Abstract
This clinical paper provides an in-depth exploration of advanced techniques for bonding orthodontic attachments under special circumstances. Challenges arise when bonding brackets to non-enamel surfaces, such as dental restorations, and in conditions such as amelogenesis imperfecta, which affect enamel integrity. Distinct approaches required for bonding to different restorative materials, including glassy ceramics, zirconia, resin composites and metals, are outlined. Moreover, we describe strategies to manage bonding in conditions including amelogenesis imperfecta, hypodontia and microdontia in a multidisciplinary context.
Key points
-
Awareness of the precise composition of existing dental restorations can be invaluable as it will inform the approach to bonding orthodontic attachments. This is especially the case with ceramic and resin composite restorations.
-
Conditions affecting the dental structure, such as fluorosis, amelogenesis imperfecta and dentinogenesis imperfecta, have highly variable presentations. The required approaches to attaching orthodontic components to these teeth range from normal enamel bonding protocols to specialised modifications.
-
Patients with existing dental restorations or enamel conditions need to be made aware of both the increased risk of attachment bond failure and risk of damage to the restorations and/or teeth as a consequence of fixed appliance orthodontic treatment.
Similar content being viewed by others
Introduction
Successful use of orthodontic fixed appliances relies on a secure and predictable attachment of the fixed components to the teeth. Bonding of orthodontic brackets to teeth has superseded banding since the acid-etch technique was adapted for direct bonding of brackets to enamel.1,2,3 The numerous advantages of bonding include reduced plaque accumulation, patient comfort and reduced chairside time. Further reduction in chairside time and delegation of the bond-up procedure may also arise with indirect bonding procedures.4
Resin composite materials have proven to be the most suitable for orthodontic applications, demonstrating sufficient shear bond strength of at least 6-8 MPa, and the threshold tensile bond strength value proposed to resist functional and masticatory forces.5 Additionally, resin composite bonding is associated with an acceptable level of attachment failure rate of up to 5% over 18 months, while higher failure rates are associated with alternative adhesives, such as glass ionomers and resin-modified glass ionomers.6,7
With an increasing number of adults pursuing orthodontic treatment, there is a growing requirement to bond brackets to dental restorations, such as composite or amalgam, porcelain veneers, and crowns made of ceramic or metal alloys. Beyond these restorations, orthodontic practitioners also encounter patients who have undergone teeth whitening or with distinct dental conditions, including amelogenesis imperfecta. These conditions can result in anomalous tooth surfaces, presenting unique challenges for orthodontic treatment. We describe the various techniques, which can be employed to bond orthodontic attachments in these special circumstances.
Bonding to regular enamel surfaces
Chemical treatment to ‘condition' enamel with acid was first described in 1955 by Buonocore, who used an 85% concentration of orthophosphoric acid (OPhA) to bond acrylic resin restorations to enamel.1 OPhA remains the most commonly used agent to etch enamel in dentistry, typically using at a 37% concentration for 15-30 seconds before orthodontic bonding.8,9 This acid etching (AE) creates microporosities and an uneven surface on the enamel by demineralising inter-prismatic enamel and prismatic enamel at different rates, which facilitates strong micromechanical bonding.9 Other methods to roughen the enamel surface before bonding orthodontic attachments, though not widely used, are described in the literature and include air abrasion, lasers and conditioning with maleic acid.9,10,11
Self-etch primers (SEPs) are bonding agents, which do not require a separate etching or conditioning step. Instead, dissolution of calcium from the hydroxyapatite crystals is followed by incorporation of the calcium in the polymerised resin by means of a methacrylated phosphoric acid ester.12 Various systematic reviews and meta-analyses have either concluded that there is weak evidence of a higher bracket bond failure rate with SEP compared to the conventional AE bonding technique, or that there is no clinical difference in terms of failure between the techniques.7,13,14
Bonding to restorative materials
The specific challenges and associated modifications relating to each restorative material are outlined and summarised in Table 1.
Ceramic restorations
Dental ceramics are widely used in restorative dentistry for their biocompatibility, natural appearance and excellent mechanical properties. Initially, dental crowns made from materials like feldspar or alumina were introduced in the early twentieth century and later, leucite was added to ceramics to reduce the thermal expansion differences between ceramics and metal alloys.9,15 Bonding orthodontic brackets to ceramic surfaces is more challenging than to enamel, requiring effective bonding techniques. Ceramics require specific etching methods due to their acid resistance and the success of bonding composite cement to ceramics mainly depends on the conditioning agent used.15 Ceramic surfaces can be treated by mechanical means, chemical, or both, using silane coupling agents to achieve chemical adhesion between the organic cement and the inorganic ceramic.
Chemical conditioning of ceramic surfaces
Hydrofluoric acid (HF) is a corrosive, inorganic acid, used widely in industry for its capability to etch glass, metal and silicon compounds, making it well-suited to overcome the acid resistance of dental ceramics. Etching with HF creates a microscopically porous surface that enhances micromechanical retention between ceramic and adhesive resins by selectively reacting with the glassy phases.9 The standard procedure involves etching the ceramic surface with 9.6% HFA for one minute, followed by rinsing with water and applying a silane coupling agent.16 It has also been recommended to use a neutralising agent, such as sodium hypochlorite (NaOCl), after the HF application to remove residual acidity.9 While HFA is effective, caution is necessary due to its corrosive nature and toxicity to living tissues. This risk relates to its toxicity, particularly to soft tissues, rather than its acidity.9,17 It is therefore critical to protect all soft tissues and to use a minimal amount of HF product to treat the ceramic surface and exercise care when rinsing the agent, employing high-volume suction.
It has been demonstrated that using silane coupling agents, composed of hybrid inorganic-organo-functional trialkoxysilane monomers, significantly enhances the adhesion of orthodontic brackets to porcelain surfaces.9,18 Silane operates by establishing weak chemical connections with both organic and inorganic materials, facilitating adhesion between the porcelain and the composite resin.3 Following application of the silane coupling agent, a standard bonding agent and composite bracket adhesive is used (Fig. 1).
The widely accepted view that phosphoric acid is ineffective as a ceramic etching agent has also been challenged.9,19,20 One laboratory-based study demonstrated a shear bond strength of 6-8 MPa for orthodontic brackets bonded to naturally glazed, feldspathic, porcelain-fused-to-metal blanks, with 37% OPhA etching and silane priming.19 Another laboratory study comparing molar tubes bonded to porcelain molar crowns when conditioned with 9.38% HF or 37% OPhA found both techniques produced comparable shear bond strengths when a silane primer was used before bonding.20
Mechanical conditioning of ceramic surfaces
The bond strength can be further increased by mechanically roughening the porcelain surface before etching, with either a diamond bur or sandblasting with aluminium oxide particles of <50 μm.9 Removal of the surface glaze will allow the acid to directly etch the underlying ceramic, but will also compromise the appearance of the ceramic at the debond stage.3 It is therefore recommended to reserve mechanical conditioning for cases where bracket bonding has failed or in joint orthodontic-restorative cases where replacement restorations are planned. Patients need to be aware of the potential compromise to the ceramic surface, which can occur when any bonding technique is used, but especially when mechanical conditioning is employed. An alternative to mechanically condition ceramic surfaces is to use CO2 or Nd:YAG (neodymium-doped yttrium aluminium garnet) lasers to condition the ceramic surface before silane application.9
Zirconia restorations
Zirconia is a polycrystalline material with no glass content. It can be used clinically as a monolithic (full-contour) restoration or veneered facially with a glassy ceramic to improve aesthetics.21 If there is facial ceramic, the methods described above for bonding orthodontic brackets to ceramic can be applied.22 Full-contour restorations are commonly less aesthetic but have extremely high shear strength and are therefore generally reserved for posterior teeth. Monolithic zirconia, which will only have a surface glaze, can be clinically distinguished from a ceramic surface by its lack of translucency and monochrome appearance (Fig. 2).
Bonding to monolithic zirconia is problematic due to the absence of etchable glass and traditionally, the APC bonding concept is employed when bonding is desired. This method uses air particle abrasion (A), zirconia primer (P) and adhesive composite resin (C) steps to create adhesion.23
A systematic review has proposed the use of airborne particle abrasion with 50 µm Al2O3 at 0.1-0.25 MPa in combination with a phosphate monomer containing adhesive resin to condition monolithic zirconia for bonding.24 A zirconia primer may only offer increased bond strength for orthodontic attachment bonding when the zirconia substructure is definitely exposed and the only predictable method to remove the glaze is with a diamond bur.25 Dental dam isolation is also advocated during zirconia bonding.
Resin composite restorations
Similar challenges arise when attempting to bond orthodontic attachments to surfaces, such as resin composite restorations or composite veneers. Suggested strategies to increase bond strength to existing composite include extending the etching time with OPhA to 30 − 60 seconds or mechanical roughening, either with intra-oral sandblasting or tungsten carbide or diamond burs.9,26 HF and silane coupling agents have been found to be ineffective and it can be expected that different composite materials will respond variably to the same conditioning technique, similar to ceramic responses.9,27 Simply roughening the composite surface with a bur before using a standard bonding primer has been shown to be the most effective method to achieving adequate bond strength, except in the case of nanofilled composites. In the latter, roughening by sandblasting followed by application of a plastic conditioner may be effective.26 Eslamian et al.27 have advocated for the use of ceramic brackets as a mitigation due to their significantly higher bond strengths. However, there is some concern that this increased bond strength could risk damaging the composite surface during bracket removal.9
Amalgam restorations
Zachrisson et al.28 pioneered the practice of bonding orthodontic brackets to amalgam, recommending air abrasion with 50 μm aluminium oxide particles followed by the application of resin adhesives. Although this approach yielded a significantly lower mean tensile bond strength to that of metal brackets bonded to etched enamel, shear bond strength may be sufficient for orthodontic purposes.9,29,30 Attempts to enhance amalgam adhesion by surface roughening with diamond burs resulted in lower bond strengths compared to sandblasting.9,30 The use of a 4-META (4-methacryloxyethyl trimellitate anhydride) metal primer has been recommended to increase bond strength to existing amalgam restorations.3 However, the evidence to support its use for this purpose is lacking.9,31
Small amalgam restorations on molar buccal surfaces may not require any special treatment if sufficient enamel is available to contact the molar tube.3 Conversely, teeth with larger amalgam restorations are potentially more suited to banding, especially if the facility to use intra-oral sandblasting is unavailable.
Gold and stainless-steel restorations
Using 4-META in conjunction with surface conditioning may be an effective method for bonding to various metal surfaces, such as gold and stainless steel, with sandblasting being the most effective conditioning method.3,9 Nonetheless, the bond strength to these metals is notably lower compared to that achieved with amalgam.32 Another method researched for bonding to gold, which yielded higher shear bond strength, was sandblasting the gold surface with 30 μm silicon dioxide followed by silane application. The silicon dioxide both roughens the gold surface and embeds a fine silica layer on the surface.33 These tests were, however, conducted in laboratory settings using standardised gold alloy.9 As a result, successfully bonding orthodontic appliances to these materials continues to be difficult, and banding remains a more predictable alternative.
Whitened teeth
Tooth whitening is becoming increasingly popular as a cosmetic procedure, available both through professional in-clinic services and do-it-yourself home kits. The active constituents in dental bleaching formulae are hydrogen peroxide or carbamide peroxide. The whitening effect is achieved through an oxidative process, where peroxide compounds penetrate the tooth enamel, generating oxygen-free radicals and hydrogen peroxide ions.34 These agents then move through the enamel and into the dentine layer, where they act to lighten the tooth by breaking down both surface-level and deeper stains into colourless substances.35 After whitening, oxygen-free radicals can linger on the tooth enamel for several days. Oxygen acts as an inhibitor to the hardening process of dental composite materials. Consequently, during this period, the bonding of composite materials can be affected during the polymerisation phase.
Adjustments to orthodontic bonding for whitened teeth
Laboratory studies suggest waiting up to three weeks after bleaching for optimal bonding but real-world studies show varied results, making it hard to pinpoint a recommended hiatus.3 Comparing the traditional AE technique with self-etching primers, evidence suggests that the conventional approach results in stronger bonds.36 One explanation is that bleaching agents may reduce the calcium and phosphate levels in enamel.3,36 It is, therefore, recommended to use separate etching and bonding steps, rather than self-etch priming when bonding to bleached enamel and although the evidence is equivocal, it seems prudent to advise patients not to use bleaching products for 1-2 weeks before the placement of fixed appliances.
Dental fluorosis
Dental fluorosis results from high fluoride intake during tooth development, causing disturbances in tooth formation, with drinking water being the most common source of excessive exposure.37,38 Excessive fluoride during enamel formation impedes elimination of enamel proteins such as amelogenins, resulting in subsurface porosities and areas of hypomineralisation. Affected enamel can vary in appearance from white striations to brown stains or pits in severe forms.39 Severity can be quantified using the 10-point Thystrup and Fejerskov Index (TFI).40 It has been shown that resin bond strength is reduced in fluorosed teeth.41
Adjustments to orthodontic bonding for teeth with fluorosis
The two mains challenges presented by severely fluorosed enamel are achieving an adequate bond strength and the risk of surface enamel fracture at debond.41 There is a lack of clinical evidence available to inform the best approach while the quality of the enamel varies markedly both between patients and within individual patients teeth.3 It is generally accepted the more fluorosed the enamel surface, the longer the etch time needs to be. Specifically, it has been demonstrated that in milder forms (TFI 1-3) enamel reacts in a similar manner to etching regular enamel with 37% OPhA, whilst more severely affected enamel (TFI 4) requires doubling the etch time (to 30-60 seconds) to produce a similar depth and pattern. For more severe forms (TFI 5+), increasing the etching time correlates poorly with the etch pattern produced and it is suggested that the superficial enamel may need to be ground away before etching the subsurface enamel to 15-30 seconds.42 An alternate technique is to ‘deproteinase' the surface enamel with a 5.25% NaOCl solution for 60 seconds before regular etching with 37% OPhA. This has been shown to increase shear bond strength by an average of 50% for teeth with a TFI of 4.43
It is important to consider that an increased bond strength may increase the risk to enamel fracture at debond.41 Therefore, a cautious approach would be to only employ the more aggressive techniques on a case-by-case basis and possibly only after a standard approach has failed to adequately retain brackets in patients with dental fluorosis.
Amelogenesis imperfecta
Amelogenesis imperfecta (AI) is a hereditary condition that affects the enamel of both primary and permanent teeth. It has four principal forms:44
-
Hypoplastic
-
Hypocalcified
-
Hypomature and
-
Hypomaturation/hypoplastic with taurodontism.
These main forms can then be further subdivided based on mode of inheritance and clinical appearance.44 There is large variability in the clinical presentation from light colour change and surface irregularities to extensive enamel breakdown. Orthodontic treatment in AI presents significant challenges due to inferior bond strengths, fragile enamel, surface irregularities and lack of evidence-based clinical protocols.45,46,47 This can lead to multiple unwanted debonds and further breakdown of enamel during bracket removal.
The effect on enamel is different for each form of AI but abnormal crystallite formation and decreased mineral content have been reported, which may negatively affect resin bonding.47,48 Normal enamel is harder and has higher resin-bonding strength than hypocalcified AI enamel.49 Direct restorations, such as resin composite strip crowns and less-than-four-surface composite resin restorations are also more prone to failure than on unaffected teeth.50
Adjustments to orthodontic bonding for teeth with amelogenesis imperfecta
Hypocalcified enamel is more porous and has lower mineral content than sound enamel.49,50 There is some evidence that deproteinisation with pre-treatment of 5% NaOCl may increase the bond strength to hypocalcified enamel.51,52,53 However, Faria-e-Silva et al.47 investigated the bond strength to extracted permanent hypocalcified AI molars and reported inferior bond strength compared to sound teeth and no improvement with the use of 5% NaOCl. Others have suggested the use of glass ionomer cement-based adhesives to improve bracket retention and prevent further enamel demineralisation.45 Ceramic or plastic orthodontic brackets may reduce the risk of cohesive failure of fragile enamel during debond procedures as they can be removed with a handpiece instead of pliers.3,45
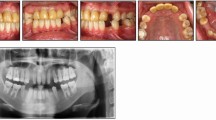
A predictable bond can be expected to the available enamel in hypoplastic AI (Fig. 3) as it represents a quantitative rather than a qualitative defect.46 It is practical, in an AI patient, to etch an easily accessible surface and examine if a clinical etch pattern is observed. However, in cases where large areas of dentine are exposed, either due to enamel breakdown or inherent quantitative defects, bonding challenges may arise. AI-affected dentine has a superficial, hypermineralised, acid-resistant layer impairing the penetration of resin adhesive, preventing resin tag formation in the tubule leading to a weaker hybrid layer.54,55 Epasinghe and Yiu examined the effect of additional AE on the bond strength of a self-etch adhesive to AI-affected dentine and did not find an improvement in the microtensile bond strength.56 Provisionally restoring such teeth with composite veneers or crowns allows for more predictable bracket bonding and also ensures better expression of the bracket prescription during the orthodontic phase of treatment (Fig. 4, Fig. 5). Similarly, in patients with a high rate of previous restoration failure, augmented retention such as banding should be considered. In circumstances where there has been loss proximal tooth structure, for example in posterior teeth affected by AI, challenges will arise when these are being prepared for full-coverage restoration due to the difficulty establishing a finish line and acceptable emergence profile for the restoration on the mesial and distal aspects for the tooth. In these cases, an addition benefit of banding the tooth (Fig. 6) is the increased interproximal distance afforded by the band space on debond.
a, b) Example of a patient with hypoplastic amelogenesis imperfecta where fixed appliances are bonded directly to enamel in the mandibular arch and to provisional direct composite resin veneers in the maxillary arch. The patient is being prepared for orthognathic surgery followed by definitive indirect maxillary arch restorations
Dentinogenesis imperfecta
Dentinogenesis imperfecta (DI) is a rare hereditary disorder of dentine formation following an autosomal dominant pattern of transmission. It affects both formation and mineralisation of dentine in both primary teeth and permanent teeth and affected teeth appear to have altered colour and transparency - typically, amber or grey-blue, or opalescent.57 In mild forms, the enamel remains intact, while in more severe cases, the enamel fractures from the underlying affected dentine.
The condition is often described using the classification by Shields et al.,58 who divided DI into three subtypes:
-
Type I, associated with osteogenesis imperfecta (OI) and resulting from mutations in the collagen type I genes
-
Type II, presents clinically similar to Type I but in the absence of OI (though radiographically, the crowns appear bulbous with a characteristic cervical constriction and accompanied by short and slender roots)
-
Type III, a phenotype characterised by large pulp chambers.
Type II is the most common variant and more recently, it has been demonstrated that Type II and Type III are essentially the same disease, associated with mutation of the dentine sialophosphoprotein gene located on the chromosome 4q21.57
Adjustments to orthodontic bonding for teeth with dentinogenesis imperfecta
Research in the area of orthodontic bonding to DI teeth is limited to case reports.59,60 Experience suggests the enamel of intact DI teeth can be treated the same as regular enamel, though there is a risk of enamel fracturing or shearing off.59,60 If this is a concern, the teeth may be banded.59 At the other extreme, some teeth may lack sufficient structure for bonding of attachments and may require indirect restoration before orthodontic treatment.61 Where dentine is exposed and there is a need to bond attachments directly to dentine, a self-etching dentine bonding agent is recommended.
Microdontia and hypodontia
Bonding orthodontic brackets to teeth with aberrant crown morphologies exposes a limitation of pre-adjusted edgewise appliance systems. Microdontia of maxillary incisor teeth is reasonably common, with the prevalence of peg-shaped maxillary permanent lateral incisors being 1.6% in the general population, rising to 2.7% in orthodontic patients.62 Generally, the crowns of these teeth have a reduced size in all three dimensions. Building these teeth up to a more correct size and form with resin composite before bonding fixed appliances has several advantages apart from the immediate aesthetic improvement (Fig. 7), allowing for faithful expression of the bracket prescription and also providing a definite end-point to guide space closure.
a, b) Example of a patient with microdontia affecting the upper incisor teeth. Restoring these teeth with resin composite veneers before fixed appliance treatment provides not only an immediate aesthetic improvement, but also will allow for correct expression of the bracket prescription and provide a definite end-point when closing the spaces on an arch wire
Conclusion
A range of special circumstances which require modification of standard techniques for bonding orthodontic attachments have been outlined. Adjuncts such as intra-oral sandblasters, along with additional conditioning etchants, bonding and coupling agents, when used appropriately, will help to increase bonding strength. Clinicians should be mindful of the variations between patients with the same developmental condition and indeed, the subtle differences between similar restorative materials in order to mitigate the increased risk of bond failure in these unique situations.
References
Buonocore M G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res 1955; 34: 849-853.
Newman G V. Epoxy adhesives for orthodontic attachments: progress report. Am J Orthod 1965; 51: 901-912.
Prado N, Caldwell S, Ashley M. Orthodontic bonding to atypical tooth surfaces. Orthod Update 2020; 13: 57-62.
Czolgosz I, Cattaneo P M, Cornelis M A. Computer-aided indirect bonding versus traditional direct bonding of orthodontic brackets: bonding time, immediate bonding failures, and cost-minimization. A randomized controlled trial. Eur J Orthod 2021; 43: 144-151.
Reynolds I R, von Fraunhofer J A. Direct bonding of orthodontic brackets - a comparative study of adhesives. Br J Orthod 1976; 3: 143-146.
Millett D T, McCabe J F. Orthodontic bonding with glass ionomer cement - a review. Eur J Orthod 1996; 18: 385-399.
Dudás C, Czumbel L M, Kiss S et al. Clinical bracket failure rates between different bonding techniques: a systematic review and meta-analysis. Eur J Orthod 2023; 45: 175-185.
Sadowsky P L, Retief D H, Cox P R, Hernández-Orsini R, Rape W G, Bradley E L. Effects of etchant concentration and duration on the retention of orthodontic brackets: an in vivo study. Am J Orthod Dentofacial Orthop 1990; 98: 417-421.
Alzainal A H, Majud A S, Al-Ani A M, Mageet A O. Orthodontic Bonding: Review of the Literature. Int J Dent 2020; 2020: 8874909.
Berk N, Başaran G, Ozer T. Comparison of sandblasting, laser irradiation, and conventional acid etching for orthodontic bonding of molar tubes. Eur J Orthod 2008; 30: 183-189.
Olsen M E, Bishara S E, Damon P, Jakobsen J R. Evaluation of Scotchbond Multipurpose and maleic acid as alternative methods of bonding orthodontic brackets. Am J Orthod Dentofacial Orthop 1997; 111: 498-501.
Chu C H, Ou K L, Dong de R, Huang H M, Tsai H H, Wang W N. Orthodontic bonding with self-etching primer and self-adhesive systems. Eur J Orthod 2011; 33: 276-281.
Fleming P S, Johal A, Pandis N. Self-etch primers and conventional acid-etch technique for orthodontic bonding: a systematic review and meta-analysis. Am J Orthod Dentofacial Orthop 2012; 142: 83-94.
Kerayechian N, Bardideh E, Bayani S. Comparison of self-etch primers with conventional acid-etch technique for bonding brackets in orthodontics: a systematic review and meta-analysis. Eur J Orthod 2022; 44: 385-395.
Ramakrishnaiah R, Alkheraif A A, Divakar D D, Matinlinna J P, Vallittu P K. The Effect of Hydrofluoric Acid Etching Duration on the Surface Micromorphology, Roughness, and Wettability of Dental Ceramics. Int J Mol Sci 2016; 17: 822.
Karan S, Toroglu M S. Porcelain refinishing with two different polishing systems after orthodontic debonding. Angle Orthod 2008; 78: 947-953.
Bajraktarova-Valjakova E, Korunoska-Stevkovska V, Georgieva S et al. Hydrofluoric Acid: Burns and Systemic Toxicity, Protective Measures, Immediate and Hospital Medical Treatment. Open Access Maced J Med Sci 2018; 6: 2257-2269.
Barceló Santana H F, Hernández Medina R, Acosta Torres S L, Sánchez Herrera L M, Fernández Pedrero A J, Ortíz González R. Evaluation of bond strength of metal brackets by a resin to ceramic surfaces. J Clin Dent 2006; 17: 5-9.
Pannes D D, Bailey D K, Thompson J Y, Pietz D M. Orthodontic bonding to porcelain: a comparison of bonding systems. J Prosthet Dent 2003; 89: 66-69.
Purmal K, Alam M K, Sukumaran P. Shear bond strength of orthodontic buccal tubes to porcelain. Dent Res J 2013; 10: 81-86.
Heffernan M J, Aquilino S A, Diaz-Arnold A M, Haselton D R, Stanford C M, Vargas M A. Relative translucency of six all-ceramic systems. Part I. core materials. J Prosthet Dent 2002; 88: 4-9.
Heffernan M J, Aquilino S A, Diaz-Arnold A M, Haselton D R, Stanford C M, Vargas M A. Relative translucency of six all-ceramic systems. Part II. core and veneer materials. J Prosthet Dent 2002; 88: 10-15.
Blatz M B, Alvarez M, Sawyer K, Brindis M. How to Bond Zirconia: The APC Concept. Compend Contin Educ Dent 2016; 37: 611-617.
Quigley N P, Loo D S S, Choy C, Ha W N. Clinical efficacy of methods for bonding to zirconia: A systematic review. J Prosthet Dent 2021; 125: 231-240.
Kwak J Y, Jung H K, Choi I K, Kwon T Y. Orthodontic bracket bonding to glazed full-contour zirconia. Restor Dent Endod 2016; 41: 106-113.
Viwattanatipa N, Jermwiwatkul W, Chintavalakorn R, Nanthavanich N. The effect of different surface preparation techniques on the survival probabilities of orthodontic brackets bonded to nanofill composite resin. J Orthod 2010; 37: 162-173.
Eslamian L, Borzabadi-Farahani A, Mousavi N, Ghasemi A. The effects of various surface treatments on the shear bond strengths of stainless steel brackets to artificially-aged composite restorations. Aust Orthod J 2011; 27: 28-32.
Zachrisson B U, Buyukyilmaz T. Recent advances in bonding to gold, amalgam and porcelain. J Clin Orthod 1993; 27: 661-675.
Sperber R L, Watson P A, Rossouw P E, Sectakof P A. Adhesion of bonded orthodontic attachments to dental amalgam: In vitro study. Am J Orthod Dentofacial Orthop 1999; 116: 506-513.
Skilton J W, Tyas M J, Woods M G. Effects of surface treatment on orthodontic bonding to amalgam. Aust Orthod J 2006; 22: 59-66.
Isman E, Ozsevık S, Yavan M A, Tosun S, Surmelioglu D. Effects of two metal primers on the shear bond strength of orthodontic molar tubes bonded to silver amalgam restorations of different dimensions. J Adhes Sci Technol 2016; 30: 1109-1118.
Zachrisson B U. Orthodontic bonding to artificial tooth surfaces: clinical versus laboratory findings. Am J Orthod Dentofacial Orthop 2000; 117: 592-594.
Ryu M J, Gang S N, Lim S H. Effect of silica coating on bond strength between a gold alloy and metal bracket bonded with chemically cured resin. Korean J Orthod 2014; 44: 105-112.
Pimentel A H, Valente L L, Isolan C P, Münchow E A, Piva E, de Moraes R R. Effect of waiting time for placing resin composite restorations after bleaching on enamel bond strength. Appl Adhes Sci 2015; 3: 23.
McEvoy S A. Chemical agents for removing intrinsic stains from vital teeth. II. Current techniques and their clinical application. Quintessence Int 1989; 20: 379-384.
Gurgan S, Alpaslan T, Kiremitci A, Cakir F Y, Yazici E, Gorucu J. Effect of different adhesive systems and laser treatment on the shear bond strength of bleached enamel. J Dent 2009; 37: 527-534.
Møller I J. Fluorides and dental fluorosis. Int Dent J 1982; 32: 135-147.
Goodarzi F, Mahvi A H, Hosseini M et al. The prevalence of dental fluorosis and exposure to fluoride in drinking water: a systematic review. J Dent Res Dent Clin Dent Prospects 2016; 10: 127-135.
DenBesten P K, Thariani H. Biological mechanisms of fluorosis and level and timing of systemic exposure to fluoride with respect to fluorosis. J Dent Res 1992; 71: 1238-1243.
Thylstrup A, Fejerskov O. Clinical appearance of dental fluorosis in permanent teeth in relation to histologic changes. Community Dent Oral Epidemiol 1978; 6: 315-328.
Wiltshire W A, Noble J. Clinical and laboratory perspectives of improved orthodontic bonding to normal, hypoplastic, and fluorosed enamel. Semin Orthod 2010; 16: 55-65.
Al-Sugair M H, Akpata E S. Effect of fluorosis on etching of human enamel. J Oral Rehabil 1999; 26: 521-528.
Sharma R, Kumar D, Verma M. Deproteinization of Fluorosed Enamel with Sodium Hypochlorite Enhances the Shear Bond Strength of Orthodontic Brackets: An In vitro Study. Contemp Clin Dent 2017; 8: 20-25.
Witkop C J Jr. Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. J Oral Pathol 1988; 17: 547-553.
Arkutu N, Gadhia K, McDonald S, Malik K, Currie L. Amelogenesis imperfecta: the orthodontic perspective. Br Dent J 2012; 212: 485-489.
Seow W K, Amaratunge A. The effects of acid-etching on enamel from different clinical variants of amelogenesis imperfecta: an SEM study. Pediatr Dent 1998; 20: 37-42.
Faria-e-Silva A L, De Moraes R R, Menezes Mde S, Capanema R R, De Moura A S, Martelli H Jr. Hardness and microshear bond strength to enamel and dentin of permanent teeth with hypocalcified amelogenesis imperfecta. Int J Paediatr Dent 2011; 21: 314-320.
Gopinath V K, Al-Salihi K A, Yean C Y, Ann M C, Ravichandran M. Amelogenesis imperfecta: enamel ultra-structure and molecular studies. J Clin Pediatr Dent 2004; 28: 319-322.
Wright J T, Deaton T G, Hall K I, Yamauchi M. The mineral and protein content of enamel in amelogenesis imperfecta. Connect Tissue Res 1995; 32: 247-252.
Chen C F, Hu J C, Estrella M R, Peters M C, Bresciani E. Assessment of restorative treatment of patients with amelogenesis imperfecta. Pediatr Dent 2013; 35: 337-342.
Saroğlu I, Aras S, Oztaş D. Effect of deproteinization on composite bond strength in hypocalcified amelogenesis imperfecta. Oral Dis 2006; 12: 305-308.
Venezie R D, Vadiakas G, Christensen J R, Wright J T. Enamel pretreatment with sodium hypochlorite to enhance bonding in hypocalcified amelogenesis imperfecta: case report and SEM analysis. Pediatr Dent 1994; 16: 433-436.
Ekambaram M, Yiu C K. Bonding to hypomineralized enamel - a systematic review. Int J Adhes Adhes 2016; 69: 27-32.
Van Meerbeek B, Braem M, Lambrechts P, Vanherle G. Morphological characterization of the interface between resin and sclerotic dentine. J Dent 1994; 22: 141-146.
Sánchez-Quevedo M C, Ceballos G, García J M, Luna J D, Rodríguez I A, Campos A. Dentine structure and mineralization in hypocalcified amelogenesis imperfecta: a quantitative X-ray histochemical study. Oral Dis 2004; 10: 94-98.
Epasinghe D J, Yiu C K Y. Effect of etching on bonding of a self-etch adhesive to dentine affected by amelogenesis imperfecta. J Investig Clin Dent 2018; DOI: 10.1111/jicd.12276.
De La Dure-Molla M, Philippe Fournier B, Berdal A. Isolated dentinogenesis imperfecta and dentin dysplasia: revision of the classification. Eur J Hum Genet 2015; 23: 445-451.
Shields E D, Bixler D, el-Kafrawy A M. A proposed classification for heritable human dentine defects with a description of a new entity. Arch Oral Biol 1973; 18: 543-553.
Kindelan J, Tobin M, Roberts-Harry D, Loukota R A. Orthodontic and orthognathic management of a patient with osteogenesis imperfecta and dentinogenesis imperfecta: a case report. J Orthod 2003; 30: 291-296.
Fan F, Li N, Huang S, Ma J. A multidisciplinary approach to the functional and esthetic rehabilitation of dentinogenesis imperfecta type II. A clinical report. J Prosthet Dent 2019; 122: 95-103.
Soliman S, Meyer-Marcotty P, Hahn B, Halbleib K, Krastl G. Treatment of an Adolescent Patient with Dentinogenesis Imperfecta Using Indirect Composite Restorations - A Case Report and Literature Review. J Adhes Dent 2018; 20: 345-354.
Hua F, He H, Ngan P, Bouzid W. Prevalence of peg-shaped maxillary permanent lateral incisors: A meta-analysis. Am J Orthod Dentofacial Orthop 2013; 144: 97-109.
Acknowledgments
The authors would like to thank Professor P Fleming and Mr Giovanni Franco for providing the clinical photographs in Figure 1 and the ceramic restoration examples in Figure 2 respectively.
Funding
Open Access funding provided by the IReL Consortium.
Author information
Authors and Affiliations
Contributions
Angus Burns: overall design of the piece. Majority of the background literature researching and manuscript writing. Treatment (orthodontics) of clinical cases provided. Annie Hughes: contribution to background literature research and writing. Treatment (restorative dentistry) of clinical cases provided. Michael O'Sullivan: contribution to writing in area of amelogenesis imperfect and editing manuscript. Treatment planning and supervision of treatment (restorative dentistry) of clinical some of the clinical cases provided.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0.© The Author(s) 2024.
About this article
Cite this article
Burns, A., Hughes, A. & O’Sullivan, M. Orthodontic bonding in special circumstances. Br Dent J 237, 400–406 (2024). https://doi.org/10.1038/s41415-024-7791-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41415-024-7791-z