Abstract
Members of the Bcl-2 family are essential regulators of cell fate. Some of them (proapoptotic) promote cell death, while others (antiapoptotic) support cell survival. Bcl-2 modifying factor (Bmf) is an understudied BH3-only protein of this family that is widely expressed in many normal and cancer tissues. Bmf’s proapoptotic activity is essential in physiological and pathological processes, including hematopoiesis, gametogenesis, diabetes, tumorigenesis, etc. However, Bmf has remained in the shadow of other BH3-only proteins for many years. This review aims to rectify this injustice and elucidate the multifaceted functions of Bmf, its regulation, and its significance in both normal and pathological contexts.
Similar content being viewed by others
Facts
-
The BH3-only protein Bmf is a key regulator of anoikis
-
Bmf is involved in hematopoiesis and gametogenesis
-
Dysregulation of Bmf contributes to the development of cancer and non-cancer diseases
Open questions
-
Are the functions of Bmf redundant in normal and malignant cells?
-
Could Bmf be a prognostic biomarker for cancer progression?
-
Which mechanisms underlie Bmf’s dysregulation in various processes?
-
Could Bmf regulation be exploited in anticancer therapy?
-
Is Bmf an “activator” or “sensitizer” of the BH3-only protein?
Introduction
Apoptosis, the most investigated type of programmed cell death (PCD), is a complex process that is essential for the survival of multicellular organisms. Two main pathways of apoptosis induction are known: the extrinsic (receptor-mediated) pathway, which is induced by binding of death ligands (e.g., FasL) to corresponding death receptors (e.g., Fas), and the intrinsic (mitochondrial) pathway, controlled by the proteins of the Bcl-2 family [1, 2]. The members of this family possess anti- and proapoptotic properties. The prosurvival proteins (Bcl-2, Mcl-1, Bcl-xL, Bfl-1, Bcl-w, and Bcl-B) are responsible for neutralizing their proapoptotic counterparts, and they are commonly upregulated in tumor tissues and promote malignant progression. The proteins of the Bcl-2 family that trigger apoptosis are divided into two subsets: effectors and regulators. The first subgroup contains multidomain proteins (Bak and Bax) that can undergo conformational changes, oligomerize, form pores, and cause mitochondrial outer membrane permeabilization (MOMP). MOMP results in the activation of caspase signaling and subsequent apoptotic cell death. The second subgroup consists of single Bcl-2 Homology (BH) domain proteins and that is reflected in their name—BH3-only proteins. This subset is the most numerous of the Bcl-2 family proteins and comprises at least eight members (Bim, Noxa, Puma, Bad, Bid, Hrk, Bik, and Bmf) [3,4,5,6,7,8,9]. Some of them, like Bim, are well-studied and discussed in detail [10,11,12,13]. At the same time, the other BH3-only proteins, like Bmf, are poorly explored. Bmf was characterized in 2008 as a “minor brother” of Bim due to similarities in structure and function, but ever since, it has remained in the background [14]. Here, we attempt to remedy this omission and highlight the existing data concerning Bmf, which is now an underappreciated member of the Bcl-2 family proteins.
General aspects of Bmf
Bmf protein, encoded by the BMF gene, which is located on chromosome 15q14/15q15.1, was identified as a novel BH3-only protein in 2001 using Mcl-1 as bait in a yeast two-hybrid (Y2H) screen [15]. The similarity between Bmf and Bim was quickly discovered [14]. Indeed, among other BH3-only proteins, these members of the Bcl-2 family have a lot in common: both proteins are constitutively expressed and active [14], in contrast to Bid, whose activation requires proteolytic cleavage [16] or Noxa and Puma, whose expression is mediated by p53 in response to stressful stimuli [17]. The unique feature of Bim and Bmf is the presence of motifs for binding dynein light chain proteins (DLC), which are involved in the transport of vesicles and organelles [15, 18, 19]. This circumstance determines similar localization of Bim and Bmf: under normal conditions, these proteins interact with DLC proteins (Bim – DLC1, Bmf – DLC2) and are sequestered to cytoskeletal structures (Bim – microtubules, Bmf – myosin V). In response to various stimuli, both proteins are released into the cytoplasm and, after translocation to mitochondria, endoplasmic reticulum (Bim), or only to mitochondria (Bmf), can regulate anoikis (an apoptosis-like form that will be described later) [14, 15, 20,21,22].
Three variants of alternative splicing of human Bmf (Bmf I, Bmf II, and Bmf III) have been described. Bmf I, hereafter referred to as Bmf, is the most abundant isoform and contains 184 amino acids (aa) (Fig. 1A) [18]. BMF expression was detected in various normal and cancer cells [23]. According to the human protein atlas (HPA) database, Bmf mRNA is enriched in normal and pathological tissues and organs of the immune system (especially thymus, bone marrow, spleen), gastrointestinal tract, and reproductive system (Fig. 1B, C). For instance, the high expression of Bmf and its other splice variants (Bmf II and Bmf III) was predominantly observed in healthy and malignant B-cells [18]. This occurrence may be explained by Bmf’s impact on B-cell homeostasis, as discussed below. Both shortened isoforms that are predicted to encode proteins of 163 (Bmf II) and 129 (Bmf III) aa lack the BH3 domain but retain the DLC2 binding domain. Accordingly, they could not exert proapoptotic activity and were able to increase colony formation, thereby promoting cell survival, in contrast to Bmf [14, 18]. It is worth noting that, besides mammals, Bmf orthologs were also detected in fishes, frogs, rats, and mice, and their BH3 domain, as well as the DLC2 binding motif, are conserved in all these evolutionary distinct species [9, 24].
A The scheme of the Bmf domain structure was obtained from www.uniprot.org (Q96LC9). DLC2 - dynein light chain 2 motif; BH3 - Bcl-2 Homology (BH) domain 3. P – phosphosites of Bmf. Blue and orange colors of kinases indicate their impact on Bmf-mediated apoptotic activity discussed below – “pro-“ and “antiapoptotic” ones, respectively. The thin and thick arrows of ERK2 indicate “minor” and “major” phosphosites, respectively. B The consensus dataset of normalized BMF expression levels generated by mixing RNA-seq data from the HPA and GTEx projects in healthy human tissues (obtained from www.proteinatlas.org) [23, 166]. C The normalized BMF expression levels from the HPA cell line resource in human tumors (obtained from www.proteinatlas.org) [23, 166]. 20 healthy and cancer tissue types with the highest BMF expression level are visualized in the histograms. Data are presented as nTPM. HPA human protein atlas, GTE Genotype-Tissue Expression, nTPM normalized transcript per million.
Despite its discovery more than 20 years ago, the exact protein structure of Bmf has not been determined, and only several complexes, containing part of its structure, were described in the literature and protein databases [25, 26]. Like other BH3-only proteins, Bmf interacts via its single BH domain with the special hydrophobic pocket, named the BH3-binding groove, of antiapoptotic members of the Bcl-2 family proteins [5]. Notably, Bmf was found to have a low affinity for Mcl-1 and Bfl-1/A1, which is similar to Mcl-1 [27], and predominantly interacts with Bcl-2, Bcl-xL, and Bcl-w [14, 20, 25, 28, 29]. The binding of α-helical fragments of human Bmf to the BH3-binding groove of Bcl-xL, Bcl-2, and Mcl-1 (PDB IDs 8iqk, 8iql, and 8iqm, respectively) was shown in Fig. 2A–C [25]. The interactions between these proteins are determined by the formation of conserved hydrophobic contacts (Ile133, Leu137, Ile140 and Phe144 residues of Bmf) and salt bridges (Asp142 residue of Bmf), which are formed in the Bmf-Bcl-xL (Asp142–Arg139) and Bmf-Bcl-2 (Asp142–Arg146) complexes (Fig. 2A, B) [25]. On the contrary, only a water-mediated hydrogen bond (Asp142-Arg263) instead of a salt bridge is formed in the Bmf-Mcl-1 complex (Fig. 2C) [25]. Accordingly, the Bmf aspartate residue is not found to directly bind to A1 protein in a mouse Bmf-A1 complex (PDB ID 2vog) [30]. Hence, the absence of electrostatic interactions may be a reason for lower Bmf affinity to Mcl-1 and A1 compared to Bcl-2, Bcl-xL, and Bcl-w [25, 30]. Additionally, the Bmf fragment corresponding to its DLC2 binding motif was found to adopt a β-strand conformation (Fig. 2D) and form multiple intermolecular hydrophobic and polar interactions. In particular, two hydrogen bonds between Thr72 residue of Bmf and Phe62 residue of DLC2 participate in stabilization of human Bmf-DLC2 complex (PDB ID 7cnu), and its destruction was associated with Bmf phosphorylation at Thr72 by mitogen-activated protein kinase (MAPK) p38, which is discussed below [26].
Bmf in complexes with Bcl-xL (A), Bcl-2 (B), Mcl-1 (C), and DLC2 (D). Bmf fragments (green color) contain 127–147 aa (A–C) and 64–73 aa (D). The figure was prepared using VMD [167].
It should be noted that various pro- (e.g., Bim [13], Noxa [31]) and antiapoptotic (e.g., Bcl-xL [32], Mcl-1 [4]) members of the Bcl-2 family proteins are also regulated by alternative splicing. Importantly, this type of regulation could invert their functional activity in most cases: spliced isoforms of prosurvival proteins could acquire proapoptotic properties like Mcl-1S or Bcl-xS. By contrast, BH3-only proteins like Bmf II and III transcripts lose the BH3 domain, missing the ability to activate apoptosis. Unfortunately, these protein alterations are poorly studied, and the biological significance of many isoforms of the members of the Bcl-2 family, including Bmf, remains unclear. Nevertheless, alternative splicing seems to modulate the activity of Bcl-2 proteins, thereby determining cell fate.
It should be noted that alternative splicing is common for eukaryotic cells compared to non-AUG initiation translation [33]. Some time ago, a new alternative translation start site (CUG) was discovered in mouse Bmf mRNA, which resulted in two major isoforms: BmfS (an ortholog of the human protein, containing 185 aa) and BmfCUG, containing 24 extra aa at its N-terminal end [34, 35]. Importantly, both mouse variants were proapoptotic and possessed similar properties, including subcellular localization and a pattern of interactions with Bcl-2 prosurvival proteins [34].
Notably, according to their functional activity, BH3-only proteins are commonly divided into two subgroups: “activators” (Bid-like), which can directly bind and activate effector proteins, and “direct sensitizers” (Bad-like), which can only disrupt complexes between pro- and antiapoptotic proteins to release Bax/Bak or “activators” [36]. However, this classification is somewhat controversial, as proteins vary in their ability to activate effectors, which is confirmed by the facts regarding Bmf. According to some reports, this protein was suggested to be a “sensitizer” [37, 38]. However, other studies indicate that Bmf might possess modest Bax/Bak-“activating capacity” [39,40,41]. Thus, Bmf could be considered a very mild “activator,” which is rather weaker than Bid or Bim, and its limited potency to activate Bax/Bak is likely to depend on the cell context or stimuli.
Functions of Bmf in physiology and pathology
Anoikis
Anoikis is an apoptotic-like type of cell death that is activated due to loss or inadequate cell adhesion [42]. This PCD subtype is of great importance in maintaining cell homeostasis in physiological and pathological conditions. As the first option, anoikis prevents carcinogenesis by eradicating cells that lose attachment to the extracellular matrix or adhere to inappropriate locations, thereby inhibiting the invasion of tumor cells. Anoikis suppression was found to be linked with cancer progression and metastasis in many reports [43,44,45]. At the same time, this process is also crucial for the development of normal tissues and organs. Particularly, anoikis-mediated regulation of cell rearrangement is associated with embryonic development, organ formation, tissue damage and repair, inflammatory response, and stem cell differentiation. The latter is especially important for further improvement of different stem cell‐based therapeutic applications, which are now intensively studied [46]. Therefore, besides cancer progression, anoikis dysregulation could be associated with various abnormalities that promote the development of cardiovascular diseases [47], diabetes [48, 49], brain injury [50, 51], tissue fibrosis [52, 53], and other non-cancer pathologies [46]. As mentioned above, apoptosis activation is under the tight control of multiple regulators, including members of the Bcl-2 family. The hallmarks of Bmf regulation at various levels will be discussed below in detail.
Importantly, starting with the first publication in 2001 [15], numerous studies reported that Bmf is one of the main mediators of anoikis, and as was mentioned above, Bmf’s liberation from the cytoskeleton leads to anoikis activation [21, 26, 54, 55]. Nevertheless, it should be noted that Bmf-lacking cells could also undergo anoikis, which illustrates the redundancy of Bcl-2 family proteins [56]. This phenomenon has great biological significance: a loss of one protein does not disrupt cell homeostasis, and its function could be substituted by others.
Other PCD types
According to several papers, Bmf might be associated with the other PCD modes—autophagy and necroptosis. First, Bmf was also proposed to regulate autophagy via stabilization of Bcl-2/Beclin-1 interactions. In particular, Bmf suppression in a p53-dependent manner might facilitate the release of Beclin-1, one of the key autophagy mediators [57] from a complex with Bcl-2 and promote this PCD type [35, 58]. Furthermore, cells that lacked Bmf were associated with increased autophagy [35]. Thus, like other Bcl-2 family proteins, Bmf seems to be involved in the modulation of autophagic processes, acting as a “switch” between two PCD forms: upregulation of BMF expression or its release from microfilaments stimulates anoikis and suppresses autophagy.
Second, Bmf was assumed to participate in necroptosis. Initially, Bmf was suggested to be related to necroptosis regulation using genome-wide screening in L929 cells [59]. However, another report failed to confirm the participation of Bmf in necroptosis regulation. Tumor necrosis factor alpha (TNFα) and zVAD.fmk-mediated (a pan-caspase inhibitor) necroptosis was shown not to be altered in Bmf-deficient cells in comparison with parental L929 cells or mouse embryonic fibroblasts (MEFs) [60]. At the same time, Bmf knockout was shown to partially abate cadmium-induced necroptosis [60]. Thus, Bmf’s impact on necroptosis modulation is now controversial.
Bmf in physiology
Analysis of Bmf knockout mice revealed that this protein, as well as the other BH3-only proteins, is dispensable for embryogenesis [56, 61]. However, Bmf suppression was shown to be engaged in the survival of hematopoietic stem and progenitor cells (HSPCs) [62]. It should be noted that Bmf plays an important role in B-cell development and maturation, and loss of Bmf causes abnormalities in B-cell homeostasis. In particular, it impaired apoptosis at various stages of B-cell development and resulted in the accumulation of pre- and mature B-cells [56, 61, 63]. Bmf knockout was also discovered to promote gamma-radiation-induced thymic lymphoma formation, which is in line with the HPA data (Fig. 1C, D) [56]. Moreover, double suppression of Bmf/Bim enhanced Bim-mediated alterations in B-cell homeostasis [64, 65] and could lead to premature lethality (~50% of mice were born alive) [65]. Thus, Bmf and Bim work in concert to regulate PCD and enhance each other’s actions.
Bmf was found to be involved in spermatogenesis and oogenesis regulation. On the one hand, Bmf expression was induced by a decline in testosterone levels in rat testis. Thus, cell death of spermatids following loss of cell attachment in response to lowered testosterone might be Bmf-dependent [24, 66]. On the other hand, this BH3-only protein could also regulate oogenesis: BMF depletion caused an increase in the number of primordial follicles from 100 postnatal days onward and extended fertility in mice [67]. Moreover, Bmf knockout led to enlarged germ cell numbers in embryonic and early postnatal mouse ovaries [68]. Interestingly, in contrast with males, which did not show any obvious developmental defects, female Bmf knockout mice are characterized by abnormalities of uterovaginal development [21, 64], including hydrometrocolpos and an imperforate vagina in about every fifth case [64]. The causes of this circumstance are probably associated with defects in apoptosis, which is essential to developing the female reproductive tract [69, 70]. In addition, Bmf is also implicated in mammary [55, 71] and intestinal epithelium morphogenesis [21]. For instance, elevated BMF expression was shown during anoikis in mammary [55] and intestinal epithelial cells (IEC), and Bmf knockout was found to rescue IECs in in vitro and in vivo models [21].
Bmf in pathology
Bmf might also participate in the regulation of neurological disorders. First, its expression was disclosed to be significantly increased during oligodendroglia differentiation [72]. Second, Bmf could contribute to neuronal injury: Bmf deficiency resulted in neuroprotection of cortical neurons in response to excitotoxic or ischemic conditions [73] and NGF deprivation or amyloid beta toxicity [74]. Finally, Bmf-lacking mice were more susceptible to seizure-induced neuronal death compared to wild-type controls. As seizure activity could cause energy depletion, and apoptosis is an energy-dependent process, it might be speculated that this effect was not associated with apoptotic functions of Bmf, and could be explained, for instance, by necrotic death activation [75].
Bmf is likely to be involved in the pathogenesis of diabetes, particularly mediating apoptosis of renal proximal tubular cells [76, 77]. Interestingly, AMPK, a sensor of “bioenergetic stress”, was demonstrated to induce the expression of Bmf in pancreatic beta cells, as well as in neurons [75, 78, 79]. Moreover, Bmf could be activated in response to glucocorticoids and engaged in glucocorticoid-induced apoptosis [80]. At the same time, BMF depletion was observed to protect beta cells from apoptosis and enhance hyperglycemia in diabetic mice [79]. The exact mechanism of this phenomenon remains unclear. However, it should be noted that the overexpression of antiapoptotic proteins (e.g., Bcl-2 or Bcl-xL) led to similar effects (survival of beta-cells and augmented hyperglycemia) that might be explained by impaired glucose signaling [79, 81, 82]. Additionally, the Bmf level was disclosed to positively correlate with vascular calcification and aging in diabetic models, and BMF ablation was found to abrogate this effect, diminishing calcification and senescence of vascular smooth muscle cells [83, 84]. Generally, these findings suggest an important and understudied role for Bmf in the maintenance of glucose homeostasis.
As can be seen from the above, Bmf is implicated in the regulation of multifarious physiological (hematopoiesis, gametogenesis, tissue remodeling) and pathological (neuronal damage, diabetes) processes that are predominantly associated with apoptosis/anoikis modulation. Moreover, all findings presented here demonstrate non-redundant functions of Bmf compared to Bim and other BH3-only proteins, confirming its biological significance.
The regulation of Bmf in normal and pathological conditions
The transcriptional regulation of Bmf
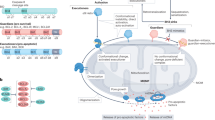
Various positive (e.g., FOXO3) and negative (e.g., YAP/TEAD/SLUG) transcription regulators, summarized in Table 1, have been proposed to modulate BMF expression (Fig. 3). Notably, besides that, a balance between histone acetylation and deacetylation, which is a part of epigenetic regulation, determines the expression levels of many genes in normal and cancer cells, and hyperacetylation of histones mediated by histone deacetylase inhibitors (HDACi) could induce transcription of various genes. Interestingly, numerous articles reported that several HDACi, including vorinostat (a drug approved by the FDA for the treatment of cutaneous T-cell lymphoma), were discovered to enhance BMF expression [85,86,87,88,89,90,91,92]. Specifically, HDACi-mediated apoptosis was associated with an elevation in the Bmf protein level, and Bmf knockout attenuated this effect. Moreover, HDAC1 [85] or HDAC8 [87] overexpression led to a decline in Bmf expression. Thus, HDACs serve as important transcriptional regulators of Bmf, and their inhibition could enhance cancer cell death through, among other matters, Bmf upregulation.
P - phosphorylated form of the Bmf protein. The details and the other regulators, which are not presented here to simplify the scheme, are discussed in the text. The blue and red arrows indicate “positive” and “negative” influence on the participants of Bmf regulation and anoikis/apoptosis. This figure was generated by using the Servier Medical Art images, that is licensed under a Creative Commons Attribution 4.0 Unported License.
The post-transcriptional regulation of Bmf
In contrast to the abovementioned alternative splicing of Bmf, which remains understudied, the regulation of mRNA levels of this BH3-only protein has become clearer during recent years. Above all, plenty of microRNAs (miRNAs) were shown to target Bmf (Fig. 2). These regulatory elements represent small (about 21–23 nucleotides) non-coding RNAs, which bind to corresponding mRNAs through the 3′-untranslated region (UTR), thereby causing their degradation [93]. Table 2 includes information about several miRNAs that participate in the negative control of BMF expression.
Besides miRNAs, other post-transcriptional regulators were reported to orchestrate Bmf mRNA stability, which is responsible for the modulation of miRNA activity. These modulators mainly include long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs). LncRNAs comprise molecules containing more than 200 nucleotides without protein-coding ability [94]. Moreover, there is a subgroup of antisense lncRNAs, which are transcribed in an antisense orientation compared to corresponding protein-coding or non-coding genes [95]. CircRNAs are single-stranded molecules, in which the 3′ and 5′ ends, unlike those in linear RNA, are covalently closed [96]. LncRNAs, circRNAs, and other regulators that contribute to the control of Bmf mRNA levels are presented in Table 3.
In addition, the RelB/p52 complex mentioned earlier was found to downregulate the Bmf level not only at the transcriptional level but also at the post-transcriptional stage. Specifically, this complex was able to interact with the promoter region of the miR-221 gene and augment its expression, while this miRNA was shown to target Bmf mRNA in different models, including multiple myeloma [92].
The posttranslational regulation of Bmf
Above all, the level of Bmf can be altered in response to various stimuli. First, Bmf induction was observed upon cytokine withdrawal in granulocytes [97]. Later, Bmf suppression was found to reduce apoptotic cell death in response to impairment of CAP-dependent translation, in particular, under serum deprivation [34]. Besides that, Bmf was shown to mediate anoikis/apoptosis upon UV irradiation [15], paclitaxel (microtubule inhibitor) [98], and arsenic trioxide [99], which can also disturb microtubule dynamics [100]. Specifically, UV exposure resulted in a release of Bmf from cytoskeleton [15], and paclitaxel treatment led to a disruption of Bim complexes with prosurvival Bcl-2 proteins by a cooperation of Bmf and Puma in breast cancer [98]. At the same time, arsenic trioxide led to an increase in BMF expression in multiple myeloma [99]. Next, chemokines (CXCL12 and CCL21) and their corresponding receptors (CXCR4 and CCR7) were disclosed to inhibit anoikis and promote metastasis and tumorigenesis via the downregulation of Bmf levels. The precise mechanism of this phenomenon remains unknown and might be linked to the activation of several signaling pathways [101].
Furthermore, MAPK signaling cascades could contribute to the posttranslational regulation of Bmf. On the one hand, p38 MAPK, involved in a “proapoptotic” pathway [102], was found to affect Bmf and increase its apoptotic activity via p38 MAPK-mediated Bmf phosphorylation at Thr72, which is located within the DLC2 binding domain. This modification led to the disruption of interactions between DLC2 and Bmf, causing, as mentioned above, the release of the latter from the cytoskeleton and promoting cell death (Fig. 3) [26]. Another “proapoptotic” pathway, JNK [103], was observed to phosphorylate Bmf at Ser74; however, JNK was only found to facilitate Bmf-induced anoikis, but generally, it is not essential for anoikis induction [64, 104, 105]. On the other hand, ERK1/2, which positively regulates a “prosurvival” pathway [106], was disclosed to diminish the ability of Bmf to trigger apoptosis at different regulatory levels. First, suppression of ERK1/2 positive regulators NRAS or MEK was shown to elevate both Bmf mRNA and protein levels [107]. Second, activation of PI3K/AKT or MEK/ERK signaling pathways was associated with abatement of BMF transcription [55, 108]. Third, ERK2 was able to directly phosphorylate this BH3-only protein at Ser74 (“minor site”) and Ser77 (“major site”), which did not affect Bmf stability and its interaction with DLC2 or antiapoptotic Bcl-2 proteins. However, ERK2-mediated Bmf phosphorylation decreased its apoptotic activity [109]. Importantly, Ser74 was the same target site for JNK, but, obviously, the two-site phosphorylation demonstrated in this case could lead to the opposite results. Nevertheless, it might explain why Bmf was able to translocate from the cytoskeleton into the cytoplasm only in MEK inhibitor-sensitive cancer cells, while in resistant cells, this protein remained bonded to DLC2 and did not induce apoptotic cell death [110]. Finally, the EGFR/MEK/ERK pathway was also revealed to be activated and block anoikis via suppressing Bmf expression under hypoxic conditions [111]. The possible mechanism underlying this result remains to be elucidated, but the obtained findings suggest that tumors, which often survive under hypoxia due to the “Warburg effect,” can escape anoikis, proliferate, and be intolerant to Bmf-induced chemotherapeutics.
As mentioned earlier, DLC1/2 proteins can interact with Bmf and Bim, controlling their functional activity [15, 19, 38]. Recently, similarly to Bim and DLC1 [112], Bmf was found to dimerize and form a complex upon DLC2 binding, which might also be necessary for preventing its proteasomal degradation [38]. Moreover, these proteins were detected to assemble multiple complexes like Bmf-DLC-Bim, and Bmf overexpression was revealed to trigger Bim degradation [38]. Hence, these data suggest that “relative proteins” Bmf and Bim could modulate each other’s functions in the presence of DLC1/2 proteins.
The role of Bmf in tumorigenesis
According to the findings observed, Bmf, like all proteins participating in the triggering of PCD, is under strict control at different levels. This complex regulatory system is essential to prevent spontaneous cell death. However, the alterations in Bmf regulation could promote the progression of both cancer and non-cancer diseases. On the one hand, an increase in the Bmf level due to the predominance of its “activators” might lead to excessive apoptosis in some pathological conditions, e.g., ischemic stroke. On the other hand, Bmf suppression (e.g., due to increased levels of miRNAs or decreased levels of “positive” regulators) could facilitate tumor growth and carcinogenesis, confirming the oncosuppressive role of Bmf that was illustrated in this review.
Next, in agreement with the previously mentioned Bmf’s impact on B-cell homeostasis, this BH3-only protein was observed to participate in the pathogenesis of chronic lymphocytic leukemia (CLL), which is associated with B-cell malignancy. First, a low level of Bmf was found in neonatal B1 B-cells in contrast with B2 B-cells, and Bmf deficiency coupled with high expression of c-Myc in B1 B-cells might predispose to CLL development [113]. Second, Single Nucleotide Polymorphism (SNP) rs539846-A, located in the third intron of BMF, was shown to diminish BMF expression via alteration of the RELA-binding motif and its enhancer activity. Hence, the presence of this genetic variant could also intensify oncogenicity in CLL [114].
Notably, a loss of 15q14/15, containing the BMF gene, was found in several metastatic tumors (lung, breast, and colorectal carcinomas) [115]. Considering that Bmf is involved in the regulation of mammary morphogenesis [55], this protein may be a possible prognostic marker in breast cancer. Logically, cancer cells evade Bmf-mediated anoikis, an important defense mechanism, and survive after detachment from their niches to progress and metastasize.
Interestingly, novel studies have shown a link between Bmf and tumorigenicity in liver cancer [116, 117]. As mentioned above, the YAP signaling pathway acts as a negative regulator of Bmf at the transcriptional level [118, 119]. Recently, YAP activation or Bmf inhibition was found to promote the stemness of liver cancer stem cells. Moreover, in conditions that mimic raised compression in growing tumors, the YAP/Bmf axis mediated enhanced tumorigenic potential, while Bmf overexpression was discovered to diminish these effects [116, 117]. Altogether, these results support the oncosuppressive functions of Bmf. Indeed, the loss of Bmf results in the protection of cancer cells from anoikis, facilitating metastatic spreading and tumorigenesis, but its overexpression or augmented release from cytoskeletal structures could trigger cell death and prevent metastasis of detached cancer cells.
It should be noted that malignant cells can accumulate mutations during tumorigenesis. Interestingly, mutant p53 (p53-R273H), which loses its oncosuppressive activity and acquires oncogenic properties [120], was shown to inhibit Bmf expression through activation of the PI3K/AKT signaling cascade in various cancer types [121], which corresponds with the previously mentioned report concerning the negative influence of this pathway on Bmf function [55]. Hence, p53 status may be a potential biomarker that predicts the proapoptotic activity of Bmf.
Finally, Bcl-2 family proteins can also undergo mutation, which alters their properties [5, 122]. However, Bmf mutations are not common in tumors [123, 124]. According to an open database (www.cbioportal.org) containing the results of multiple cancer studies, 122 genetic alterations of the BMF gene, predominantly deep deletions and mutations, were detected in 23 cancer types among a total of 32 (10,953 patients). The alterations were found most frequently in colorectal, ovarian, and lung carcinomas in absolute number (n = 14) and uterine carcinosarcoma in relative number (8.77%), which agrees with the disturbance of Bmf mRNA in cancer tissues, presented earlier (Fig. 1D). The detailed results are presented in Table 4.
Nevertheless, despite their rarity, the appearance of genetic alterations in the Bmf gene may result in anoikis resistance and tumor progression. Thus, mutations in the DLC2 motif (Leu73Ile) or the BH3 domain (Arg135Gln/*; Gln138*) might lead to a loss of its ability to interact with other proteins, including members of the Bcl-2 family. For example, the mutation of Arg135 could change the spatial position of Ile133 and Leu137 in the BH3 domain of Bmf, which are important for the hydrophobic contacts with the BH3-binding groove of antiapoptotic Bcl-2 family proteins. Additionally, Leu73 directly binds with Thr72 in the DLC2 motif, which promotes stabilization of the human Bmf-DLC2 complex (Fig. 2D); consequently, the substitution Leu73Ile could destroy this complex. Taken together, Bmf mutations could serve as possible prognostic markers in patients with various malignant diseases.
Concluding remarks
Here, we summarize various aspects of Bmf functions and regulation in normal and pathological conditions. This BH3-only protein of the Bcl-2 family plays a crucial role not only in “classical” apoptosis but also in anoikis regulation, contributing to normal development and cancer prevention. Despite numerous reports concerning Bmf, its significance in various states remains undefined, which is associated with an abundance of BH3-only proteins. Thus, mice lacking Bmf could survive, indicating that BH3-only (mainly Bim) members of the Bcl-2 family could be replaced by compensate the functions of each other. Hence, it is difficult to assess the exact impact of Bmf on specific processes. Nevertheless, the presence of several abnormalities in Bmf knockout models mentioned earlier [21, 61,62,63,64,65] illustrates its importance for normal development. Therefore, these observations suggest that there was no complete substitute for Bmf. This assumption may also be supported by the multiplex regulation of Bmf. It would be a bit strange that an “auxiliary” protein is under the strict control of most modulators. Moreover, some of the Bmf regulators presented here, for instance, the YAP/TEAD/SLUG complex, were observed to have an exclusive influence on Bmf, but not on its “relative” Bim [118].
Next, loss of Bmf was found to promote tumorigenesis and drug resistance, suggesting that this protein may be a prognostic marker of some cancer diseases. As mentioned, Bmf is involved in HDACi-mediated apoptosis, and its rate could predict the response of cancer cells to them. Moreover, considering the negative impact of the MEK/ERK signaling pathway on Bmf, the use of MEK inhibitors could enhance the efficacy of cancer therapy in some cases; meanwhile, dysregulation of Bmf might be linked with the development of resistance to MEK inhibitors. Finally, the investigations of all BH3-only proteins and their interactions with prosurvival Bcl-2 proteins at appropriate times allowed the development of a novel class of drugs – BH3-mimetics, including Venetoclax (a selective Bcl-2 inhibitor), approved by the FDA [5].
Notably, targeting members of the Bcl-2 family is an attractive therapeutic approach that has been intensively studied over the last decades. Despite achieving some success in this field (Venetoclax approval), several problems remain unresolved. In addition to low therapeutic efficacy in most cases due to the abundance and “compensatory effect” of Bcl-2 family proteins, its blocking could lead to undesirable side effects. Specifically, thrombocytopenia and cardiotoxicity are associated with Bcl-xL and Mcl-1 inhibition, respectively [5, 125]. Similar concerns may also arise regarding the Bmf target regulation. Hence, a possible anoikis/apoptosis induction in healthy tissues and promotion of neurodegeneration or diabetes in response to drug-mediated Bmf upregulation should be taken in consideration in the future. However, Bmf may be considered as a predictive factor for assessing the effectiveness of cancer treatment, such as CLL. To conclude, “minor does not mean useless,” and this statement is true for the Bmf protein. Besides that, other functions of Bmf are likely to be unknown at this moment. Are they worth studying in the future? The answer is probably “yes” because, at least, it could shed light on the novel mechanisms underlying the physiological and pathological processes and, at most, improve existing therapeutic approaches. Altogether, research on distinct members of the Bcl-2 family proteins is of fundamental and applied significance, which is clearly shown for the Bmf protein here.
References
Mustafa M, Ahmad R, Tantry IQ, Ahmad W, Siddiqui S, Alam M, et al. Apoptosis: a comprehensive overview of signaling pathways, morphological changes, and physiological significance and therapeutic implications. Cells. 2024;13:1838.
Moyer A, Tanaka K, Cheng EH. Apoptosis in cancer biology and therapy. Annu Rev Pathol. 2025;20:303–28.
Vogler M, Braun Y, Smith VM, Westhoff M-A, Pereira RS, Pieper NM, et al. The BCL2 family: from apoptosis mechanisms to new advances in targeted therapy. Signal Transduct Target Ther. 2025;10:91.
Pervushin NV, Senichkin VV, Zhivotovsky B, Kopeina GS. Mcl-1 as a ‘barrier’ in cancer treatment: can we target it now? Int Rev Cell Mol Biol. 2020;351:23–55.
Senichkin VV, Pervushin NV, Zuev AP, Zhivotovsky B, & Kopeina GS. Targeting Bcl-2 family proteins: What, Where, When? Biochemistry. 2020;85:1210–26.
Pervushin NV, Kopeina GS, Zhivotovsky B. Bcl-B: an “unknown” protein of the Bcl-2 family. Biol Direct. 2023;18:69.
Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249–57.
Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6.
Coultas L, Huang DCS, Adams JM, Strasser A. Pro-apoptotic BH3-only Bcl-2 family members in vertebrate model organisms suitable for genetic experimentation. Cell Death Differ. 2002;9:1163–6.
Faber AC, Ebi H, Costa C, Engelman JA. Apoptosis in targeted therapy responses. Role Bim Adv Pharm. 2012;65:519–42.
Ramesh S, Wildey GM, Howe PH. Transforming growth factor beta (TGFbeta)-induced apoptosis: the rise & fall of Bim. Cell Cycle. 2009;8:11–7.
Shukla S, Saxena S, Singh BK, Kakkar P. BH3-only protein BIM: an emerging target in chemotherapy. Eur J Cell Biol. 2017;96:728–38.
Sionov RV, Vlahopoulos SA, Granot Z. Regulation of bim in health and disease. Oncotarget. 2015;6:23058–134.
Piñon JD, Labi V, Egle A, Villunger A. Bim and Bmf in tissue homeostasis and malignant disease. Oncogene. 2008;27:S41–52.
Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–32.
Billen LP, Shamas-Din A, Andrews DW. Bid: a Bax-like BH3 protein. Oncogene. 2008;27:S93–104.
Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63.
Morales AA, Olsson A, Celsing F, Osterborg A, Jondal M, Osorio LM. Expression and transcriptional regulation of functionally distinct Bmf isoforms in B-chronic lymphocytic leukemia cells. Leukemia. 2004;18:41–47.
Day CL, Puthalakath H, Skea G, Strasser A, Barsukov I, Lian L-Y, et al. Localization of dynein light chains 1 and 2 and their pro-apoptotic ligands. Biochem J. 2004;377:597–605.
Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80.
Hausmann M, Leucht K, Ploner C, Kiessling S, Villunger A, Becker H, et al. BCL-2 modifying factor (BMF) is a central regulator of anoikis in human intestinal epithelial cells. J Biol Chem. 2011;286:26533–40.
Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein bim. Cell. 2007;129:1337–49.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Show MD, Folmer JS, Anway MD, Zirkin BR. Testicular expression and distribution of the rat bcl2 modifying factor in response to reduced intratesticular testosterone. Biol Reprod. 2004;70:1153–61.
Wang H, Guo M, Wei H, Chen Y. Structural basis of the specificity and interaction mechanism of Bmf binding to pro-survival Bcl-2 family proteins. Comput Struct Biotechnol J. 2023;21:3760–7.
Zhi Z, Ouyang Z, Ren Y, Cheng Y, Liu P, Wen Y, et al. Non-canonical phosphorylation of Bmf by p38 MAPK promotes its apoptotic activity in anoikis. Cell Death Differ. 2022;29:323–36.
Simmons MJ, Fan G, Zong W-X, Degenhardt K, White E, Gélinas C. Bfl-1/A1 functions, similar to Mcl-1, as a selective tBid and Bak antagonist. Oncogene. 2008;27:1421–8.
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403.
Kong W, Zhou M, Li Q, Fan W, Lin H, Wang R. Experimental characterization of the binding affinities between proapoptotic BH3 peptides and antiapoptotic Bcl-2 proteins. ChemMedChem. 2018;13:1763–70.
Smits C, Czabotar PE, Hinds MG, Day CL. Structural plasticity underpins promiscuous binding of the prosurvival protein A1. Structure. 2008;16:818–29.
Wang Z, Sun Y. Identification and characterization of two splicing variants of human Noxa. Anticancer Res. 2008;28:1667–74.
Chen W, Li J. Alternative splicing of BCL-X and implications for treating hematological malignancies. Oncol Lett. 2021;22:670.
Asano K. Why is start codon selection so precise in eukaryotes? Translation. 2014;2:e28387.
Grespi F, Soratroi C, Krumschnabel G, Sohm B, Ploner C, Geley S, et al. BH3-only protein Bmf mediates apoptosis upon inhibition of CAP-dependent protein synthesis. Cell Death Differ. 2010;17:1672–83.
Contreras AU, Mebratu Y, Delgado M, Montano G, Hu C-AA, Ryter SW, et al. Deacetylation of p53 induces autophagy by suppressing Bmf expression. J Cell Biol. 2013;201:427–37.
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–92.
Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35.
Singh PK, Roukounakis A, Weber A, Das KK, Sohm B, Villunger A, et al. Dynein light chain binding determines complex formation and posttranslational stability of the Bcl-2 family members Bmf and Bim. Cell Death Differ. 2020;27:434–50.
Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. BH3 domains other than Bim and Bid can directly activate Bax/Bak. J Biol Chem. 2011;286:491–501.
Hockings C, Anwari K, Ninnis RL, Brouwer J, O’Hely M, Evangelista M, et al. Bid chimeras indicate that most BH3-only proteins can directly activate Bak and Bax, and show no preference for Bak versus Bax. Cell Death Dis. 2015;6:e1735.
Ye K, Meng WX, Sun H, Wu B, Chen M, Pang Y-P, et al. Characterization of an alternative BAK-binding site for BH3 peptides. Nat Commun. 2020;11:3301.
Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26.
Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833:3481–98.
Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226:380–93.
Dai Y, Zhang X, Ou Y, Zou L, Zhang D, Yang Q, et al. Anoikis resistance–protagonists of breast cancer cells survive and metastasize after ECM detachment. Cell Commun Signal. 2023;21:190.
Mei J, Jiang X-Y, Tian H-X, Rong D-C, Song J-N, Wang L, et al. Anoikis in cell fate, physiopathology, and therapeutic interventions. MedComm. 2024;5:e718.
Imamaki R, Ogawa K, Kizuka Y, Komi Y, Kojima S, Kotani N, et al. Glycosylation controls cooperative PECAM-VEGFR2-β3 integrin functions at the endothelial surface for tumor angiogenesis. Oncogene. 2018;37:4287–99.
An T, Zhang Z, Li Y, Yi J, Zhang W, Chen D, et al. Integrin β1-mediated cell−cell adhesion augments metformin-induced anoikis. Int J Mol Sci. 2019;20:1161.
Lin J, Lin Y, Li X, He F, Gao Q, Wang Y, et al. Uncovering the role of anoikis-related genes in modulating immune infiltration and pathogenesis of diabetic kidney disease. J Inflamm Res. 2024;17:4975–91.
Koda M, Someya Y, Nishio Y, Kadota R, Mannoji C, Miyashita T, et al. Brain-derived neurotrophic factor suppresses anoikis-induced death of Schwann cells. Neurosci Lett. 2008;444:143–7.
Sater AP, Rael LT, Tanner AH, Lieser MJ, Acuna DL, Mains CW, et al. Cell death after traumatic brain injury: Detrimental role of anoikis in healing. Clin Chim Acta. 2018;482:149–54.
Yin J, Wang J, Zhang X, Liao Y, Luo W, Wang S, et al. A missing piece of the puzzle in pulmonary fibrosis: anoikis resistance promotes fibroblast activation. Cell Biosci. 2022;12:21.
Lu Q, Harrington EO, Rounds S. Apoptosis and lung injury. Keio J Med. 2005;54:184–9.
Nagaprashantha LD, Vatsyayan R, Lelsani PCR, Awasthi S, Singhal SS. The sensors and regulators of cell-matrix surveillance in anoikis resistance of tumors. Int J Cancer. 2011;128:743–52.
Schmelzle T, Mailleux AA, Overholtzer M, Carroll JS, Solimini NL, Lightcap ES, et al. Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. Proc Natl Acad Sci USA. 2007;104:3787–92.
Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L, et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med. 2008;205:641–55.
Tran S, Fairlie WD, Lee EF. BECLIN1: protein structure, function and regulation. Cells. 2021;10:1522.
Delgado M, Tesfaigzi Y. BH3-only proteins, Bmf and Bim, in autophagy. Cell Cycle. 2013;12:3453–4.
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–23.
Tischner D, Manzl C, Soratroi C, Villunger A, Krumschnabel G. Necrosis-like death can engage multiple pro-apoptotic Bcl-2 protein family members. Apoptosis. 2012;17:1197–209.
Baumgartner F, Woess C, Pedit V, Tzankov A, Labi V, Villunger A. Minor cell-death defects but reduced tumor latency in mice lacking the BH3-only proteins Bad and Bmf. Oncogene. 2013;32:621–30.
Labi V, Bertele D, Woess C, Tischner D, Bock FJ, Schwemmers S, et al. Haematopoietic stem cell survival and transplantation efficacy is limited by the BH3-only proteins Bim and Bmf. EMBO Mol Med. 2013;5:122–36.
Frenzel A, Labi V, Chmelewskij W, Ploner C, Geley S, Fiegl H, et al. Suppression of B-cell lymphomagenesis by the BH3-only proteins Bmf and Bad. Blood. 2010;115:995–1005.
Hübner A, Cavanagh-Kyros J, Rincon M, Flavell RA, Davis RJ. Functional cooperation of the proapoptotic Bcl2 family proteins Bmf and Bim in vivo. Mol Cell Biol. 2010;30:98–105.
Labi V, Woess C, Tuzlak S, Erlacher M, Bouillet P, Strasser A, et al. Deregulated cell death and lymphocyte homeostasis cause premature lethality in mice lacking the BH3-only proteins Bim and Bmf. Blood. 2014;123:2652–62.
Show MD, Hill CM, Anway MD, Wright WW, Zirkin BR. Phosphorylation of mitogen-activated protein kinase 8 (MAPK8) is associated with germ cell apoptosis and redistribution of the Bcl2-modifying factor (BMF). J Androl. 2008;29:338–44.
Liew SH, Vaithiyanathan K, Cook M, Bouillet P, Scott CL, Kerr JB, et al. Loss of the proapoptotic BH3-only protein BCL-2 modifying factor prolongs the fertile life span in female mice. Biol Reprod. 2014;90:77.
Vaithiyanathan K, Liew SH, Zerafa N, Gamage T, Cook M, O’Reilly LA, et al. BCL2-modifying factor promotes germ cell loss during murine oogenesis. Reproduction. 2016;151:553–62.
Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–99.
Rodriguez I, Araki K, Khatib K, Martinou JC, Vassalli P. Mouse vaginal opening is an apoptosis-dependent process which can be prevented by the overexpression of Bcl2. Dev Biol. 1997;184:115–21.
Sakamoto K, Wehde BL, Yoo KH, Kim T, Rajbhandari N, Shin HY, et al. Janus kinase 1 is essential for inflammatory cytokine signaling and mammary gland remodeling. Mol Cell Biol. 2016;36:1673–90.
Itoh T, Itoh A, Pleasure D. Bcl-2-related protein family gene expression during oligodendroglial differentiation. J Neurochem. 2003;85:1500–12.
Pfeiffer S, Anilkumar U, Chen G, Ramírez-Peinado S, Galindo-Moreno J, Muñoz-Pinedo C, et al. Analysis of BH3-only proteins upregulated in response to oxygen/glucose deprivation in cortical neurons identifies Bmf but not Noxa as potential mediator of neuronal injury. Cell Death Dis. 2014;5:e1456.
Akhter R, Saleem S, Saha A, Biswas SC. The pro-apoptotic protein Bmf co-operates with Bim and Puma in neuron death induced by β-amyloid or NGF deprivation. Mol Cell Neurosci. 2018;88:249–57.
Moran C, Sanz-Rodriguez A, Jimenez-Pacheco A, Martinez-Villareal J, McKiernan RC, Jimenez-Mateos EM, et al. Bmf upregulation through the AMP-activated protein kinase pathway may protect the brain from seizure-induced cell death. Cell Death Dis. 2013;4:e606.
Lau GJ, Godin N, Maachi H, Lo C-S, Wu S-J, Zhu J-X, et al. Bcl-2-modifying factor induces renal proximal tubular cell apoptosis in diabetic mice. Diabetes. 2012;61:474–84.
Ghosh A, Zhao S, Lo C-S, Maachi H, Chenier I, Lateef MA, et al. Heterogeneous nuclear ribonucleoprotein F mediates insulin inhibition of Bcl2-modifying factor expression and tubulopathy in diabetic kidney. Sci Rep. 2019;9:6687.
Kilbride SM, Farrelly AM, Bonner C, Ward MW, Nyhan KC, Concannon CG, et al. AMP-activated protein kinase mediates apoptosis in response to bioenergetic stress through activation of the pro-apoptotic Bcl-2 homology domain-3-only protein BMF. J Biol Chem. 2010;285:36199–206.
Pfeiffer S, Halang L, Düssmann H, Byrne MM, Prehn J. BH3-Only protein bmf is required for the maintenance of glucose homeostasis in an in vivo model of HNF1α-MODY diabetes. Cell Death Discov. 2015;1:15041.
Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S, et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia. 2008;22:370–7.
Zhou YP, Pena JC, Roe MW, Mittal A, Levisetti M, Baldwin AC, et al. Overexpression of Bcl-x(L) in beta-cells prevents cell death but impairs mitochondrial signal for insulin secretion. Am J Physiol Endocrinol Metab. 2000;278:E340–351.
Luciani DS, White SA, Widenmaier SB, Saran VV, Taghizadeh F, Hu X, et al. Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic β-cells. Diabetes. 2013;62:170–82.
Lin X, Xiang Q-Y, Li S, Song W-L, Wang Y-J, Ni Y-Q, et al. BMF-AS1/BMF promotes diabetic vascular calcification and aging both in vitro and in vivo. Aging Dis. 2023;14:170–83.
Lin X, Zhan J-K, Zhong J-Y, Wang Y-J, Wang Y, Li S, et al. lncRNA-ES3/miR-34c-5p/BMF axis is involved in regulating high-glucose-induced calcification/senescence of VSMCs. Aging. 2019;11:523–35.
Zhang Y, Adachi M, Kawamura R, Imai K. Bmf is a possible mediator in histone deacetylase inhibitors FK228 and CBHA-induced apoptosis. Cell Death Differ. 2006;13:129–40.
Zhang Y, Adachi M, Kawamura R, Zou HC, Imai K, Hareyama M, et al. Bmf contributes to histone deacetylase inhibitor-mediated enhancing effects on apoptosis after ionizing radiation. Apoptosis. 2006;11:1349–57.
Kang Y, Nian H, Rajendran P, Kim E, Dashwood WM, Pinto JT, et al. HDAC8 and STAT3 repress BMF gene activity in colon cancer cells. Cell Death Dis. 2014;5:e1476.
Xargay-Torrent S, López-Guerra M, Saborit-Villarroya I, Rosich L, Campo E, Roué G, et al. Vorinostat-induced apoptosis in mantle cell lymphoma is mediated by acetylation of proapoptotic BH3-only gene promoters. Clin Cancer Res. 2011;17:3956–68.
Laporte AN, Poulin NM, Barrott JJ, Wang XQ, Lorzadeh A, Vander Werff R, et al. Death by HDAC Inhibition in Synovial Sarcoma Cells. Mol Cancer Ther. 2017;16:2656–67.
Enßle JC, Boedicker C, Wanior M, Vogler M, Knapp S, Fulda S. Co-targeting of BET proteins and HDACs as a novel approach to trigger apoptosis in rhabdomyosarcoma cells. Cancer Lett. 2018;428:160–72.
Jenkins LJ, Luk IY, Fairlie WD, Lee EF, Palmieri M, Schoffer KL, et al. Genotype-tailored ERK/MAPK pathway and HDAC inhibition rewires the apoptotic rheostat to trigger colorectal cancer cell death. Mol Cancer Ther. 2023;22:52–62.
Vallabhapurapu SD, Noothi SK, Pullum DA, Lawrie CH, Pallapati R, Potluri V, et al. Transcriptional repression by the HDAC4-RelB-p52 complex regulates multiple myeloma survival and growth. Nat Commun. 2015;6:8428.
Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–9.
Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen L-L, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24:430–47.
Liu B, Xiang W, Liu J, Tang J, Wang J, Liu B, et al. The regulatory role of antisense lncRNAs in cancer. Cancer Cell Int. 2021;21:459.
Huang A, Zheng H, Wu Z, Chen M, Huang Y. Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics. 2020;10:3503–17.
Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101:2393–400.
Kutuk O, Letai A. Displacement of Bim by Bmf and Puma rather than increase in Bim level mediates paclitaxel-induced apoptosis in breast cancer cells. Cell Death Differ. 2010;17:1624–35.
Morales AA, Gutman D, Lee KP, Boise LH. BH3-only proteins Noxa, Bmf, and Bim are necessary for arsenic trioxide-induced cell death in myeloma. Blood. 2008;111:5152–62.
Gao L, Xue B, Xiang B, Liu KJ. Arsenic trioxide disturbs the LIS1/NDEL1/dynein microtubule dynamic complex by disrupting the CLIP170 zinc finger in head and neck cancer. Toxicol Appl Pharmacol. 2020;403:115158.
Kochetkova M, Kumar S, McColl SR. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009;16:664–73.
Gräb J, Rybniker J. The expanding role of p38 mitogen-activated protein kinase in programmed host cell death. Microbiol Insights. 2019;12:1178636119864594.
Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–51.
Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432–7.
Girnius N, Davis RJ. JNK promotes epithelial cell anoikis by transcriptional and post-translational regulation of BH3-only proteins. Cell Rep. 2017;21:1910–21.
Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–77.
Dolgikh N, Hugle M, Vogler M, Fulda S. NRAS-mutated rhabdomyosarcoma cells are vulnerable to mitochondrial apoptosis induced by coinhibition of MEK and PI3Kα. Cancer Res. 2018;78:2000–13.
Cook SJ, Stuart K, Gilley R, Sale MJ. Control of cell death and mitochondrial fission by ERK1/2 MAP kinase signalling. FEBS J. 2017;284:4177–95.
Shao Y, Aplin AE. ERK2 phosphorylation of serine 77 regulates Bmf pro-apoptotic activity. Cell Death Dis. 2012;3:e253.
VanBrocklin MW, Verhaegen M, Soengas MS, Holmen SL. Mitogen-activated protein kinase inhibition induces translocation of Bmf to promote apoptosis in melanoma. Cancer Res. 2009;69:1985–94.
Whelan KA, Caldwell SA, Shahriari KS, Jackson SR, Franchetti LD, Johannes GJ, et al. Hypoxia suppression of Bim and Bmf blocks anoikis and luminal clearing during mammary morphogenesis. Mol Biol Cell. 2010;21:3829–37.
Singh PK, Roukounakis A, Frank DO, Kirschnek S, Das KK, Neumann S, et al. Dynein light chain 1 induces assembly of large Bim complexes on mitochondria that stabilize Mcl-1 and regulate apoptosis. Genes Dev. 2017;31:1754–69.
Hayakawa K, Formica AM, Brill-Dashoff J, Shinton SA, Ichikawa D, Zhou Y, et al. Early generated B1 B cells with restricted BCRs become chronic lymphocytic leukemia with continued c-Myc and low Bmf expression. J Exp Med. 2016;213:3007–24.
Kandaswamy R, Sava GP, Speedy HE, Beà S, Martín-Subero JI, Studd JB, et al. Genetic predisposition to chronic lymphocytic leukemia is mediated by a BMF super-enhancer polymorphism. Cell Rep. 2016;16:2061–7.
Wick W, Petersen I, Schmutzler RK, Wolfarth B, Lenartz D, Bierhoff E, et al. Evidence for a novel tumor suppressor gene on chromosome 15 associated with progression to a metastatic stage in breast cancer. Oncogene. 1996;12:973–8.
Ma D, Luo Q, Song G. Matrix stiffening facilitates stemness of liver cancer stem cells by YAP activation and BMF inhibition. Biomater Adv. 2024;163:213936.
Ma D, Liang R, Luo Q, Song G. Pressure loading regulates the stemness of liver cancer stem cells via YAP/BMF signaling axis. J Cell Physiol. 2025;240:e31451.
Kurppa KJ, Liu Y, To C, Zhang T, Fan M, Vajdi A, et al. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell. 2020;37:104–122.e12.
Yang W, Zhang M, Zhang T-X, Liu J-H, Hao M-W, Yan X, et al. YAP/TAZ mediates resistance to KRAS inhibitors through inhibiting proapoptosis and activating the SLC7A5/mTOR axis. JCI Insight. 2024;9:e178535.
Alvarado-Ortiz E, de la Cruz-López KG, Becerril-Rico J, Sarabia-Sánchez MA, Ortiz-Sánchez E, García-Carrancá A. Mutant p53 gain-of-function: role in cancer development, progression, and therapeutic approaches. Front Cell Dev Biol. 2020;8:607670.
Tan BS, Tiong KH, Choo HL, Chung FF-L, Hii L-W, Tan SH, et al. Mutant p53-R273H mediates cancer cell survival and anoikis resistance through AKT-dependent suppression of BCL2-modifying factor (BMF). Cell Death Dis. 2015;6:e1826.
Packham G. Mutation of BCL-2 family proteins in cancer. Apoptosis. 1998;3:75–82.
Soung YH, Lee JW, Park WS, Nam SW, Lee JY, Yoo NJ, et al. BH3 domain mutation of proapoptotic genes Bad, Bmf and Bcl-G is rare in transitional cell carcinomas of the urinary bladder. Pathology. 2006;38:33–34.
Yoo NJ, Soung YH, Lee SH, Jeong EG, Lee SH. Mutational analysis of the BH3 domains of proapoptotic Bcl-2 family genes Bad, Bmf and Bcl-G in laryngeal squamous cell carcinomas. Tumori. 2007;93:195–7.
Tantawy SI, Timofeeva N, Sarkar A, Gandhi V. Targeting MCL-1 protein to treat cancer: opportunities and challenges. Front Oncol. 2023;13:1226289.
Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J. Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene. 2007;26:970–81.
Hornsveld M, Tenhagen M, van de Ven RA, Smits AMM, van Triest MH, van Amersfoort M, et al. Restraining FOXO3-dependent transcriptional BMF activation underpins tumour growth and metastasis of E-cadherin-negative breast cancer. Cell Death Differ. 2016;23:1483–92.
Wang R, Kang Y, Löhr CV, Fischer KA, Bradford CS, Johnson G, et al. Reciprocal regulation of BMF and BIRC5 (Survivin) linked to Eomes overexpression in colorectal cancer. Cancer Lett. 2016;381:341–8.
Fedele PL, Liao Y, Gong J, Yao Y, Van Delft MF, Low MSY, et al. The transcription factor IRF4 represses proapoptotic BMF and BIM to licence multiple myeloma survival. Leukemia. 2021;35:2114–8.
Fahl SP, Daamen AR, Crittenden RB, Bender TP. c-Myb coordinates survival and the expression of genes that are critical for the Pre-BCR checkpoint. J Immunol. 2018;200:3450–63.
Banerjee D, Boboila S, Okochi S, Angelastro JM, Kadenhe-Chiweshe AV, Lopez G, et al. Activating transcription factor 5 promotes neuroblastoma metastasis by inducing anoikis resistance. Cancer Res Commun. 2023;3:2518–30.
Vaz S, Ferreira FJ, Macedo JC, Leor G, Ben-David U, Bessa J, et al. FOXM1 repression increases mitotic death upon antimitotic chemotherapy through BMF upregulation. Cell Death Dis. 2021;12:542.
Xia H-F, He T-Z, Liu C-M, Cui Y, Song P-P, Jin X-H, et al. MiR-125b expression affects the proliferation and apoptosis of human glioma cells by targeting Bmf. Cell Physiol Biochem. 2009;23:347–58.
Ooi AGL, Sahoo D, Adorno M, Wang Y, Weissman IL, Park CY. MicroRNA-125b expands hematopoietic stem cells and enriches for the lymphoid-balanced and lymphoid-biased subsets. Proc Natl Acad Sci USA. 2010;107:21505–10.
Fan Y-X, Bian X-H, Qian P-D, Chen Z-Z, Wen J, Luo Y-H, et al. MicroRNA-125b inhibits cell proliferation and induces cell apoptosis in esophageal squamous cell carcinoma by targeting BMF. Oncol Rep. 2018;40:61–72.
Kozlova A, Pachera E, Maurer B, Jüngel A, Distler JHW, Kania G, et al. Regulation of fibroblast apoptosis and proliferation by microRNA-125b in systemic sclerosis. Arthritis Rheumatol. 2019;71:2068–80.
Wu Y, Guo Z, Yao K, Miao Y, Liang S, Liu F, et al. The transcriptional foundations of Sp110-mediated macrophage (RAW264.7) resistance to Mycobacterium tuberculosis H37Ra. Sci Rep. 2016;6:22041.
Bu X, Zhao Y, Chang M, Ge X. Downregulation of lncRNA SNHG14 alleviates neurons injury by modulating the miR-181c-5p/BMF axis in ischemic stroke. Brain Res Bull. 2021;174:379–88.
Hu Q-G, Yang Z, Chen J-W, Kazobinka G, Tian L, Li W-C. MiR-183-5p-PNPT1 axis enhances cisplatin-induced apoptosis in bladder cancer cells. Curr Med Sci. 2022;42:785–96.
Liu Y, Shen Z, Wei X, Gu L, Zheng M, Zhang Y, et al. CircSLC39A8 attenuates paclitaxel resistance in ovarian cancer by regulating the miR‑185‑5p/BMF axis. Transl Oncol. 2023;36:101746.
Wang J, Xu B, Tian GG, Sun T, Wu J. Ablation of the MiR-17-92 MicroRNA cluster in germ cells causes subfertility in female mice. Cell Physiol Biochem. 2018;45:491–504.
Wang J-T, Wang Z-H. Role of miR-193a-5p in the proliferation and apoptosis of hepatocellular carcinoma. Eur Rev Med Pharm Sci. 2018;22:7233–9.
Fiori ME, Barbini C, Haas TL, Marroncelli N, Patrizii M, Biffoni M, et al. Antitumor effect of miR-197 targeting in p53 wild-type lung cancer. Cell Death Differ. 2014;21:774–82.
Gramantieri L, Fornari F, Ferracin M, Veronese A, Sabbioni S, Calin GA, et al. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res. 2009;15:5073–81.
He X-X, Guo A-Y, Xu C-R, Chang Y, Xiang G-Y, Gong J, et al. Bioinformatics analysis identifies miR-221 as a core regulator in hepatocellular carcinoma and its silencing suppresses tumor properties. Oncol Rep. 2014;32:1200–10.
Xie X, Huang Y, Chen L, Wang J. miR-221 regulates proliferation and apoptosis of ovarian cancer cells by targeting BMF. Oncol Lett. 2018;16:6697–704.
Zhao X, Wang P, Liu J, Zheng J, Liu Y, Chen J, et al. Gas5 exerts tumor-suppressive functions in human glioma cells by targeting miR-222. Mol Ther. 2015;23:1899–911.
Xia H-F, Jin X-H, Cao Z-F, Hu Y, Ma X. MicroRNA expression and regulation in the uterus during embryo implantation in rat. FEBS J. 2014;281:1872–91.
Sun Y, Lu C-M, Song Z, Xu K-K, Wu S-B, Li Z-J. Expression and regulation of microRNA-29a and microRNA-29c in early diabetic rat cataract formation. Int J Ophthalmol. 2016;9:1719–24.
Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25:125–30.
Sul O-J, Rajasekaran M, Park H-J, Suh J-H, Choi H-S. MicroRNA-29b enhances osteoclast survival by targeting BCL-2-modifying factor after lipopolysaccharide stimulation. Oxid Med Cell Longev. 2019;2019:6018180.
Qin Z, Wang X, Zhou Y, Zheng J, Li H, Li L. Upregulation of miR-29b-3p alleviates coronary microembolization-induced myocardial injury via regulating BMF and GSK-3β. Apoptosis. 2023;28:210–21.
Klatt CL, Theis V, Hahn S, Theiss C, Matschke V. Deregulated miR-29b-3p correlates with tissue-specific activation of intrinsic apoptosis in an animal model of amyotrophic lateral sclerosis. Cells. 2019;8:1077.
Zhang T-J, Xu Z-J, Wen X-M, Gu Y, Ma J-C, Yuan Q, et al. SLIT2 promoter hypermethylation-mediated SLIT2-IT1/miR-218 repression drives leukemogenesis and predicts adverse prognosis in myelodysplastic neoplasm. Leukemia. 2022;36:2488–98.
Catuogno S, Cerchia L, Romano G, Pognonec P, Condorelli G, de Franciscis V. miR-34c may protect lung cancer cells from paclitaxel-induced apoptosis. Oncogene. 2013;32:341–51.
Xiong W, Zhang A, Xiao X, Liu W. CircSETD3 (hsa_circ_0000567) inhibits proliferation and induces apoptosis in cholangiocarcinoma cells via downregulation of microRNA-421 expression. Bioengineered. 2022;13:10191–201.
Feng G, Liu J, Lu Z, Li Y, Deng M, Liu G, et al. miR-450-5p and miR-202-5p synergistically regulate follicle development in black goat. Int J Mol Sci. 2022;24:401.
Jiang S, Luo C, Chen Y, Chen J, Tao S, Zou Q, et al. MicroRNA-640 inhibition enhances the chemosensitivity of human glioblastoma cells to temozolomide by targeting Bcl2 modifying factor. Biochem Genet. 2023;61:538–50.
Lu F, Mo L, Liu A. Circ_0001360 absence alleviates oxygen-glucose deprivation/reoxygenation-induced SK-N-SH cell injury via controlling the miR-671-5p/BMF pathway. Int J Neurosci. 2024;134:492–502.
Jiang D, Sun X, Wang S, Man H. Upregulation of miR-874-3p decreases cerebral ischemia/reperfusion injury by directly targeting BMF and BCL2L13. Biomed Pharmacother. 2019;117:108941.
Xu F, Xia T, Xu Q-T, Zhang X, Huang Y-Z, Sun X, et al. RBMS2 chemosensitizes breast cancer cells to doxorubicin by regulating BMF expression. Int J Biol Sci. 2022;18:1724–36.
Guo X, Xiang C, Zhang Z, Zhang F, Xi T, Zheng L. Displacement of Bax by BMF mediates STARD13 3’UTR-induced breast cancer cells apoptosis in an miRNA-depedent manner. Mol Pharm. 2018;15:63–71.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1.
de Bruijn I, Kundra R, Mastrogiacomo B, Tran TN, Sikina L, Mazor T, et al. Analysis and visualization of longitudinal genomic and clinical data from the AACR project GENIE biopharma collaborative in cBioPortal. Cancer Res. 2023;83:3861–7.
Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:eaan2507.
Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38.
Funding
This study was supported by a grant from the non-commercial organization “The Russian Science Foundation” (23-74-30006). Work in the authors’ laboratories was supported by grants from the Swedish (222013) and Stockholm (181301) Cancer Societies (to BZ). Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
Conceptualization—NVP, GSK, and BZ; original draft preparation, figures and tables—NVP and DKN; review and editing—NVP, GSK, and BZ. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors have approved this publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pervushin, N.V., Nilov, D.K., Zhivotovsky, B. et al. Bcl-2 modifying factor (Bmf): “a mysterious stranger” in the Bcl-2 family proteins. Cell Death Differ 33, 3–14 (2026). https://doi.org/10.1038/s41418-025-01562-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-025-01562-z