Abstract
Background
Steroid hormones influence breast morphology and cellular proliferation and are associated with breast carcinogenesis. However, their associations with mammographic breast density (MBD) are less studied, particularly in premenopausal women. We, therefore, investigated the associations of steroid hormone metabolites with MBD in premenopausal women.
Methods
Our study included 700 premenopausal women scheduled for screening mammograms. We analyzed 54 steroid hormone metabolites (Metabolon®) and assessed volumetric measures of MBD including volumetric percent density (VPD), dense volume (DV), and non-dense volume (NDV) using Volpara. We investigated associations using linear regression modeling to estimate the covariate-adjusted means of VPD, NDV, and DV, corresponding to each steroid hormone metabolite tertile and on a continuous scale. Models were adjusted for age, body fat percentage, age at menarche, race, alcohol consumption, family history of breast cancer, oral contraceptive use, body shape at age 10, and parity/age at first birth. We applied false discovery rate (FDR) to control multiple testing and determined significance at FDR-adjusted p-value ≤ 0.05.
Results
One corticosteroid (cortolone glucuronide (1)) and four androgenic steroid metabolites (androstenediol (3beta,17beta) monosulfate (2), androstenediol (3beta,17beta) disulfate (1), 5alpha-androstan-3alpha,17beta-diol monosulfate (2), and 5alpha-androstan-3alpha,17beta-diol disulfate) were inversely associated with VPD. For instance, VPD was lower monotonically across tertiles (T) of cortolone glucuronide (1) (T1 = 8.9%, T2 = 8.3%, and T3 = 7.3%; p-trend=7.55 × 10−5, FDR p-value = 0.01); androstenediol (3beta,17beta) monosulfate (2), (T1 = 8.8%, T2 = 8.6% and T3 = 7.5%; p-trend=8.89 × 10−4, FDR p-value = 0.03), and androstenediol (3beta,17beta) disulfate (1) (T1 = 9.0%, T2 = 8.4% and T3 = 7.6%; p-trend=8.41 × 10−4, FDR p-value = 0.03). Five progestin steroid metabolites were positively associated with VPD, but only 5alpha-pregnan-3beta,20alpha-diol monosulfate (2) was marginally significant after FDR correction (T1 = 7.5%, T2 = 8.2%, T3 = 8.8%; p-trend=4.56 × 10−3, FDR p-value = 0.06). Two corticosteroid metabolites, tetrahydrocortisol glucuronide and cortolone glucuronide (1), were positively associated with NDV. For instance, NDV was higher across tertiles of cortolone glucuronide (1) (T1 = 744.3 cm3, T2 = 829.0 cm3, and T3 = 931.8 cm3; p-trend=4.64 × 10−6, FDR p-value = 7.51 × 10−4). No metabolites were associated with DV.
Conclusion
We identified novel inverse associations of cortolone glucuronide (1) and four androgenic steroid metabolites with VPD, underscoring the importance of steroid hormone metabolites in MBD and the potential for modulating these in reducing MBD.
Similar content being viewed by others
Introduction
Steroid hormones play important roles in breast development and function throughout a woman’s life [1]. Changes in the structure and morphology of female breasts occur during transitions between different reproductive phases and are mainly driven by steroid hormones [2, 3]. This underscores the importance of steroid hormones in breast tissue development and proliferation, which could potentially precede breast carcinogenesis [4].
Mammographic breast density (MBD) is a known risk factor for breast cancer [5, 6]. Steroid hormones stimulate proliferation of breast epithelial and stromal cells, resulting in more fibroglandular tissue content. Estrogens upregulate growth factors and mitogenic signaling in breast tissue, and progestogens further enhance epithelial proliferation and may act synergistically with estrogens [7, 8]. This mechanistic link is supported by clinical evidence, as demonstrated by observations of reduction in MBD with gonadotropin-releasing hormone agonists or the use of tamoxifen [9, 10], while hormone replacement therapy in postmenopausal women is associated with increased MBD [11]. Additionally, factors such as nulliparity and later age at first childbirth are positively associated with MBD [12].
However, epidemiological studies investigating associations between steroid hormones and MBD have yielded mixed results [13,14,15,16,17,18]. Some studies have found positive associations of progesterone and 17-hydroxyprogesterone with MBD [13, 19, 20]. Likewise, estradiol, estrone, and estrone sulfate have been reported to be positively associated with MBD [13, 19, 21], although inverse or null associations have also been reported in some studies [22, 23]. Androgens such as testosterone and dehydroepiandrosterone (DHEA) have shown inverse associations with MBD in several studies [13, 14, 24]. These associations often differ between pre- and postmenopausal women. For instance, progesterone has shown stronger positive associations with MBD in premenopausal women [19]. These prior studies have limitations, including inconsistent results, inadequate control for potential confounders, and use of older mammography techniques and non-volumetric MBD assessment. Furthermore, most previous studies have focused on steroid hormones rather than their metabolites, potentially overlooking important downstream pathways in hormone metabolism that could influence MBD and breast cancer risk. Overall, while there is evidence linking steroid hormones to MBD, little is known about the associations of steroid hormone metabolites with MBD.
To address these limitations and gain a more comprehensive understanding of the role of steroid hormones in MBD, we investigated the associations of steroid hormone metabolites with MBD in premenopausal women. We evaluated metabolites from different steroid hormone sub-pathways, including those from sterol, pregnanolone, corticosteroid, progestin, estrogenic, and androgenic steroids. Additionally, we leveraged previously collected circulating progesterone data to examine correlations with steroid hormone metabolites. This approach provided biological context for the metabolite measurements. Our aim was to identify specific metabolites associated with MBD and reveal new insights into the complex interplay between steroid hormone metabolism and MBD.
Methods
Study population
Participants in this study included 705 premenopausal women who had screening mammograms at the Joanne Knight Breast Health Center of Washington University School of Medicine (WUSM), St. Louis, MO. Details of the study population and recruitment process have been previously published [25]. In summary, study materials were mailed to women who had their mammogram screening scheduled and were followed up with calls providing more details and answering questions relating to the study. Interested participants were screened in line with our inclusion and exclusion criteria. Inclusion criteria were: (i) premenopausal at the time of screening, (ii) not pregnant at the time of screening. Exclusion criteria were: (i) previous history of breast cancer, (ii) current or previous use of selective estrogen receptor modulators in the past 6 months, (iii) history of breast augmentation or reduction. Our analytic sample included 700 women after excluding 5 women whose MBD measures were unavailable. Participants filled out surveys providing information about their demographic, reproductive, and social history. On the day of their screening mammogram, height and weight were measured, fasting blood draws were performed, and body fat percentage was assessed using bioelectrical impedance with the OMRON Full Body Sensor Body Composition Monitor and Scale (model HBF-514C) [26]. Body shape at age 10 was self-reported using the Stunkard nine-figure somatotype pictogram (scale 1–9), where 1–2 represents the leanest body shapes and 6–9 the heaviest [27]. This scale has been previously validated for retrospective assessment of childhood body shape [28]. Within 30 min of blood draws, we obtained the plasma component of the samples and stored them at −80 °C at the Tissue Procurement Core of Siteman Cancer Center. Study approval was granted by the Institutional Review Board of WUSM, and all participants provided informed consent for the study.
Mammographic breast density
Volpara version 1.5 was used to assess volumetric measures of MBD: volumetric percent density (VPD, %), dense volume (DV, cm3), and non-dense volume (NDV, cm3). Volpara averaged the craniocaudal and mediolateral oblique views of both breasts in its assessment.
Steroid hormone metabolites
Plasma samples were processed at Metabolon Inc. (Morrisville, NC), following a methodology similar to a prior investigation [29]. In a concise overview, samples were analyzed using ultrahigh-performance liquid chromatography/mass spectrometry (UHPLC/MS) after methanol extraction. The profiling analysis comprised four arms. Two of these involve positive ionization methods of reverse phase chromatography optimized for both hydrophilic (LC/MS Pos Polar) and hydrophobic/lipid compounds (LC/MS Pos Lipid). The third arm involves negative ionization methods of reverse phase chromatography (LC/MS Neg), and the fourth involves negative ionization (LC/MS Neg Polar) coupled with hydrophilic interaction liquid chromatography method [30]. All methods alternated between full scan MS and data-dependent MSn scans, with the scan range generally spanning 70–1000 m/z. Metabolon’s proprietary software was used to identify spectral peaks in plasma samples, using area under the curve (AUC). Samples were distributed randomly during the platform’s analytical run. A composite quality control sample, made by merging aliquots from all study samples, was included for quality monitoring. To identify metabolites, the ion features in experimental samples were compared to a reference library of purified chemical standard entries [31]. To address potential batch variations, laboratory values for each metabolite were normalized by dividing them by the median values of metabolites within its instrument batch.
Circulating progesterone
In a subset of women (N = 335), we leveraged circulating progesterone levels that had been previously assayed at the Department of Laboratory Medicine, Boston Children’s Hospital (Boston, MA) using a competitive electrochemiluminescence immunoassay on the FDA-approved Roche E Modular system (Roche Diagnostics) [32].
Statistical analysis
For metabolite data preprocessing, a total of 1074 metabolites were initially identified. Metabolites with missing observations in ≥300 women and those with coefficients of variations (CV) ≥ 0.25 were excluded, leaving 828 metabolites (mean CV = 0.13). Missing values in the remaining metabolites were imputed via the “impute” package in R using the 10-nearest neighbor method [33]. This approach identifies 10-nearest neighbors using Euclidean distance and averages the values from those observations to impute the missing value [34]. We then applied ComBat normalization, a model-based approach that uses empirical Bayes shrinkage to adjust the mean and variance for each metabolite [35,36,37,38]. We included 54 steroid hormone metabolites for this study.
We used linear regression models to examine the associations of metabolites with MBD. Metabolite levels were categorized into tertiles, and the least square means (LSM) and 95% confidence intervals (CI) of VPD, DV, and NDV across these tertiles were estimated. Tertiles of a metabolite were operationalized as an ordinal variable in linear models to test for linear trends by assigning each participant the mean value of their tertile group, with a trend test p-value calculated. We also analyzed metabolites in continuous scale to examine changes in MBD corresponding to one standard deviation (SD) unit increase in the metabolite. MBD measures were log10-transformed before analysis to meet the normality and homoscedasticity assumptions, and the β-coefficients from linear modeling, as well as 95% CIs, were back-transformed to original scale for ease of interpretation. To determine whether age at menarche or body fat percentage modified the associations between steroid hormone metabolites and MBD, the interaction term between each metabolite and body fat percentage or age at menarche was added to the multivariable linear regression models. Stratified analyses were additionally performed for body fat percentage and age at menarche ( < vs. ≥ median). We also performed a sensitivity analysis restricted to women with a history of oral contraceptive (OC) use (N = 625) to assess associations between steroid hormone metabolites and MBD in a hormonally more homogeneous subgroup.
We accounted for several potential confounders in our models, including age (continuous), race (non-Hispanic white, non-Hispanic black, others), family history of breast cancer in first-degree relatives (yes, no), age at menarche (continuous), body fat percentage (continuous), body shape at age 10 (1&2, 3&4, 5, 6–9), alcohol consumption (never, <1 drink per week, 1–2 drinks per week, 3–5 drinks per week, and ≥6 drinks per week), OC use (never, <1 year, 1–4 years, 5–9 years, ≥10 years), parity and age at first birth (nulliparous, 1–2 children & <25 years, 1–2 children & 25–29 years, 1–2 children & ≥30 years, ≥3 children & <25 years, ≥3 children & ≥25 years). We adjusted for body fat percentage rather than body mass index (BMI) because they were both highly correlated (r = 0.88), and body fat percentage explained slightly more variability in VPD (R2 = 0.45) compared to BMI (R2 = 0.43). For missing covariates, we performed multivariate imputation by chain equations using the “mice” package in R [39]. All linear regression models included adjustment for all covariates listed above.
In a subset of women with available progesterone measurements (N = 335), we performed partial Spearman rank correlation analysis between steroid hormone metabolites and circulating progesterone levels. Correlations were adjusted for age and BMI using the “ppcor” package in R [40]. In addition, we assessed the associations of circulating progesterone levels alone with MBD measures (VPD, DV, NDV) using the same covariate-adjusted linear regression models applied for metabolite analyses. Progesterone was evaluated both as a continuous variable and across tertiles.
To complement our metabolite-level analysis, we performed principal component analysis (PCA) for each steroid hormone metabolite sub-pathway. Because the estrogenic steroids sub-pathway contained only one metabolite, it was excluded. For each sub-pathway, we extracted the first principal component (PC1), and PC1 scores were correlated with MBD outcomes (VPD, DV, NDV) using Spearman rank correlation.
To address multiple testing, we applied the Benjamini-Hochberg procedure to control the false discovery rate (FDR). Statistical significance was determined by FDR-adjusted p-value ≤ 0.05 for both the correlation and regression analyses. Statistical analyses were conducted using version 4.2.1 of R statistical software.
Results
The study population had a mean age of 46.0 years and a mean BMI of 30.0 kg/m², with 71.8% identifying as non-Hispanic White and 23.1% as non-Hispanic Black. The majority (76.6%) had no family history of breast cancer, and 39.9% reported ≥10 years of OC use (Table 1).
Steroid hormone metabolite determinants of MBD
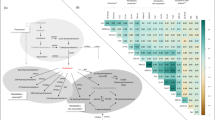
Five metabolites (one corticosteroid and four androgenic steroids) were inversely associated with VPD. The mean VPD was lower across tertiles of cortolone glucuronide (1) from 8.9% in the first tertile T1, to 8.3% in T2, and 7.3% in T3 [p-trend=7.55 × 10−5, FDR p-value = 0.01] (Table 2). One SD higher cortolone glucuronide (1) was associated with 7% lower VPD [FDR p-value = 0.01] (Fig. 1A; Supplemental Table 1). The four androgenic steroid metabolites inversely associated with VPD were androstenediol (3beta,17beta) monosulfate (2), androstenediol (3beta,17beta) disulfate (1), 5alpha-androstan-3alpha,17beta-diol monosulfate (2), and 5alpha-androstan-3alpha,17beta-diol disulfate. One SD higher 5alpha-androstan-3alpha,17beta-diol monosulfate (2) was associated with 7% lower VPD [FDR p-value = 0.01] (Fig. 1A; Supplemental Table 1). Across tertiles of androstenediol (3beta,17beta) monosulfate (2), the mean VPD was 8.8% in T1, 8.6% in T2, and 7.5% in T3 [p-trend=8.89 × 10−4, FDR p-value = 0.03]. Similarly, the mean VPD was 9.0% in T1, 8.4% in T2, and 7.6% in T3 of androstenediol (3beta,17beta) disulfate (1) (Table 2). Five of the seven progestin steroid metabolites were positively associated with VPD, although only 5alpha-pregnan-3beta,20alpha-diol monosulfate (2) was marginally significant after FDR correction [FDR p-value = 0.06]. The mean VPD across tertiles of 5alpha-pregnan-3beta,20alpha-diol monosulfate (2) was 7.5% in T1, 8.2% in T2, and 8.8% in T3 [p-trend=4.56 × 10−3, FDR p-value = 0.06] (Table 2). On the other hand, none of the 9 pregnenolone steroid metabolites were associated with VPD, even before FDR correction.
No steroid hormone metabolite was significantly associated with DV (Fig. 1B; Table 3).
Two corticosteroid metabolites, tetrahydrocortisol glucuronide and cortolone glucuronide (1), were positively associated with NDV. The mean NDV across tertiles of cortolone glucuronide (1) was 744.3 cm3 in T1, 829.0 cm3 in T2, and 931.8 cm3 in T3 [p-trend = 4.64 × 10−6, FDR p-value = 7.51 × 10−4] (Table 4). One SD higher cortolone glucuronide (1) was associated with 8 cm3 higher NDV [FDR p-value = 0.01] (Fig. 1C; Supplemental Table 2). Similarly, NDV was 785.2 cm3 in T1, 807.9 cm3 in T2, and 900.8 cm3 in T3 of tetrahydrocortisol glucuronide [p-trend=1.86 × 10−3, FDR p-value = 0.05]. No androgenic steroid metabolite was significantly associated with NDV, but 5alpha-androstan-3beta,17beta-diol disulfate was marginally significant after FDR correction [FDR p-value = 0.06] (Table 4). Cortolone glucuronide (1) was significantly associated with both VPD and NDV, but not DV.
Body fat percentage did not modify the associations of steroid hormone metabolites and MBD (data not shown). Age at menarche modified the association of 17alpha-hydroxypregnanolone glucuronide with VPD (FDR p-interaction=0.02). Across tertiles of 17alpha-hydroxypregnanolone glucuronide, VPD was 7.9% in T1, 7.3% in T2, and 7.6% in T3 in early menarche group ( < 13 years), and 9.3% in T1, 10.3% in T2, and 11.6% in T3 in late menarche group ( ≥ 13 years).
Sensitivity analysis
We observed no notable differences from the analysis of the whole study population in the analyses limited to women with a history of OC use (N = 625), except that the two androgenic steroid metabolites (androstenediol (3beta,17beta) disulfate (1) and 5alpha-androstan-3alpha,17beta-diol disulfate) which were inversely associated with VPD in the whole study population were not statistically significant in the subset (Supplemental Table 3). However, the directions and magnitude of their associations remained unchanged (Table 2; Supplemental Table 3).
Correlation with circulating progesterone
Of the 54 steroid hormone metabolites, 21 were significantly and positively correlated with circulating progesterone levels (FDR p-value ≤ 0.05) in the subset with available progesterone measurements (N = 335). Metabolites in the progestin steroids sub-pathway had the strongest positive correlations. The top five metabolites positively correlated with circulating progesterone were 5alpha-pregnan-3beta,20beta-diol monosulfate (1) [r = 0.82, FDR p-value = 3.59 × 10–81], pregnanediol-3-glucuronide [r = 0.82, FDR p-value = 1.38 × 10–80], 5alpha-pregnan-3beta,20alpha-diol monosulfate (2) [r = 0.81, FDR p-value = 6.11 × 10–76], 5alpha-pregnan-3beta,20alpha-diol disulfate [r = 0.78, FDR p-value = 2.32 × 10–67], and 17alpha-hydroxypregnanolone glucuronide [r = 0.74, FDR p-value = 9.10 × 10–57] (Supplemental Fig. 1; Supplemental Table 4).
Circulating progesterone and MBD
No significant associations were observed between circulating progesterone and VPD, DV, or NDV (all p-values > 0.1, data not shown). These results suggest that, within this subset (N = 335), circulating progesterone alone was not associated with MBD.
Sub-pathway analysis
In PCA of steroid hormone metabolites sub-pathways, corticosteroid PC1 was inversely correlated with VPD [r = –0.27, p-value = 9.75×10–13] and positively correlated with NDV [r = 0.30, p-value = 1.49 × 10–15], consistent with findings from regression models for individual corticosteroid metabolites. Progestin PC1 was positively correlated with VPD [r = 0.19, p-value = 3.40 × 10–7] and inversely correlated with NDV [r = –0.17, p-value = 1.04 × 10–5]. In contrast, androgenic steroid PC1 was not strongly correlated with MBD outcomes [r ≈ –0.05 for VPD], despite several androgenic steroid metabolites showing significant inverse associations with VPD in regression analyses. Pregnenolone and sterol steroid PC1 were not correlated with MBD, consistent with null results in regression analyses (Supplemental Fig. 2).
Discussion
We found significant inverse associations between five steroid hormone metabolites (one corticosteroid and four androgenic steroids): cortolone glucuronide (1), androstenediol (3beta,17beta) monosulfate (2), androstenediol (3beta,17beta) disulfate (1), 5alpha-androstan-3alpha,17beta-diol monosulfate (2), and 5alpha-androstan-3alpha,17beta-diol disulfate and VPD in premenopausal women. Among these, cortolone glucuronide (1) was inversely associated with VPD and positively associated with NDV. These findings provide novel insights into the association of steroid hormone metabolism with MBD in premenopausal women. Our findings highlight the role of downstream steroid hormone metabolites, extend the understanding of hormonal regulation of MBD beyond prior studies that focused predominantly on steroid hormones, and suggest potential pathways for identifying new biomarkers to improve breast cancer risk stratification and prevention strategies in premenopausal women.
The pathway for steroid hormone synthesis begins with the conversion of cholesterol to pregnenolone by side-chain cleavage enzyme [41]. Subsequent enzymatic reactions convert pregnenolone to progesterone, initiating a cascade that leads to the synthesis of other progestogens, androgens, estrogens, and corticosteroids [42]. The observed associations of cortolone glucuronide (1) with both VPD and NDV are a significant and notable finding, as no prior study, to the best of our knowledge, has reported this metabolite in association with MBD. This association was confirmed in the analysis restricted to OC users. There is very limited information regarding this metabolite and how it may be related to MBD and/or breast cancer risk. However, few previous studies have reported observations about other corticosteroids and MBD. For instance, Gabrielson et al. reported an inverse association between 11-deoxycortisol, a precursor in cortisol biosynthesis, and percent MBD. Although 11-deoxycortisol was not profiled in our study, the downstream metabolite cortolone glucuronide (1) offers additional insights into the role of corticosteroid metabolism. Cortolone glucuronide (1) is formed through the reduction and subsequent glucuronidation of cortisol. Its association with BMI and fat mass index in a previous study [43] suggests that it could reflect metabolic processes linked to adiposity. However, the observed associations of cortolone glucuronide (1) with VPD and NDV in our study, which persisted after adjusting for body fat percentage, indicate additional mechanisms, independent of adiposity. Future research should investigate the potential biological mechanisms that might explain the association of cortolone glucuronide (1) with MBD.
We observed that progestin steroid metabolites were positively associated with VPD, although only 5alpha-pregnan-3beta,20alpha-diol monosulfate (2) was marginally significant after multiple testing correction. Notably, this metabolite was strongly correlated with circulating progesterone (r = 0.81). To our knowledge, no previous study has investigated the association of the specific progestin steroid metabolites that we profiled (n = 7, Table 2) with MBD measures. In contrast, circulating progesterone itself was not significantly associated with VPD, DV, or NDV. The smaller sample size for progesterone (N = 335 vs. 700 for metabolite analysis) may have limited our ability to detect associations. Prior studies examining progesterone alone have reported mixed results, with some showing positive associations with MBD [24, 44, 45], while others observed null associations [22, 46]. Considering these inconsistencies, our investigation of progestin steroid metabolites expands the current knowledge considerably. By examining these metabolites, we provide a deeper and more detailed view of the hormone activities at the cellular level, thereby providing a better opportunity to understand its metabolic and biochemical associations with MBD [47]. The strong positive correlations observed between these metabolites and circulating progesterone levels validate our methodology and support the biological relevance of studying these metabolites in relation to MBD, highlighting those that may serve as proxies for endogenous progesterone activity. Experimental evidence supports a role for progesterone metabolites in breast tissue proliferation. Wiebe et al. in an experiment on human breast cancer tissue observed that progesterone can fulfill its roles through the effect of two functionally distinct groups of its metabolites: 5alpha-pregnanes and 4-pregnenes [48]. Through 5alpha-reductase and 3alpha-hydroxysteroid oxidoreductase enzymes, breast tissue can convert progesterone irreversibly to 5alpha-pregnanes and reversibly to 4-pregnenes, respectively. Further experimental evidence in support of these findings was reported in two follow-up studies [49, 50]. Notably, breast tissue proliferation increases the stroma and epithelial cells of the breast which are represented as dense tissue on mammogram [51, 52]. Therefore, it is possible that the positive association between 5alpha-pregnane metabolites and VPD observed in our study is driven by their proliferation-stimulating effects on breast tissue. Alternatively, these findings may also implicate the receptor activator of nuclear factor-κB (RANK) signaling pathway. The RANK system is a mediator of progesterone-induced breast proliferation [53]. Higher serum levels of RANK-ligand (RANKL) have also been positively associated with VPD [54] and breast cancer risk [55]. Therefore, our findings on progestin metabolites might have been driven, in part, by the RANKL signaling system or by a combined effect of the proliferative effects of the 5alpha-pregnane metabolites and the RANKL system. Future research could determine whether these metabolites interact with the RANKL signaling system or operate through independent mechanisms to influence MBD.
We found novel inverse associations between four androgenic steroid metabolites and VPD, which have not been previously reported. Prior studies have mainly focused on androgenic hormones rather than the broader androgenic steroid metabolites (n = 23) examined in our analysis. Gabrielson et al. evaluated seven androgenic hormones and reported mostly null or weak associations of DHEA, DHEA sulfate (DHEA-S), androstenedione, testosterone, and free testosterone with MBD in premenopausal women [24]. However, they observed inverse associations of testosterone and free testosterone with MBD change [24]. Another study found an inverse association between free testosterone and percent MBD [13]. These findings indicate the complexity of androgen effects on breast tissue and the importance of considering specific androgenic steroid metabolites in addition to circulating hormones. Our findings provide additional insights by focusing on downstream androgenic steroid metabolites that may reflect more direct tissue exposure to androgens. Notably, the first principal component of the androgenic steroids sub-pathway (PC1) showed only a weak inverse correlation with VPD, indicating that pathway-level measures may mask the effects of individual metabolites with different biochemical activities or tissue-specific metabolism. Metabolites like 5alpha-androstan-3alpha,17beta-diol disulfate are products of 5alpha-reduction and sulfation, key metabolic processes that occur after androgenic hormones are synthesized and may influence stromal and epithelial components of breast tissue differently [56]. Androgens have shown positive associations with estrogen receptor positive (ER + ) breast cancer and inverse associations with ER− breast cancer [57]. However, the mechanisms behind these associations are not well understood. The inverse associations we observed between these androgenic steroid metabolites and VPD suggest that these metabolites may reflect metabolic pathways with potential relevance to ER− breast cancer. It is also possible that MBD and androgenic steroid metabolites be independent predictors of breast cancer risk. Overall, the association between these metabolites, MBD, and breast cancer risk is complex and not fully understood. Future studies are needed to clarify these associations and characterize the underlying biological mechanisms.
There are some limitations of this study. We cannot establish causality because our analysis is cross-sectional. We did not adjust for phases of the menstrual cycle. The steroid hormone metabolites most affected by cyclical fluctuations in estrogen and progesterone across the menstrual cycle in premenopausal women are primarily the estrogen metabolites, including estradiol and estrone [58], progesterone and its metabolites, notably allopregnanolone and pregnanediol [59], and to a lesser extent, androgens such as testosterone and 17-hydroxyprogesterone [60]. Other androgens such as dihydrotestosterone remain relatively stable across the cycle [58]. The variability in these metabolites may partly explain the inconsistent associations across studies in premenopausal women. Among the specific metabolites we evaluated, prior evidence is limited. However, based on our data, we hypothesize that the lack of cycle adjustment may mostly impact progestin metabolites, particularly the ones strongly correlated with circulating progesterone levels (Supplemental Table 4). This study has notable strengths. Our study population is large and racially diverse, hence, enhancing generalizability. We assessed volumetric measures of MBD. We analyzed metabolites that provide a reliable representation of endogenous hormone production. Our focus on metabolite-level associations captures downstream hormonal effects and reveals the activity of enzymatic pathways, providing mechanistic insights into MBD that cannot be inferred from circulating hormone levels alone. We performed sensitivity analysis among OC users, and the results were consistent with the overall analysis. OC use suppresses the cyclical variation seen in natural menstrual cycles, resulting in more stable steroid hormone metabolite levels. Restricting the analysis to OC users allowed us to assess whether observed associations were consistent in a hormonally homogeneous subgroup, thereby improving internal validity and interpretability. The consistency of results in this subgroup further reduces concerns about variability in endogenous steroid hormone metabolites.
In conclusion, we report inverse associations of cortolone glucuronide (1) and several androgenic steroid metabolites, as well as positive associations of progestin steroid metabolites, with VPD in premenopausal women. The novel associations observed, particularly for cortolone glucuronide (1) with both VPD and NDV, underscore the need for a more nuanced and comprehensive understanding of steroid hormone metabolism in relation to MBD and breast cancer risk. These findings offer insight into biomarkers of steroid hormones in premenopausal women and suggest potential targets for interventions aimed at influencing MBD, potentially informing future research on breast cancer risk and prevention strategies.
Data availability
The data and analytical code of this study will be made available upon reasonable request to the corresponding author.
References
Lange CA, Yee D. Progesterone and breast cancer. Women’s Health. 2008;4:151–62.
Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2:a003178–a.
Yu JH, Kim MJ, Cho H, Liu HJ, Han S-J, Ahn T-G. Breast diseases during pregnancy and lactation. Obstet Gynecol Sci. 2013;56:143–59.
Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8.
Pettersson A, Graff RE, Ursin G, dos Santos Silva I, McCormack V, Baglietto L, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106:dju078.
McCormack VA, Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15:1159–69.
Eigeliene N, Härkönen P, Erkkola R. Effects of estradiol and medroxyprogesterone acetate on morphology, proliferation and apoptosis of human breast tissue in organ cultures. BMC Cancer. 2006;6:246.
Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84:4559–65.
Howell A, Ashcroft L, Fallowfield L, Eccles DM, Eeles RA, Ward A, et al. RAZOR: a phase II open randomized trial of screening plus goserelin and raloxifene versus screening alone in premenopausal women at increased risk of breast cancer. Cancer Epidemiol, Biomark Prev. 2018;27:58–66.
Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–52.
McTiernan A, Martin CF, Peck JD, Aragaki AK, Chlebowski RT, Pisano ED, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J Natl Cancer Inst. 2005;97:1366–76.
Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control. 2000;11:653–62.
Bertrand KA, Eliassen AH, Hankinson SE, Rosner BA, Tamimi RM. Circulating hormones and mammographic density in premenopausal women. Horm Cancer. 2018;9:117–27.
Walker K, Fletcher O, Johnson N, Coupland B, McCormack VA, Folkerd E, et al. Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer Res. 2009;69:6490–9.
Jung S, Stanczyk FZ, Egleston BL, Snetselaar LG, Stevens VJ, Shepherd JA, et al. Endogenous sex hormones and breast density in young women. Cancer Epidemiol Biomark Prev. 2015;24:369–78.
Chen JH, Chen WP, Chan S, Yeh DC, Su MY, McLaren CE. Correlation of endogenous hormonal levels, fibroglandular tissue volume and percent density measured using 3D MRI during one menstrual cycle. Ann Oncol. 2013;24:2329–35.
Meyer F, Brisson J, Morrison AS, Brown JB. Endogenous sex hormones, prolactin, and mammographic features of breast tissue in premenopausal women2. JNCI: J Natl Cancer Inst. 1986;77:617–20.
Ahern TP, Sprague BL, Farina NH, Tsai E, Cuke M, Kontos D, et al. Lifestyle, behavioral, and dietary risk factors in relation to mammographic breast density in women at high risk for breast cancer. Cancer Epidemiol, Biomark Prev. 2021;30:936–44.
Gabrielson M, Azam S, Hardell E, Holm M, Ubhayasekera KA, Eriksson M, et al. Hormonal determinants of mammographic density and density change. Breast Cancer Res. 2020;22:95.
Noh JJ, Maskarinec G, Pagano I, Cheung LWK, Stanczyk FZ. Mammographic densities and circulating hormones: a cross-sectional study in premenopausal women. Breast. 2006;15:20–8.
Yong M, Atkinson C, Newton KM, Aiello Bowles EJ, Stanczyk FZ, Westerlind KC, et al. Associations between endogenous sex hormone levels and mammographic and bone densities in premenopausal women. Cancer Causes Control. 2009;20:1039–53.
Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, et al. The association of breast mitogens with mammographic densities. Br J Cancer. 2002;87:876–82.
Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomark Prev. 2005;14:2641–7.
Gabrielson M, Ubhayasekera KA, Acharya SR, Franko MA, Eriksson M, Bergquist J, et al. Inclusion of endogenous plasma dehydroepiandrosterone sulfate and mammographic density in risk prediction models for breast cancer. Cancer Epidemiol Biomark Prev. 2020;29:574–81.
Matthew KA, Getz KR, Jeon MS, Luo C, Luo J, Toriola AT. Associations of vitamins and related cofactor metabolites with mammographic breast density in premenopausal women. J Nutr. 2024;154:424–34.
Ning YS, Getz KR, Kyeyune JK, Jeon MS, Luo C, Luo J, et al. PFAS levels, early life factors, and mammographic breast density in premenopausal women. Environ Health Perspect. 2024;132:097008.
Stunkard AJ, Sørensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis. 1983;60:115–20.
Yochum L, Tamimi RM, Hankinson SE. Birthweight, early life body size and adult mammographic density: a review of epidemiologic studies. Cancer Causes Control. 2014;25:1247–59.
Collet T-H, Sonoyama T, Henning E, Keogh JM, Ingram B, Kelway S, et al. A metabolomic signature of acute caloric restriction. J Clin Endocrinol Metab. 2017;102:4486–95.
Evans AM, Bridgewater B, Liu Q, Mitchell M, Robinson R, Dai H, et al. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. 2014;4:1.
DeHaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminformatics. 2010;2:1–12.
Toriola AT, Appleton CM, Zong X, Luo J, Weilbaecher K, Tamimi RM, et al. Circulating receptor activator of nuclear factor-κB (RANK), RANK ligand (RANKL), and mammographic density in premenopausal women. Cancer Prev Res (Philos). 2018;11:789–96.
Hastie T, Tibshirani R, Narasimhan B, Chu G. impute: impute: imputation for microarray data [Internet]. Bioconductor version: Release. 2022.
Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, et al. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17:520–5.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27.
Fortin J-P, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–70.
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–3.
Yu Y, Zhang N, Mai Y, Ren L, Chen Q, Cao Z, et al. Correcting batch effects in large-scale multiomics studies using a reference-material-based ratio method. Genome Biol. 2023;24:201.
Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
Kim S. ppcor: An R package for a fast calculation to semi-partial correlation coefficients. Commun Stat Appl Methods. 2015;22:665–74.
Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. 1996;17.
Pregnenolone—an overview|ScienceDirect Topics.
McClain KM, Friedenreich CM, Matthews CE, Sampson JN, Check DP, Brenner DR, et al. Body composition and metabolomics in the Alberta Physical Activity and Breast Cancer Prevention Trial. J Nutr. 2021;152:419–28.
Hada M, Oh H, Fan S, Falk RT, Geller B, Vacek P, et al. Relationship of serum progesterone and progesterone metabolites with mammographic breast density and terminal ductal lobular unit involution among women undergoing diagnostic breast biopsy. J Clin Med. 2020;9:245.
Iversen A, Frydenberg H, Furberg A-S, Flote VG, Finstad SE, McTiernan A, et al. Cyclic endogenous estrogen and progesterone vary by mammographic density phenotypes in premenopausal women. Eur J Cancer Prev. 2016;25:9–18.
Jung S, Egleston BL, Chandler DW, Van Horn L, Hylton NM, Klifa CC, et al. Adolescent endogenous sex hormones and breast density in early adulthood. Breast Cancer Res. 2015;17:77.
Patti GJ, Yanes O, Siuzdak G. Metabolomics: the apogee of the omics trilogy. Nat Rev Mol cell Biol. 2012;13:263–9.
Wiebe JP, Muzia D, Hu J, Szwajcer D, Hill SA, Seachrist JL. The 4-pregnene and 5α-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion1. Cancer Res. 2000;60:936–43.
Wiebe JP. Progesterone metabolites in breast cancer. Endocr-Relat Cancer. 2006;13:717–38.
Wiebe JP, Rivas MA, Mercogliano MF, Elizalde PV, Schillaci R. Progesterone-induced stimulation of mammary tumorigenesis is due to the progesterone metabolite, 5α-dihydroprogesterone (5αP) and can be suppressed by the 5α-reductase inhibitor, finasteride. J Steroid Biochem Mol Biol. 2015;149:27–34.
Macias H, Hinck L. Mammary gland development. WIREs Dev Biol. 2012;1:533–57.
Haslam SZ, Woodward TL. Host microenvironment in breast cancer development: Epithelial-cell–stromal-cell interactions and steroid hormone action in normal and cancerous mammary gland. Breast Cancer Res. 2003;5:208.
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–7.
Toriola AT, Appleton CM, Zong X, Luo J, Weilbaecher K, Tamimi RM, et al. Circulating receptor activator of nuclear factor-κB (RANK), RANK ligand (RANKL) and mammographic density in premenopausal women. Cancer Prev Res. 2018;11:789–96.
Kiechl S, Schramek D, Widschwendter M, Fourkala E-O, Zaikin A, Jones A, et al. Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer. Oncotarget. 2016;8:3811–25.
Hilborn E, Stål O, Jansson A. Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: with a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget. 2017;8:30552–62.
Farhat GN, Cummings SR, Chlebowski RT, Parimi N, Cauley JA, Rohan TE, et al. Sex hormone levels and risks of estrogen receptor-negative and estrogen receptor-positive breast cancers. J Natl Cancer Inst. 2011;103:562–70.
Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, et al. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids. 2011;76:177–82.
Hamidovic A, Davis J, Soumare F, Datta A, Naveed A. Trajectories of allopregnanolone and allopregnanolone to progesterone ratio across the six subphases of menstrual cycle. Biomolecules. 2023;13.
Wang Y, Davis SR, Gialouris J, Desai R, Handelsman DJ. Evaluation of reference ranges of four circulating sex steroids from dried blood spots in women aged 18-40 years. J Steroid Biochem Mol Biol. 2025;253:106796.
Acknowledgements
We would like to thank all the women who participated in this study.
Funding
This study was supported by NIH/NCI R01CA246592. The funders had no role in the design of the study, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
ATT conceptualized the study and acquired the data. MSJ, CL, JL, and GP analyzed the data. GP, KAM, and GJP interpreted the data. GP and KAM wrote the first draft of the manuscript. All authors critically revised the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Ethics approval and consent to participate
Study approval was granted by the Institutional Review Board (IRB) (20200559) of Washington University School of Medicine in St. Louis. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pourali, G., Matthew, K.A., Jeon, M.S. et al. Steroid hormone metabolites and mammographic breast density in premenopausal women. Br J Cancer 134, 225–236 (2026). https://doi.org/10.1038/s41416-025-03246-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03246-4