Abstract
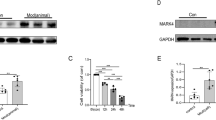
Mitochondrial dysfunction and cell death play important roles in diabetic cardiomyopathy, but the underlying mechanisms remain unclear. Here, we report that mitochondrial dysfunction and cell apoptosis are prominent features of primary cardiomyocytes after exposure to high glucose/palmitate conditions. The protein level of MIC60, a core component of mitochondrial cristae, is decreased via ubiquitination and degradation under these conditions. Exogenous expression of MIC60 alleviates cristae disruption, mitochondrial dysfunction and apoptosis. Moreover, we identified MARCH5 as an E3 ubiquitin ligase that specifically targets MIC60 in this process. Indeed, MARCH5 mediates K48-linked ubiquitination of MIC60 at Lys285 to promote its degradation. Mutation of the ubiquitination site in MIC60 or the MIC60-interacting motifs in MARCH5 abrogates MARCH5-mediated MIC60 ubiquitination and degradation. Silencing MARCH5 significantly alleviates high glucose/palmitate-induced mitochondrial dysfunction and apoptosis in primary cardiomyocytes. In addition to E3 ubiquitin ligases, molecular chaperones also play important roles in protein stability. We previously reported that the mitochondrial chaperone TRAP1 inhibits the ubiquitination of MIC60, but the detailed mechanism is unknown. Here, we find that TRAP1 performs this function by competing with MARCH5 for binding to MIC60. Our findings provide new insights into the mechanism underlying mitochondrial dysfunction in cardiomyocytes in diabetic cardiomyopathy.

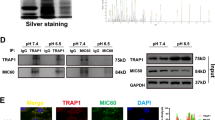
MARCH5 promotes ubiquitination of MIC60 to induce MIC60 degradation, mitochondrial dysfunction and apoptosis in cardiomyocytes under diabetic conditions. TRAP1 inhibits MARCH5-mediated ubiquitination by competitively interacting with MIC60.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All the data used during the study are available from the corresponding author on request.
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Dillmann WH. Diabetic cardiomyopathy. Circ Res. 2019;124:1160–2.
Wang L, Cai Y, Jian L, Cheung CW, Zhang L, Xia Z. Impact of peroxisome proliferator-activated receptor-alpha on diabetic cardiomyopathy. Cardiovasc Diabetol. 2021;20:2.
Dewanjee S, Vallamkondu J, Kalra RS, John A, Reddy PH, Kandimalla R. Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev. 2021;68:101338.
Gao P, Cao M, Jiang X, Wang X, Zhang G, Tang X, et al. Cannabinoid receptor 2-centric molecular feedback loop drives necroptosis in diabetic heart injuries. Circulation. 2023;147:158–74.
Kyriazis ID, Hoffman M, Gaignebet L, Lucchese AM, Markopoulou E, Palioura D, et al. KLF5 is induced by FOXO1 and causes oxidative stress and diabetic cardiomyopathy. Circ Res. 2021;128:335–57.
Kolleritsch S, Kien B, Schoiswohl G, Diwoky C, Schreiber R, Heier C, et al. Low cardiac lipolysis reduces mitochondrial fission and prevents lipotoxic heart dysfunction in Perilipin 5 mutant mice. Cardiovasc Res. 2020;116:339–52.
Jin L, Geng L, Ying L, Shu L, Ye K, Yang R, et al. FGF21-Sirtuin 3 axis confers the protective effects of exercise against diabetic cardiomyopathy by governing mitochondrial integrity. Circulation. 2022;146:1537–57.
Chang X, Li Y, Cai C, Wu F, He J, Zhang Y, et al. Mitochondrial quality control mechanisms as molecular targets in diabetic heart. Metabolism. 2022;137:155313.
Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol. 2021;18:809–23.
Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P, et al. Hyperglycemia-driven inhibition of AMP-activated protein kinase alpha2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation. 2019;139:1913–36.
Gomez-Valades AG, Pozo M, Varela L, Boudjadja MB, Ramirez S, Chivite I, et al. Mitochondrial cristae-remodeling protein OPA1 in POMC neurons couples Ca(2+) homeostasis with adipose tissue lipolysis. Cell Metab. 2021;33:1820–35.e1829.
Tombo N, Imam Aliagan AD, Feng Y, Singh H, Bopassa JC. Cardiac ischemia/reperfusion stress reduces inner mitochondrial membrane protein (mitofilin) levels during early reperfusion. Free Radic Biol Med. 2020;158:181–94.
Kondadi AK, Anand R, Reichert AS. Cristae membrane dynamics - a paradigm change. Trends Cell Biol. 2020;30:923–36.
Chen L, Dong J, Liao S, Wang S, Wu Z, Zuo M, et al. Loss of Sam50 in hepatocytes induces cardiolipin-dependent mitochondrial membrane remodeling to trigger mtDNA release and liver injury. Hepatology. 2022;76:1389–408.
Thapa D, Nichols CE, Lewis SE, Shepherd DL, Jagannathan R, Croston TL, et al. Transgenic overexpression of mitofilin attenuates diabetes mellitus-associated cardiac and mitochondria dysfunction. J Mol Cell Cardiol. 2015;79:212–23.
Takeda K, Nagashima S, Shiiba I, Uda A, Tokuyama T, Ito N, et al. MITOL prevents ER stress-induced apoptosis by IRE1alpha ubiquitylation at ER-mitochondria contact sites. EMBO J. 2019;38:e100999.
Park YY, Nguyen OT, Kang H, Cho H. MARCH5-mediated quality control on acetylated Mfn1 facilitates mitochondrial homeostasis and cell survival. Cell Death Dis. 2014;5:e1172.
Chen Z, Siraj S, Liu L, Chen Q. MARCH5-FUNDC1 axis fine-tunes hypoxia-induced mitophagy. Autophagy. 2017;13:1244–5.
Eldeeb MA, Bayne AN, Trempe JF, Fon EA. Fine-tuning TOM-mitochondrial import via ubiquitin. Trends Cell Biol. 2020;30:425–7.
Arai S, Varkaris A, Nouri M, Chen S, Xie L, Balk SP. MARCH5 mediates NOXA-dependent MCL1 degradation driven by kinase inhibitors and integrated stress response activation. Elife. 2020;9:e54954.
Gu H, Li Q, Huang S, Lu W, Cheng F, Gao P, et al. Mitochondrial E3 ligase March5 maintains stemness of mouse ES cells via suppression of ERK signalling. Nat Commun. 2015;6:7112.
Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H. Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci. 2010;123:619–26.
Cannino G, Urbani A, Gaspari M, Varano M, Negro A, Filippi A, et al. The mitochondrial chaperone TRAP1 regulates F-ATP synthase channel formation. Cell Death Differ. 2022;29:2335–46.
Amoroso MR, Matassa DS, Laudiero G, Egorova AV, Polishchuk RS, Maddalena F, et al. TRAP1 and the proteasome regulatory particle TBP7/Rpt3 interact in the endoplasmic reticulum and control cellular ubiquitination of specific mitochondrial proteins. Cell Death Differ. 2012;19:592–604.
Lettini G, Sisinni L, Condelli V, Matassa DS, Simeon V, Maddalena F, et al. TRAP1 regulates stemness through Wnt/beta-catenin pathway in human colorectal carcinoma. Cell Death Differ. 2016;23:1792–803.
Masgras I, Cannino G, Ciscato F, Sanchez-Martin C, Darvishi FB, Scantamburlo F, et al. Tumor growth of neurofibromin-deficient cells is driven by decreased respiration and hampered by NAD(+) and SIRT3. Cell Death Differ. 2022;29:1996–2008.
Liu L, Zhang L, Zhao J, Guo X, Luo Y, Hu W, et al. Tumor necrosis factor receptor-associated protein 1 protects against mitochondrial injury by preventing high glucose-induced mPTP opening in diabetes. Oxid Med Cell Longev. 2020;2020:6431517.
Joshi A, Dai L, Liu Y, Lee J, Ghahhari NM, Segala G, et al. The mitochondrial HSP90 paralog TRAP1 forms an OXPHOS-regulated tetramer and is involved in mitochondrial metabolic homeostasis. BMC Biol. 2020;18:10.
Zhang L, Su N, Luo Y, Chen S, Zhao T. TRAP1 inhibits MIC60 ubiquitination to mitigate the injury of cardiomyocytes and protect mitochondria in extracellular acidosis. Cell Death Discov. 2021;7:389.
Shiiba I, Takeda K, Nagashima S, Ito N, Tokuyama T, Yamashita SI, et al. MITOL promotes cell survival by degrading Parkin during mitophagy. EMBO Rep. 2021;22:e49097.
Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155:160–71.
Jestin M, Kapnick SM, Tarasenko TN, Burke CT, Zerfas PM, Diaz F, et al. Mitochondrial disease disrupts hepatic allostasis and lowers the threshold for immune-mediated liver toxicity. Mol Metab. 2020;37:100981.
Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100.
Tang J, Zhang K, Dong J, Yan C, Hu C, Ji H, et al. Sam50-Mic19-Mic60 axis determines mitochondrial cristae architecture by mediating mitochondrial outer and inner membrane contact. Cell Death Differ. 2020;27:146–60.
Tsai PI, Lin CH, Hsieh CH, Papakyrikos AM, Kim MJ, Napolioni V, et al. PINK1 phosphorylates MIC60/Mitofilin to control structural plasticity of mitochondrial crista junctions. Mol Cell. 2018;69:744–56.e746.
Gorr MW, Wold LE. Mitofilin: key factor in diabetic cardiomyopathy? J Mol Cell Cardiol. 2015;85:292–3.
Li R, Liu Y, Shan YG, Gao L, Wang F, Qiu CG. Bailcalin protects against diabetic cardiomyopathy through Keap1/Nrf2/AMPK-mediated antioxidative and lipid-lowering effects. Oxid Med Cell Longev. 2019;2019:3206542.
He J, Quintana MT, Sullivan J, T LP, T JG, Schisler JC, et al. MuRF2 regulates PPARgamma1 activity to protect against diabetic cardiomyopathy and enhance weight gain induced by a high fat diet. Cardiovasc Diabetol. 2015;14:97.
Deshwal S, Fiedler KU, Langer T. Mitochondrial proteases: multifaceted regulators of mitochondrial plasticity. Annu Rev Biochem. 2020;89:501–28.
Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42.
Lavie J, De Belvalet H, Sonon S, Ion AM, Dumon E, Melser S, et al. Ubiquitin-dependent degradation of mitochondrial proteins regulates energy metabolism. Cell Rep. 2018;23:2852–63.
Haschka MD, Karbon G, Soratroi C, O’Neill KL, Luo X, Villunger A. MARCH5-dependent degradation of MCL1/NOXA complexes defines susceptibility to antimitotic drug treatment. Cell Death Differ. 2020;27:2297–312.
Tokuyama T, Uosaki H, Sugiura A, Nishitai G, Takeda K, Nagashima S, et al. Protective roles of MITOL against myocardial senescence and ischemic injury partly via Drp1 regulation. iScience. 2022;25:104582.
Phu L, Rose CM, Tea JS, Wall CE, Verschueren E, Cheung TK, et al. Dynamic regulation of mitochondrial import by the ubiquitin system. Mol Cell. 2020;77:1107–23.e1110.
Chen Z, Liu L, Cheng Q, Li Y, Wu H, Zhang W, et al. Mitochondrial E3 ligase MARCH5 regulates FUNDC1 to fine-tune hypoxic mitophagy. EMBO Rep. 2017;18:495–509.
Zheng J, Chen X, Liu Q, Zhong G, Zhuang M. Ubiquitin ligase MARCH5 localizes to peroxisomes to regulate pexophagy. J Cell Biol. 2022;221:e202103156.
Serapian SA, Sanchez-Martin C, Moroni E, Rasola A, Colombo G. Targeting the mitochondrial chaperone TRAP1: strategies and therapeutic perspectives. Trends Pharmacol Sci. 2021;42:566–76.
Landriscina M, Laudiero G, Maddalena F, Amoroso MR, Piscazzi A, Cozzolino F, et al. Mitochondrial chaperone Trap1 and the calcium binding protein Sorcin interact and protect cells against apoptosis induced by antiblastic agents. Cancer Res. 2010;70:6577–86.
Funding
This work were supported in part by the National Natural Science Foundation of China (Nos. 82270872, 82171604, 81971759 and 82300909), Natural Science Foundation of Guangdong (No. 2022A1515012249 and 2023B1515020108) and Science and Technology Planning Project of Guangzhou (No. 202206010089).
Author information
Authors and Affiliations
Contributions
LZ, GL, and TZ conceived of the project. LZ, SC, GL, TZ supervised the experiments, analyzed the data, and wrote the manuscript. LZ, YL, LL performed cellular and molecular experiments. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Luo, Y., Lv, L. et al. TRAP1 inhibits MARCH5-mediated MIC60 degradation to alleviate mitochondrial dysfunction and apoptosis of cardiomyocytes under diabetic conditions. Cell Death Differ 30, 2336–2350 (2023). https://doi.org/10.1038/s41418-023-01218-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-023-01218-w
This article is cited by
-
TRAP1 inhibits KANSL3 acetylation to alleviate mitochondrial dysfunction by promoting mitophagy in cardiomyocytes under diabetic conditions
Cell Communication and Signaling (2025)
-
March5-mediated Trim28 degradation preserves islet β-cell function in mice
Nature Communications (2025)
-
Antler stem cell-derived exosomes restore periodontal homeostasis in a rat model with diabetic periodontitis through enhancing ROS scavenging and osteogenesis
Cell Death Discovery (2025)
-
Macrod1 suppresses diabetic cardiomyopathy via regulating PARP1-NAD+-SIRT3 pathway
Acta Pharmacologica Sinica (2024)