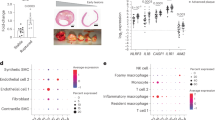
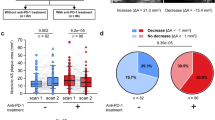
Abstract
Focal inflammation and arterial damage driven by macrophages are key pathogenic processes in atherosclerosis. However, the mechanisms that regulate these processes remain poorly understood. In this study, we demonstrate that formyl peptide receptor 1 (FPR1) agonist, a mitochondrial N-formyl peptide, is elevated in the blood of patients with atherosclerosis and correlates with carotid stenosis. Macrophages expressing FPR1 were found in atherosclerotic lesions. Conditional deletion of Fpr1 in macrophages reduced plaque formation, local inflammation, and aortic atherosclerosis in apolipoprotein E (ApoE)−/− mice. FPR1 activates protein kinase C (PKC) in macrophages, promoting the production of reactive oxygen species (ROS), tumor necrosis factor alpha (TNF-α) and interleukin-1beta (IL-1β), which accelerates the apoptosis of endothelial cells and smooth muscle cells. To inhibit FPR1 bioactivity, we developed an antagonist, T0080. Therapeutic administration of T0080 attenuates atherosclerotic progression in ApoE−/− mice. Our findings highlight the pivotal role of FPR1 in macrophage-mediated atherosclerotic plaque formation and support further investigation of T0080-mediated FPR1 inhibition as a potential treatment for atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-Seq data were obtained from NCBI’s Gene Expression Omnibus (GEO) database (GEO GSE155512, https://www.ncbi.nlm.nih.gov). Codes were implemented in R (version R-4.4.1) and are deposited in GitHub - snowinghere/R-code-for-FPR1-in-atherosclerosis. All other data are available from the corresponding author upon reasonable request.
References
Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the management of patients with chronic coronary disease: a report of the american heart association/american college of cardiology joint committee on clinical practice guidelines. Circulation 2023. 2023;148:e9–119.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Hou P, Fang J, Liu Z, Shi Y, Agostini M, Bernassola F, et al. Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis. 2023;14:691.
Bjorkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185:1630–45.
Hwang SJ, Ahn BJ, Shin MW, Song YS, Choi Y, Oh GT, et al. miR-125a-5p attenuates macrophage-mediated vascular dysfunction by targeting Ninjurin1. Cell Death Differ. 2022;29:1199–210.
Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41.
Swirski FK, Robbins CS, Nahrendorf M. Development and function of arterial and cardiac macrophages. Trends Immunol. 2016;37:32–40.
Clyburn C, Birren SJ. Crosstalk between nerves, immune cells and plaques drives atherosclerosis. Nature. 2022;605:32–4.
Yu EP, Bennett MR. The role of mitochondrial DNA damage in the development of atherosclerosis. Free Radic Biol Med. 2016;100:223–30.
Satish M, Agrawal DK. Atherothrombosis and the NLRP3 inflammasome - endogenous mechanisms of inhibition. Transl Res. 2020;215:75–85.
Khwaja B, Thankam FG, Agrawal DK. Mitochondrial DAMPs and altered mitochondrial dynamics in OxLDL burden in atherosclerosis. Mol Cell Biochem. 2021;476:1915–28.
Dorward DA, Lucas CD, Doherty MK, Chapman GB, Scholefield EJ, Conway Morris A, et al. Novel role for endogenous mitochondrial formylated peptide-driven formyl peptide receptor 1 signalling in acute respiratory distress syndrome. Thorax. 2017;72:928–36.
Li Z, Li Y, Han J, Zhu Z, Li M, Liu Q, et al. Formyl peptide receptor 1 signaling potentiates inflammatory brain injury. Sci Transl Med. 2021;13:eabe9890.
Liu M, Zhao J, Chen K, Bian X, Wang C, Shi Y, et al. G protein-coupled receptor FPR1 as a pharmacologic target in inflammation and human glioblastoma. Int Immunopharmacol. 2012;14:283–28.
Tsai YF, Yang SC, Hwang TL. Formyl peptide receptor modulators: a patent review and potential applications for inflammatory diseases (2012-2015). Expert Opin Ther Pat. 2016;26:1139–56.
Dorward DA, Lucas CD, Chapman GB, Haslett C, Dhaliwal K, Rossi AG. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol. 2015;185:1172–84.
Lee HY, Lee M, Bae YS. Formyl peptide receptors in cellular differentiation and inflammatory diseases. J Cell Biochem. 2017;118:1300–7.
Chen G, Wang X, Liao Q, Ge Y, Jiao H, Chen Q, et al. Structural basis for recognition of N-formyl peptides as pathogen-associated molecular patterns. Nat Commun. 2022;13:5232.
Petri MH, Laguna-Fernandez A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK, Back M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc Res. 2015;105:65–74.
Drechsler M, de Jong R, Rossaint J, Viola JR, Leoni G, Wang JM, et al. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res. 2015;116:827–35.
Gemperle C, Schmid M, Herova M, Marti-Jaun J, Wuest SJ, Loretz C, et al. Regulation of the formyl peptide receptor 1 (FPR1) gene in primary human macrophages. PloS One. 2012;7:e50195.
Rhoads JP, Major AS. How Oxidized Low-density lipoprotein activates inflammatory responses. Crit Rev Immunol. 2018;38:333–42.
Zhang Y, Fatima M, Hou S, Bai L, Zhao S, Liu E. Research methods for animal models of atherosclerosis (Review). Mol Med Rep. 2021;24:871.
Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–40.
Wenceslau CF, McCarthy CG, Szasz T, Goulopoulou S, Webb RC. Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome. Am J Physiol Heart Circ Physiol. 2015;308:H768–77.
Li J, Chordia MD, Zhang Y, Zong H, Pan D, Zuo Z. Critical role of FPR1 in splenocyte migration into brain to worsen inflammation and ischemic brain injury in mice. Theranostics. 2022;12:3024–44.
McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–6.
Leslie J, Millar BJ, Del Carpio Pons A, Burgoyne RA, Frost JD, Barksby BS, et al. FPR-1 is an important regulator of neutrophil recruitment and a tissue-specific driver of pulmonary fibrosis. JCI Insight. 2020;5:e125937.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Fan X, Han J, Zhong L, Zheng W, Shao R, Zhang Y, et al. Macrophage-derived GSDMD plays an essential role in atherosclerosis and cross talk between macrophages via the mitochondria-STING-IRF3/NF-kappaB axis. Arterioscler Thromb Vasc Biol. 2024;44:1365–78.
Olsen MB, Gregersen I, Sandanger O, Yang K, Sokolova M, Halvorsen BE, et al. Targeting the inflammasome in cardiovascular disease. JACC Basic Transl Sci. 2022;7:84–98.
Opoku E, Traughber CA, Zhang D, Iacano AJ, Khan M, Han J, et al. Gasdermin D mediates inflammation-induced defects in reverse cholesterol transport and promotes atherosclerosis. Front Cell Dev Biol. 2021;9:715211.
Wei Y, Lan B, Zheng T, Yang L, Zhang X, Cheng L, et al. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat Commun. 2023;14:929.
Tall AR, Bornfeldt KE. Inflammasomes and atherosclerosis: a mixed picture. Circ Res. 2023;132:1505–20.
Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol. 2017;70:2278–89.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61.
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87.
He HQ, Ye RD. The formyl peptide receptors: diversity of ligands and mechanism for recognition. Molecules. 2017;22:455.
Mormile I, Rossi FW, Prevete N, Granata F, Pucino V, de Paulis A. The N-formyl peptide receptors and rheumatoid arthritis: a dangerous liaison or confusing relationship? Front Immunol. 2021;12:685214.
van Leeuwen M, Damoiseaux J, Duijvestijn A, Tervaert JW. The therapeutic potential of targeting B cells and anti-oxLDL antibodies in atherosclerosis. Autoimmun Rev. 2009;9:53–7.
Hartley A, Haskard D, Khamis R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis - novel insights and future directions in diagnosis and therapy. Trends Cardiovasc Med. 2019;29:22–6.
Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Cayatte AJ, Rozek MM. Pathogenesis of the atherosclerotic lesion. Implications for diabetes mellitus. Diabetes Care. 1992;15:1156–67.
Xu Y, Kong X, Zhou H, Zhang X, Liu J, Yan J, et al. oxLDL/beta2GPI/anti-beta2GPI complex induced macrophage differentiation to foam cell involving TLR4/NF-kappa B signal transduction pathway. Thromb Res. 2014;134:384–92.
Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, et al. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 george lyman duff memorial lecture. Arterioscler Thromb Vasc Biol. 1996;16:831–42.
Navab M, Hama-Levy S, Van Lenten BJ, Fonarow GC, Cardinez CJ, Castellani LW, et al. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest. 1997;99:2005–19.
Back M, Yurdagul A Jr, Tabas I, Oorni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16:389–406.
Deroissart J, Porsch F, Koller T, Binder CJ. Anti-inflammatory and immunomodulatory therapies in atherosclerosis. Handb Exp Pharm. 2022;270:359–404.
Hetherington I, Totary-Jain H. Anti-atherosclerotic therapies: milestones, challenges, and emerging innovations. Mol Ther. 2022;30:3106–17.
Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and doppler US diagnosis-society of radiologists in ultrasound consensus conference. Radiology. 2003;229:340–6.
Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126:1607.
Pan H, Xue C, Auerbach BJ, Fan J, Bashore AC, Cui J, et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation. 2020;142:2060–75.
Diotallevi M, Nicol T, Ayaz F, Bailey J, Shaw A, McNeill E, et al. Isolation and in vitro culture of bone marrow-derived macrophages for the study of NO-redox biology. J Vis Exp. 2022;183:e62834.
Chen Y, Wu X, Zhang J, Pan G, Wang X, Guo X, et al. Amino acid starvation-induced LDLR trafficking accelerates lipoprotein endocytosis and LDL clearance. EMBO Rep. 2022;23:e53373.
Plaisance-Bonstaff K, Faia C, Wyczechowska D, Jeansonne D, Vittori C, Peruzzi F. Isolation, transfection, and culture of primary human monocytes. J Vis Exp. 2019;154:e59967.
Acknowledgements
We thank Drs. L. Zheng, M. Liu and Y. Zhu for fruitful discussions, and Dr. S.X. Shi for editorial assistance.
Funding
This work was supported by the National Natural Science Foundation of China (82471312, 82320108007 and 81830038) and Tianjin Science and Technology Commission Foundation (21JCYBJC00770).
Author information
Authors and Affiliations
Contributions
ZL, F-DS and SCJ formulated the conception and design of this study. YL, XZ, SW, YL, XT, XZ, YL and NY performed the experiments, YL, XZ, BG, F-DS, ZL and SW analyzed and interpreted the results. ZL, F-DS, YL, XZ and SCJ wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experiments involving human tissue samples were approved by the Medical Ethics Committee of Tianjin Medical University General Hospital (Tianjin, China). All animal experiments were approved by the Laboratory Animal Management and Ethics Committee of Tianjin Medical University General Hospital (Tianjin, China) and were performed in accordance with the “China’s Guidelines on Welfare and Ethical Review for Laboratory Animals”.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, YJ., Zhao, X., Wu, S. et al. Formyl peptide receptor 1 and its antagonist T0080 in atherosclerosis. Cell Death Differ 32, 1859–1870 (2025). https://doi.org/10.1038/s41418-025-01506-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-025-01506-7