Abstract
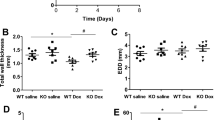
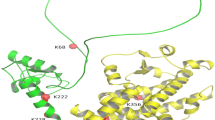
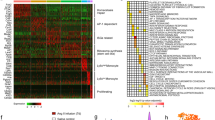
The elevated expression of poly(ADP-ribose) polymerase-1 (PARP1) and increased PARP1 activity, namely, poly(ADP-ribosyl)ation (PARylation), have been observed in cardiac remodeling, leading to extreme energy consumption and myocardial damage. However, the mechanisms underlying the regulation of PARP1 require further study. WWP2, a HECT-type E3 ubiquitin ligase, is highly expressed in the heart, but its function there is largely unknown. Here, we clarified the role of WWP2 in the regulation of PARP1 and the impact of this regulatory process on cardiac remodeling. We determined that the knockout of WWP2 specifically in myocardium decreased the level of PARP1 ubiquitination and increased the effects of isoproterenol (ISO)-induced PARP1 and PARylation, in turn aggravating ISO-induced myocardial hypertrophy, heart failure, and myocardial fibrosis. Similar findings were obtained in a model of ISO-induced H9c2 cells with WWP2 knockdown, while the reexpression of WWP2 significantly increased PARP1 ubiquitination and decreased PAPR1 and PARylation levels. Mechanistically, coimmunoprecipitation results identified that WWP2 is a novel interacting protein of PARP1 and mainly interacts with its BRCT domain, thus mediating the degradation of PARP1 through the ubiquitin–proteasome system. In addition, lysine 418 (K418) and lysine 249 (K249) were shown to be of critical importance in regulating PARP1 ubiquitination and degradation by WWP2. These findings reveal a novel WWP2–PARP1 signal transduction pathway involved in controlling cardiac remodeling and may provide a basis for exploring new strategies for treating heart disorders related to cardiac remodeling.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18.
Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7:782–94.
Henning RJ, Bourgeois M, Harbison RD. Poly(ADP-ribose) polymerase (PARP) and PARP inhibitors: mechanisms of action and role in cardiovascular disorders. Cardiovasc Toxicol. 2018;18:493–506.
Wang C, Xu W, Zhang Y, Zhang F, Huang K. PARP1 promote autophagy in cardiomyocytes via modulating FoxO3a transcription. Cell Death Dis. 2018;9:1047.
Wen JJ, Yin YW, Garg NJ. PARP1 depletion improves mitochondrial and heart function in Chagas disease: effects on POLG dependent mtDNA maintenance. PLoS Pathog. 2018;14:e1007065.
Pillai JB, Russell HM, Raman J, Jeevanandam V, Gupta MP. Increased expression of poly(ADP-ribose) polymerase-1 contributes to caspase-independent myocyte cell death during heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H486–96.
Feng GS, Zhu CG, Li ZM, Wang PX, Huang Y, Liu M, et al. Synthesis of the novel PARP-1 inhibitor AG-690/11026014 and its protective effects on angiotensin II-induced mouse cardiac remodeling. Acta Pharm Sin. 2017;38:638–50.
Lu J, Zhang R, Hong H, Yang Z, Sun D, Sun S, et al. The poly(ADP-ribosyl)ation of FoxO3 mediated by PARP1 participates in isoproterenol-induced cardiac hypertrophy. Biochim Biophys Acta. 2016;1863:3027–39.
Zhang W, Song M, Qu J, Liu GH. Epigenetic modifications in cardiovascular aging and diseases. Circ Res. 2018;123:773–86.
Nishida K, Yamaguchi O, Otsu K. Degradation systems in heart failure. J Mol Cell Cardiol. 2015;84:212–22.
Morreale FE, Walden H. Types of ubiquitin ligases. Cell. 2016;165:248.
Zou W, Chen X, Shim JH, Huang Z, Brady N, Hu D, et al. The E3 ubiquitin ligase Wwp2 regulates craniofacial development through mono-ubiquitylation of goosecoid. Nat Cell Biol. 2011;13:59–65.
Chen Z, Jiang H, Xu W, Li X, Dempsey DR, Zhang X, et al. A tunable brake for HECT ubiquitin ligases. Mol Cell. 2017;66:345–57.
Maddika S, Kavela S, Rani N, Palicharla VR, Pokorny JL, Sarkaria JN, et al. WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol. 2011;13:728–33.
Chen W, Jiang X, Luo Z. WWP2: a multifunctional ubiquitin ligase gene. Pathol Oncol Res. 2014;20:799–803.
Li H, Zhang Z, Wang B, Zhang J, Zhao Y, Jin Y. Wwp2-mediated ubiquitination of the RNA polymerase II large subunit in mouse embryonic pluripotent stem cells. Mol Cell Biol. 2007;27:5296–305.
Ishizue N, Niwano S, Niwano H, Oikawa J, Nakamura H, Hashikata T, et al. Linagliptin suppresses electrical and structural remodeling in the isoproterenol induced myocardial injury model. Int Heart J. 2019;60:411–8.
Hu H, Jiang M, Cao Y, Zhang Z, Jiang B, Tian F, et al. HuR regulates phospholamban expression in isoproterenol-induced cardiac remodeling. Cardiovasc Res. 2019;cvz205. https://doi.org/10.1093/cvr/cvz205. [Epub ahead of print].
Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, et al. Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122:280–90.
Pacher P, Szabó C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–60.
Jagtap P, Szabó C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Disco. 2005;4:421–40.
Yamazaki K, Tanaka S, Sakata R, Miwa S, Oriyanhan W, Takaba K, et al. Protective effect of cardioplegia with poly (ADP-ribose) polymerase-1 inhibitor against myocardial ischemia-reperfusion injury: in vitro study of isolated rat heart model. J Enzym Inhib Med Chem. 2013;28:143–7.
Zingarelli B, Hake PW, O’Connor M, Denenberg A, Wong HR, Kong S, et al. Differential regulation of activator protein-1 and heat shock factor-1 in myocardial ischemia and reperfusion injury: role of poly(ADP-ribose) polymerase-1. Am J Physiol Heart Circ Physiol. 2004;286:H1408–15.
Zee BM, Levin RS, DiMaggio PA, Garcia BA. Global turnover of histone post-translational modifications and variants in human cells. Epigenetics Chromatin. 2010;3:22.
Liddy KA, White MY, Cordwell SJ. Functional decorations: post-translational modifications and heart disease delineated by targeted proteomics. Genome Med. 2013;5:20.
Fessart D, Martin-Negrier ML, Claverol S, Thiolat ML, Crevel H, Toussaint C, et al. Proteomic remodeling of proteasome in right heart failure. J Mol Cell Cardiol. 2014;66:41–52.
Barac YD, Emrich F, Krutzwakd-Josefson E, Schrepfer S, Sampaio LC, Willerson JT, et al. The ubiquitin-proteasome system: a potential therapeutic target for heart failure. J Heart Lung Transpl. 2017;36:708–14.
Yang Y, Liao B, Wang S, Yan B, Jin Y, Shu HB, et al. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc Natl Acad Sci USA. 2013;110:5115–20.
Liao B, Jin Y. Wwp2 mediates Oct4 ubiquitination and its own auto-ubiquitination in a dosage-dependent manner. Cell Res. 2010;20:332–44.
Aki D, Li H, Zhang W, Zheng M, Elly C, Lee JH, et al. The E3 ligases Itch and WWP2 cooperate to limit TH2 differentiation by enhancing signaling through the TCR. Nat Immunol. 2018;19:766–75.
Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci USA. 2006;103:18308–13.
Virág L, Robaszkiewicz A, Rodriguez-Vargas JM, Oliver FJ. Poly(ADP-ribose) signaling in cell death. Mol Asp Med. 2013;34:1153–67.
Du Y, Yamaguchi H, Wei Y, Hsu JL, Wang HL, Hsu YH, et al. Blocking c-Met-mediated PARP1 phosphorylation enhances anti-tumor effects of PARP inhibitors. Nat Med. 2016;22:194–201.
Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, et al. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005;280:40450–64.
Szabo C, Pacher P, Swanson RA. Novel modulators of poly(ADP-ribose) polymerase. Trends Pharm Sci. 2006;27:626–30.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos 81900355, 81670231, 81900372, and 81970211).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by M. Piacentini
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, N., Zhang, Y., Qian, H. et al. Selective targeting of ubiquitination and degradation of PARP1 by E3 ubiquitin ligase WWP2 regulates isoproterenol-induced cardiac remodeling. Cell Death Differ 27, 2605–2619 (2020). https://doi.org/10.1038/s41418-020-0523-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-020-0523-2
This article is cited by
-
Miglustat ameliorates isoproterenol-induced cardiac fibrosis via targeting UGCG
Molecular Medicine (2025)
-
The role of HECT-type E3 ubiquitin ligases in DNA damage response and repair
Cell Death Discovery (2025)
-
The deubiquitination-PARylation positive feedback loop of the USP10-PARP1 axis promotes DNA damage repair and affects therapeutic efficacy of PARP1 inhibitor
Oncogene (2025)
-
New Types of Post-Translational Modification of Proteins in Cardiovascular Diseases
Journal of Cardiovascular Translational Research (2025)
-
CBL-b E3 ligase-mediated neddylation and activation of PARP-1 induce vascular calcification
Experimental & Molecular Medicine (2024)