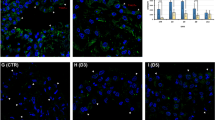
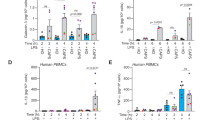
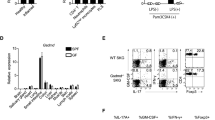
Abstract
Kinase signaling in the tiered activation of inflammasomes and associated pyroptosis is a prime therapeutic target for inflammatory diseases. While MAPKs subsume pivotal roles during inflammasome priming, specifically the MAP3K7/JNK1/NLRP3 licensing axis, their involvement in successive steps of inflammasome activation is poorly defined. Using live-cell MAPK biosensors to focus on the inflammasome triggering event allowed us to identify a subsequent process of biphasic JNK activation. We find that this biphasic post-trigger JNK signaling initially facilitates the mitochondrial reactive oxygen species generation needed to support core inflammasome formation, then supports the gasdermin-mediated cell permeation required for release of active IL-1β from human macrophages. We further identify and characterize a xanthine oxidase-ROS activated MAP3K5/JNK2 substrate licensing complex as a novel regulator of the GSDMD mobilization which precedes pyroptosis. We show that inhibitors targeting this MAP3K5 cascade alleviate morbidity in mouse models of colitis and dampen both augmented IL-1β release and cell permeation in monocytes derived from patients with gain-of-function inflammasomopathies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Please direct material requests and correspondence to fraseri@niaid.nih.gov.
References
Martinon F, Burns K, Tschopp J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-β. Mol Cell. 2002;10:417–26.
Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27.
Lamkanfi M, Dixit VM. Mechanisms and Functions of Inflammasomes. Cell 2014;157:1013–22.
Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock. Science 2013;341:1250–3.
Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 2018;48:35–44.e6.
Song N, Li T. Regulation of NLRP3 Inflammasome by Phosphorylation. Front Immunol [Internet]. 2018 [cited 2021 Apr 28];9. https://www.frontiersin.org/articles/10.3389/fimmu.2018.02305/full
Darling NJ, Cook SJ. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta BBA. Mol Cell Res. 2014;1843:2150–63.
Arthur JSC, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–92.
Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol Cell. 2017;68:185–197.e6.
Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, et al. Anthrax Toxin Induces Macrophage Death by p38 MAPK Inhibition but Leads to Inflammasome Activation via ATP Leakage. Immunity 2011;35:34–44.
Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411.
Okada M, Matsuzawa A, Yoshimura A, Ichijo H. The Lysosome Rupture-activated TAK1-JNK Pathway Regulates NLRP3 Inflammasome Activation. J Biol Chem. 2014;289:32926–36.
Regot S, Hughey JJ, Bajar BT, Carrasco S, Covert MW. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 2014;157:1724–34.
MAP kinase kinase kinases and innate immunity: Trends in Immunology [Internet]. [cited 2021 Apr 28]. https://www.cell.com/trends/immunology/comments/S1471-4906(05)00290-5
Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene 2007;26:3185–202.
Fischer FA, Mies LFM, Nizami S, Pantazi E, Danielli S, Demarco B, et al. TBK1 and IKKε act like an OFF switch to limit NLRP3 inflammasome pathway activation. Proc Natl Acad Sci [Internet]. 2021 Sep [cited 2021 Dec 20];118. https://www.pnas.org/content/118/38/e2009309118
Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, et al. NF-κB Is a Negative Regulator of IL-1β Secretion as Revealed by Genetic and Pharmacological Inhibition of IKKβ. Cell 2007;130:918–31.
Karin M, Delhase M. The IκB kinase (IKK) and NF-κB: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98.
de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. Molecular Mechanisms in Genetically Defined Autoinflammatory Diseases: Disorders of Amplified Danger Signaling. Annu Rev Immunol. 2015 ;33:823–74.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015;526:660–5.
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015;526:666–71.
Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, et al. Succination inactivates gasdermin D and blocks pyroptosis. Science 2020;369:1633–7.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;469:221–5.
Ernst O, Sun J, Lin B, Banoth B, Dorrington MG, Liang J, et al. A genome-wide screen uncovers multiple roles for mitochondrial nucleoside diphosphate kinase D in inflammasome activation. Sci Signal. 2021;14:eabe0387.
Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018;560:198–203.
Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 2009;459:433–6.
Zhang B, Wei W, Qiu J. ALK is required for NLRP3 inflammasome activation in macrophages. Biochem Biophys Res Commun. 2018;501:246–52.
Lerner AG, Upton JP, Praveen PVK, Ghosh R, Nakagawa Y, Igbaria A, et al. IRE1α Induces Thioredoxin-Interacting Protein to Activate the NLRP3 Inflammasome and Promote Programmed Cell Death under Irremediable ER Stress. Cell Metab. 2012;16:250–64.
Pereira M, Tourlomousis P, Wright JP, Monie T, Bryant CE. CARD9 negatively regulates NLRP3-induced IL-1β production on Salmonella infection of macrophages. Nat Commun. 2016;7:12874.
Ichijo H, Nishida E, Irie K, Dijke P, ten, Saitoh M, Moriguchi T, et al. Induction of Apoptosis by ASK1, a Mammalian MAPKKK That Activates SAPK/JNK and p38 Signaling Pathways. Science 1997;275:90–4.
Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–606.
Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–25.
Tartey S, Gurung P, Dasari TK, Burton A, Kanneganti TD. ASK1/2 signaling promotes inflammation in a mouse model of neutrophilic dermatosis. J Clin Investig. 2018;128:2042–7.
Place DE, Samir P, Karki R, Briard B, Vogel P, Kanneganti TD. ASK Family Kinases Are Required for Optimal NLRP3 Inflammasome Priming. Am J Pathol. 2018;188:1021–30.
Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–92.
Gong YN, Wang X, Wang J, Yang Z, Li S, Yang J, et al. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell Res. 2010;20:1289–305.
Malireddi RKS, Gurung P, Mavuluri J, Dasari TK, Klco JM, Chi H, et al. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med. 2018;215:1023–34.
Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, et al. Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death. Science 2018;362:1064–9.
Evavold CL, Hafner-Bratkovič I, Devant P, D’Andrea JM, Ngwa EM, Boršić E, et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell. 2021 Jul;S0092-8674 00796-0.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–40.
Ives A, Nomura J, Martinon F, Roger T, LeRoy D, Miner JN, et al. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat Commun. 2015;6:1–11.
Bulek K, Zhao J, Liao Y, Rana N, Corridoni D, Antanaviciute A, et al. Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis [Internet]. Am Soc Clin Investig. 2020 [cited 2021 Apr 29]. https://www.jci.org/articles/view/138103/figure/1
Stutz A, Horvath GL, Monks BG, Latz E. ASC Speck Formation as a Readout for Inflammasome Activation. In: De Nardo CM, Latz E, editors. The Inflammasome: Methods and Protocols [Internet]. Totowa, NJ: Humana Press; 2013 [cited 2021 Apr 29]. p. 91–101. (Methods in Molecular Biology). https://doi.org/10.1007/978-1-62703-523-1_8
Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21:736–45.
de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 2019;26:146–61.
Wandel MP, Kim BH, Park ES, Boyle KB, Nayak K, Lagrange B, et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat Immunol. 2020;21:880–91.
Gaudet RG, Zhu S, Halder A, Kim BH, Bradfield CJ, Huang S, et al. A human apolipoprotein L with detergent-like activity kills intracellular pathogens. Science 2021;373:eabf8113.
Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213:2113–28.
Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1β-converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci. 2001;98:13249–54.
Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol [Internet]. 2018 Aug [cited 2020 Mar 22];3. https://immunology.sciencemag.org/content/3/26/eaat2738
Xiao J, Wang C, Yao JC, Alippe Y, Xu C, Kress D, et al. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLOS Biol. 2018;16:e3000047.
Fey D, Croucher D, Kolch W, Kholodenko B. Crosstalk and Signaling Switches in Mitogen-Activated Protein Kinase Cascades. Front Physiol. 2012;3:355
Chambers JW, LoGrasso PV. Mitochondrial c-Jun N-terminal Kinase (JNK) Signaling Initiates Physiological Changes Resulting in Amplification of Reactive Oxygen Species Generation. J Biol Chem. 2011;286:16052–62.
Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK Translocation to Mitochondria Leading to Inhibition of Mitochondria Bioenergetics in Acetaminophen-induced Liver Injury*. J Biol Chem. 2008;283:13565–77.
Bauernfeind F, Bartok E, Rieger A, Franchi L, Núñez G, Hornung V. Cutting Edge: Reactive Oxygen Species Inhibitors Block Priming, but Not Activation, of the NLRP3 Inflammasome. J Immunol. 2011;187:613–7.
Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 2018;362:956–60.
Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016;535:111–6.
Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–78.
Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q, et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 2021;591:131–6.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016;535:153–8.
Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun. 2020;11:2212.
Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10:1689.
Zanoni I, Tan Y, Gioia MD, Broggi A, Ruan J, Shi J, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 2016;352:1232–6.
Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, et al. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 2016;44:833–46.
Acknowledgements
We thank the NIH Department of Transfusion Medicine for providing human blood-derived monocytes, Richard Flavell (Yale University) for generously providing ASC knockout mice, Sergi Regot (Johns Hopkins University) and Markus Covert (Stanford University) for providing KTR constructs and analysis help, colleagues in the Laboratory of Immune System Biology, and Lara Kohler for helpful discussions and critical reading of the paper.
Funding
This work was supported by the Intramural Research Program of NIAID, NIH.
Author information
Authors and Affiliations
Contributions
Conceptualization, CJB, OE, JJL, and IDCF; Methodology, CJB, SPJ, JS, SG, AAJ, CEB; Software, CJB, and SG; Validation, JS, OE, SPJ, CJB and JJL; Formal Analysis, CJB and IDCF; Investigation, CJB, OE, SPJ, JS, JL, and SG; Resources, AAJ, RGM, and IDCF; Data Curation, CJB, OE, SPJ, JS, JJL, SG, and IDCF; Writing—Original Draft, CJB and IDCF; Writing—Review & Editing, CJB, JJL, OE, SPJ, JS, CEB, RGM and IDCF; Visualization, CJB, SG and IDCF, Supervision, CEB, RGM, and IDCF; Project Administration, CJB and IDCF; Funding Acquisition, CEB, RGM and IDCF.
Corresponding author
Ethics declarations
Competing interests
CEB is on the scientific advisory board of Nodthera, Related Sciences and Lightcast, and is a consultant for Janssen. All other authors declare no competing interests.
Ethics approval
Human peripheral blood from screened, healthy donors was obtained under the NIH Clinical Center IRB-approved protocol 99-CC-0168 from the NIH Department of Transfusion Medicine. Mice were maintained in specific-pathogen-free conditions and all procedures were approved by the NIAID Animal Care and Use Committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by H Ichijo
Rights and permissions
About this article
Cite this article
Bradfield, C.J., Liang, J.J., Ernst, O. et al. Biphasic JNK signaling reveals distinct MAP3K complexes licensing inflammasome formation and pyroptosis. Cell Death Differ 30, 589–604 (2023). https://doi.org/10.1038/s41418-022-01106-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-022-01106-9
This article is cited by
-
JNK activation dynamics drive distinct gene expression patterns over time mediated by mRNA stability
npj Systems Biology and Applications (2025)
-
Updated insights into the molecular networks for NLRP3 inflammasome activation
Cellular & Molecular Immunology (2025)
-
Systematic druggable genome-wide Mendelian randomization identifies therapeutic targets for gout
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
(Pro)renin receptor mediates tubular epithelial cell pyroptosis in diabetic kidney disease via DPP4-JNK pathway
Journal of Translational Medicine (2024)
-
Multi-omics analysis reveals the genetic aging landscape of Parkinson’s disease
Scientific Reports (2024)