis had decision-making and functional capacity comparable to non-CU HC participants. Based on the data presented here, our previous findings in mice with reduced DAT functioning, and preliminary evidence of elevated HVA levels in BD, we propose that elevated dopaminergic tone (HVA) may contribute to poorer performance in decision-making tasks in people with BD (Fig. 5a). Moreover, the association between better decision-making skills and CU in people with BD appears to be either frequency or potentially dose dependent, as BD participants reporting heavy CU exhibited similar IGT performance to the no-CU group, while only those reporting moderate CU performed better in the IGT (Fig. 5b). People with BD continue to report using cannabis for therapeutic purposes, including to remediate cognitive dysfunction. Thus, greater insight into the effects of cannabis on dopamine-eCB interactions would further elucidate putative treatments for BD. The limiting nature of cross-sectional studies such as these underscores the importance of clinical trials and the use of cross-species paradigms to determine causal effects of individual cannabinoids on eCB and dopamine neurotransmitter levels. Identification of treatment targets is critical considering the concerns of adverse effects of cannabis reported on other clinical outcomes, particularly mood and psychosis symptoms. Future studies may also endeavor to collect detailed cannabinoid content and potency information (e.g., through certificate of analysis) from cannabis products that are in current use by participants. Regardless the frequency or potentially dose-dependent effects on decision-making strategies and functional capacity reported here, and the negligible differences in mood symptom severity in the BD groups, suggest that CU practices could be appropriately managed in people with BD to improve cognitive function, a possibility that merits further exploration.
a Schematic illustrating interactions between dopaminergic and endocannabinergic neurotransmission in a healthy versus reduced-dopamine transporter (DAT) system. b Differential effects of CU on risky decision-making as measured by the Iowa Gambling Task (IGT) in BD + versus BD- individuals may reflect an inverted U-shaped relationship between dopamine levels and risk-based decision-making. Decision-making impairments in BD + non-CU participants are likely driven by elevated baseline dopamine levels, which may be normalized (reduced) by chronic CU. Meanwhile, CU may impair BD- decision-making by reducing dopamine tone to sub-optimal levels. CB1R: cannabinoid receptor 1; GABA: γ-aminobutyric acid; AEA: anandamide.
Data availability
All data produced in this study is shown in manuscript and supplementary information, and unprocessed data are available from the corresponding author on reasonable request.
References
Post RM, Denicoff KD, Leverich GS, Altshuler LL, Frye MA, Suppes TM, et al. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64:680–90.
Agrawal A, Nurnberger JI, Lynskey MT. Cannabis involvement in individuals with bipolar disorder. Psychiatry Res. 2011;185:459.
Gruber SA, Sagar KA, Dahlgren MK, Olson DP, Centorrino F, Lukas SE. Marijuana impacts mood in bipolar disorder: a pilot study. Ment Health Subst Use. 2012;5:228–39.
Ashton CH, Moore PB. Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr Scand. 2011;124:250–61.
Grinspoon L, Bakalar JB. The use of cannabis as a mood stabilizer in bipolar disorder: anecdotal evidence and the need for clinical research. J Psychoact Drugs. 1998;30:171–7.
Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addiction Biol. 2008;13:264–75.
Burggren AC, Shirazi A, Ginder N, London ED. Cannabis effects on brain structure, function, and cognition: considerations for medical uses of cannabis and its derivatives. Am J Drug Alcohol Abus. 2019;45:563–79.
Ellingson JM, Hinckley JD, Ross JM, Schacht JP, Bidwell LC, Bryan AD, et al. The neurocognitive effects of cannabis across the lifespan. Curr Behav Neurosci Rep. 2021;8:124–33.
Walter TJ, Pocuca N, Young JW, Geyer MA, Minassian A, Perry W. The relationship between cannabis use and cognition in people with bipolar disorder: a systematic scoping review. Psychiatry Res 2021;297. https://doi.org/10.1016/J.PSYCHRES.2020.113695.
Braga RJ, Burdick KE, DeRosse P, Malhotra AK. Cognitive and clinical outcomes associated with cannabis use in patients with bipolar I disorder. Psychiatry Res. 2012;200:242–5.
Ringen PA, Vaskinn A, Sundet K, Engh JA, Jónsdóttir H, Simonsen C, et al. Opposite relationships between cannabis use and neurocognitive functioning in bipolar disorder and schizophrenia. Psychol Med. 2010;40:1337–47.
Halder A, Chakraborty A, Bose S Correlations of cannabis use of human cognition and bipolar disorder. Indian J Psychiatry 2015;57.
Lagerberg TV, Melle I, Ringen PA, Aminoff SR, Buchmann C, Hoegh MC, et al. The relationship between cannabis use, cognitive functioning and polygenic risk in bipolar disorder. Bipolar Disord. 2019;21:60–1.
Abush H, Ghose S, Van Enkevort EA, Clementz BA, Pearlson GD, Sweeney JA, et al. Associations between adolescent cannabis use and brain structure in psychosis. Psychiatry Res Neuroimaging. 2018;276:53–64.
Sagar KA, Dahlgren MK, Racine MT, Dreman MW, Olson DP, Gruber SA. Joint effects: a pilot investigation of the impact of bipolar disorder and marijuana use on cognitive function and mood. PLoS One. 2016;11:e0157060.
Leung J, Chan G, Stjepanović D, Chung JYC, Hall W, Hammond D. Prevalence and self-reported reasons of cannabis use for medical purposes in USA and Canada. Psychopharmacology (Berl). 2022;239:1509.
Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, et al. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–65.
Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15.
Gu Yting, Zhou C, Yang J, Zhang Q, Zhu Ghui, Sun L, et al. A transdiagnostic comparison of affective decision-making in patients with schizophrenia, major depressive disorder, or bipolar disorder. Psych J. 2020;9:199–209.
Milienne-Petiot M, Geyer MA, Arnt J, Young JW. Brexpiprazole reduces hyperactivity, impulsivity, and risk-preference behavior in mice with dopamine transporter knockdown-a model of mania. Psychopharmacology (Berl). 2017;234:1017–28.
Van Enkhuizen J, Geyer MA, Young JW. Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task: relevance to mania. Psychopharmacology (Berl). 2013;225:661–74.
Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–43.
Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the behavioral pattern monitor. Neurosci Biobehav Rev. 2007;31:882–96.
Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, et al. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–80.
Young JW, Geyer MA, Halberstadt AL, van Enkhuizen J, Minassian A, Khan A, et al. Convergent neural substrates of inattention in bipolar disorder patients and dopamine transporter-deficient mice using the 5-choice CPT. Bipolar Disord. 2020;22:46–58.
Young JW, Van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. J Psychopharmacol. 2011;25:934–43.
van Enkhuizen J, Henry BL, Minassian A, Perry W, Milienne-Petiot M, Higa KK, et al. Reduced dopamine transporter functioning induces high-reward risk-preference consistent with bipolar disorder. Neuropsychopharmacology. 2014;39:3112–22.
Anand A, Barkay G, Dzemidzic M, Albrecht D, Karne H, Zheng QH, et al. Striatal dopamine transporter availability in unmedicated bipolar disorder. Bipolar Disord. 2011;13:406–13.
Yatham LN, Liddle PF, Gonzalez M, Saraf G, Vafai N, Lam RW, et al. A positron emission tomography study of dopamine transporter density in patients with bipolar disorder with current mania and those with recently remitted mania. JAMA Psychiatry. 2022;79:1217–24.
Greenwood TA, Alexander M, Keck PE, Mcelroy S, Sadovnick AD, Remick RA, et al. Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder. American J Med Genet (Neuropsychiatr Genet). 2001;105:145–51.
Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry. 2006;11:125–33.
Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci USA. 2001;98:1982–7.
Amsterdam JD, Newberg AB. A preliminary study of dopamine transporter binding in bipolar and unipolar depressed patients and healthy controls. Neuropsychobiology. 2007;55:167–70.
van Enkhuizen J, Milienne-Petiot M, Geyer MA, Young JW. Modeling bipolar disorder in mice by increasing acetylcholine or dopamine: chronic lithium treats most, but not all features. Psychopharmacology (Berl). 2015;232:3455–67.
Kohno M, Nurmi EL, Laughlin CP, Morales AM, Gail EH, Hellemann GS, et al. Functional genetic variation in dopamine signaling moderates prefrontal cortical activity during risky decision making. Neuropsychopharmacology. 2016;41:695–703.
Glass M. The role of cannabinoids in neurodegenerative diseases. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:743–65.
Bossong MG, Mehta MA, Berckel BNM, van, Howes OD, Kahn RS, et al. Further human evidence for striatal dopamine release induced by administration of Δ9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology (Berl). 2015;232:2723.
Tournier BB, Tsartsalis S, Dimiziani A, Millet P, Ginovart N. Time-dependent effects of repeated THC treatment on dopamine D2/3 receptor-mediated signalling in midbrain and striatum. Behavioural brain Res. 2016;311:322–9.
Tournier BB, Dimiziani A, Tsartsalis S, Millet P, Ginovart N. Different effects of chronic THC on the neuroadaptive response of dopamine D2/3 receptor-mediated signaling in roman high- and roman low-avoidance rats. Synapse 2018; 72. https://doi.org/10.1002/SYN.22023.
Lukasiewicz M, Gerard S, Besnard A, Falissard B, Perrin E, Sapin H, et al. Young mania rating scale: how to interpret the numbers? determination of a severity threshold and of the minimal clinically significant difference in the EMBLEM cohort. Int J Methods Psychiatr Res. 2013;22:46.
Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton depression rating scale. J Affect Disord. 2013;150:384–8.
Haney M, Ramesh D, Glass A, Pavlicova M, Bedi G, Cooper ZD. Naltrexone maintenance decreases cannabis self-administration and subjective effects in daily cannabis smokers. Neuropsychopharmacology. 2015;40:2489–98.
Henry EA, Kaye JT, Bryan AD, Hutchison KE, Ito TA. Cannabis cue reactivity and craving among never, infrequent and heavy cannabis users. Neuropsychopharmacology. 2014;39:1214–21.
Papini S, Ruglass LM, Lopez-Castro T, Powers MB, Smits JAJ, Hien DA. Chronic cannabis use is associated with impaired fear extinction in humans. J Abnorm Psychol. 2017;126:117–24.
Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–77.
Twamley EW, Doshi RR, Nayak GV, Palmer BW, Golshan S, Heaton RK, et al. Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry. 2002;159:2013–20.
Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–43.
Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care. 2016;28:628–32.
Ayoub SM, Holloway BM, Miranda AH, Roberts BZ, Young JW, Minassian A, et al. The impact of cannabis use on cognition in people with hiv: evidence of function-dependent effects and mechanisms from clinical and preclinical studies. Curr HIV/AIDS Rep. 2024;21:87–115. https://doi.org/10.1007/S11904-024-00698-W.
Bourque J, Potvin S. Cannabis and cognitive functioning: from acute to residual effects, from randomized controlled trials to prospective designs. Front Psychiatry. 2021;12:596601.
Ashok AH, Marques TR, Jauhar S, Nour MM, Goodwin GM, Young AH, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22:666–79.
Lee FJS, Pei L, Liu F. Disruption of the dopamine transporter-dopamine D2 receptor interaction in schizophrenia. Synapse. 2009;63:710–2.
Chang TT, Yeh TL, Chiu NT, Chen PS, Huang HY, Yang YK, et al. Higher striatal dopamine transporters in euthymic patients with bipolar disorder: a SPECT study with [Tc] TRODAT-1. Bipolar Disord. 2010;12:102–6.
Hsueh Y-S, Lin C-Y, Chiu N-T, Yang YK, Chen PS, Chang HH. Changes in striatal dopamine transporters in bipolar disorder and valproate treatment. Eur Psychiatry. 2021;64:e9. https://doi.org/10.1192/J.EURPSY.2021.1.
Tsai YT, Chang CY, Wu CY, Huang YL, Chang HH, Lu TH, et al. Social cognitive deficit is associated with visuomotor coordination impairment and dopamine transporter availability in euthymic bipolar disorder. J Psychiatr Res. 2023;165:158–64.
Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–14.
Saraf G, Pinto JV, Cahn A, White AG, Shahinfard E, Vafai N, et al. Dopamine release during psychological stress in euthymic bipolar i disorder: a positron emission tomography study with [11c]raclopride. J Affect Disord. 2021;295:724–32.
Oswald LM, Wand GS, Wong DF, Brown CH, Kuwabara H, Brašić JR. Risky decision-making and ventral striatal dopamine responses to amphetamine: a positron emission tomography [(11)C]raclopride study in healthy adults. Neuroimage. 2015;113:26–36.
Linnet J, Møller A, Peterson E, Gjedde A, Doudet D. Inverse association between dopaminergic neurotransmission and iowa gambling task performance in pathological gamblers and healthy controls. Scand J Psychol. 2011;52:28–34.
Bossong MG, Van Berckel BNM, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Δ9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 2009. 2008;34:759–66.
Stokes PRA, Egerton A, Watson B, Reid A, Breen G, Lingford-Hughes A, et al. Significant decreases in frontal and temporal [11C]-raclopride binding after THC challenge. Neuroimage. 2010;52:1521–7.
Ton JMNC, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, et al. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451:59–68.
Chen J, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl). 1990;102:156–62.
Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, et al. Δ9-Tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: An in vivo microdialysis study. Brain Res. 2002;948:155–8.
Bloomfield MAP, Morgan CJA, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry. 2014;75:470–8.
Volkow ND, Wang GJ, Telang F, Fowler JS, Alexoff D, Logan J, et al. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc Natl Acad Sci USA. 2014;111:E3149–56. https://doi.org/10.1073/PNAS.1411228111.
Van De Giessen E, Weinstein JJ, Cassidy CM, Haney M, Dong Z, Ghazzaoui R, et al. Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry. 2017;22:68–75.
Bloomfield MAP, Ashok AH, Volkow ND, Howes OD. The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature. 2016;539:369.
Chetia S, Borah Gaurab, Chetia S, Borah G. Δ 9-tetrahydrocannabinol toxicity and validation of cannabidiol on brain dopamine levels: an assessment on cannabis duplicity. Natural Products Bioprospecting. 2020;10:285–96.
Whitlow CT, Liguori A, Brooke Livengood L, Hart SL, Mussat-Whitlow BJ, Lamborn CM, et al. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–11.
Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–92.
Fridberg DJ, Queller S, Ahn WY, Kim W, Bishara AJ, Busemeyer JR, et al. Cognitive mechanisms underlying risky decision-making in chronic cannabis users. J Math Psychol. 2010;54:28–38.
Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. 2011;191:51–9.
Fagundo AB, de la Torre R, Jiménez-Murcia S, Agüera Z, Pastor A, Casanueva FF, et al. Modulation of the endocannabinoids n-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) on executive functions in humans. PLoS One. 2013;8:e66387. https://doi.org/10.1371/JOURNAL.PONE.0066387.
Young JW, Goey AKL, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl). 2010;208:443–54.
Young JW, Goey AKL, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol Biochem Behav. 2010;96:7–15.
Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22.
Soehner AM, Wallace ML, Edmiston K, Chase H, Lockovich J, Aslam H, et al. Neurobehavioral reward and sleep-circadian profiles predict present and next-year mania/hypomania symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8:1251–61. https://doi.org/10.1016/J.BPSC.2023.04.012.
Henry BL, Minassian A, Perry W. Everyday functional ability across different phases of bipolar disorder. Psychiatry Res. 2013;210:850–6.
Sideli L, Quigley H, La Cascia C, Murray RM. Cannabis use and the risk for psychosis and affective disorders. J Dual Diagn. 2019;16:22–42.
Lagerberg TV, Sundet K, Aminoff SR, Berg AO, Ringen PA, Andreassen OA, et al. Excessive cannabis use is associated with earlier age at onset in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261:397–405.
Pinto JV, Medeiros LS, Santana da Rosa G, Santana de Oliveira CE, Crippa JA, de S, et al. The prevalence and clinical correlates of cannabis use and cannabis use disorder among patients with bipolar disorder: a systematic review with meta-analysis and meta-regression. Neurosci Biobehav Rev. 2019;101:78–84.
Selloni A, Bhatia G, Ranganathan M, De Aquino JP. Multimodal correlates of cannabis use among U.S. veterans with bipolar disorder: an integrated study of clinical, cognitive, and functional outcomes. J Dual Diagn. 2022;18:81–91.
Kallianpur KJ, Birn R, Ndhlovu LC, Souza SA, Mitchell B, Paul R, et al. Impact of cannabis use on brain structure and function in suppressed HIV infection. J Behav Brain Sci. 2020;10:344.
Kovács I, Richman MJ, Janka Z, Maraz A, Andó B. Decision making measured by the iowa gambling task in alcohol use disorder and gambling disorder: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;181:152–61.
Jollant F, Guillaume S, Jaussent I, Bellivier F, Leboyer M, Castelnau D, et al. Psychiatric diagnoses and personality traits associated with disadvantageous decision-making. Eur Psychiatry. 2007;22:455–61.
Balconi M, Finocchiaro R, Campanella S. Reward sensitivity, decisional bias, and metacognitive deficits in cocaine drug addiction. J Addict Med. 2014;8:399–406.
Gorlyn M, Keilp JG, Oquendo MA, Burke AK, John Mann J. Iowa gambling task performance in currently depressed suicide attempters. Psychiatry Res. 2013;207:150–7.
Zakowicz P, Skibińska M, Wasicka-Przewoźna K, Skulimowski B, Waśniewski F, Chorzepa A, et al. Impulsivity as a risk factor for suicide in bipolar disorder. Front Psychiatry. 2021;12:706933. https://doi.org/10.3389/FPSYT.2021.706933.
Sastre-Buades A, Alacreu-Crespo A, Courtet P, Baca-Garcia E, Barrigon ML. Decision-making in suicidal behavior: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;131:642–62.
Reis JP, Auer R, Bancks MP, Goff DC, Lewis CE, Pletcher MJ, et al. Cumulative lifetime marijuana use and incident cardiovascular disease in middle age: the coronary artery risk development in young adults (CARDIA) study. Am J Public Health. 2017;107:601.
Teigland C, Mohammadi I, Agatep BC, Boskovic DH, Sajatovic M. Relationship between social determinants of health and hospitalizations and costs among patients with bipolar disorder 1. J Manag Care Spec Pharm. 2023;30:72.
O’Donnell S, Scott-Storey K, Malcolm J, Vincent CD, Wuest J. Cumulative lifetime violence, social determinants of health, and cannabis use disorder post-cannabis legalization in a community sample of men: an intersectional perspective. Res Nurs Health. 2024;47:460–74.
Acknowledgements
We would like to thank Ms. Mahalah R. Buell and Mr. Richard F. Sharp for their support. This work was funded by NIDA R01DA043535 (Perry/Young) and NIDA R01DA051295 (Minassian/Young) and T32MH018399 (Salary support for BMH).
Author information
Authors and Affiliations
Contributions
AHM: writing-original draft, formal analysis, data curation; BZR: writing-original draft, revision, BMH: writing-review and editing, formal analysis, data curation; EP: writing-review and editing, data curation; HR: data curation, writing-review and editing; SMA: writing-review and editing; DP: writing-review and editing, conceptualization; KJ: writing-review and editing, SAB: formal analysis, writing-review and editing, data curation; SR: writing-review and editing, formal analysis; MAG: writing-review and editing, conceptualization; WP, AM and JWY: Investigation, conceptualization, supervision, project administration, funding acquisition, methodology, writing-review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miranda, A., Roberts, B.Z., Holloway, B.M. et al. Chronic cannabis use in people with bipolar disorder is associated with comparable decision-making and functional outcome to healthy participants. Transl Psychiatry 15, 506 (2025). https://doi.org/10.1038/s41398-025-03718-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03718-4