Abstract
Diseases of the female reproductive system, especially malignant tumors, pose a serious threat to women’s health worldwide. One of the key factors limiting research progress in this area is the lack of representative models. Organoid technology, especially tumor organoids, has been increasingly applied in the study of female reproductive system tumors due to their high heterogeneity, close resemblance to the physiological state, easy acquisition and cultivation advantages. They play a significant role in understanding the origin and causes of tumors, drug screening, and personalized treatment and more. This article reviews the organoid models for the female reproductive system, focusing on the cancer research advancements. It discusses the methods for constructing tumor organoids of the female reproductive tract and summarizes the limitations of current research. The aim is to offer a reference for future development and application of these organoid models, contributing to the advancement of anti-tumor drugs and treatment strategies for female reproductive tract cancer patients.
Similar content being viewed by others
Facts
-
Stem cell-derived organoids function both as models for disease and as tools for studying tissue regeneration. In contrast to 2D cell lines and expensive, time-consuming animal models, organoids more accurately replicate the three-dimensional structure and microenvironment of tumors, preserve tumor heterogeneity and patient-specific genetic profiles, and are well-suited for high-throughput drug screening and personalized medicine research.
-
Patient-derived organoids (PDOs) often retain key cellular subgroups from the original tumor, preserving its heterogeneity. This characteristic is crucial for disease modeling, as seen in fallopian tube organoids, which provide insights into the progression of ovarian cancer.
-
Organoids are pivotal in high-throughput drug screening and personalized treatment approaches. Ovarian cancer PDOs, for example, have been used to predict patient responses to chemotherapy, such as carboplatin/paclitaxel, and to investigate resistance mechanisms, particularly in HRR-deficient cases.
-
Organoids co-cultured with immune cells, such as tumor-infiltrating lymphocytes (TILs), offer a valuable platform for studying immune responses in cancer. In cervical cancer, organoid co-culture with immune cells has shown potential in identifying therapeutic targets and developing immunotherapy strategies.
-
Emerging technologies, including CRISPR-Cas9 gene editing, organ-on-a-chip systems, and advanced ECM-based scaffolds, are enhancing the precision of organoid models. These innovations facilitate better replication of the tumor microenvironment, supporting research in gynecological cancers such as cervical, endometrial, and ovarian cancer.
Open questions
-
How can integrating more components of the tumor microenvironment, such as immune cells and fibroblasts, improve the replication of real tumor conditions in organoid models of female reproductive tract cancers?
-
What new insights can be gained by co-culturing organoids with microbiota, particularly for understanding endometrial and ovarian cancer development? How can gene-editing technologies like CRISPR-Cas9 further refine organoid models for studying cancer progression and drug resistance?
-
What are the key challenges in improving the efficiency of generating organoids from different stages of female reproductive cancers for high-throughput drug screening and personalized treatment?
Introduction
Despite recent advancements in molecular typing and treatments like targeted therapies and immunotherapy [1, 2], gynecologic cancers affecting organs like the ovaries, uterus, and cervix remain a significant cause of morbidity and mortality in women’s health. This critical challenge underscores the urgent need for more advanced research tools to enhance understanding and improve the treatment of female reproductive tract malignancies.
Currently, commonly used gynecologic tumor models, including in vitro cell cultures and in vivo animal models, each have inherent limitations. Two-dimensional (2D) cell lines are far removed from the biological characteristics of real cells due to their inability to reflect the interactions between different cell subtypes and between cells and the extracellular matrix [3,4,5]. Animal models are limited by long cycles, high costs, and ethical concerns, which preclude their use in high-throughput screening, and there are shortcomings in reproducing the genetic characteristics of patients’ tumors. Organoids, as efficient and stable ex vivo models that closely resemble native tissue in cellular composition, morphology, function, metabolism, gene expression, and response, offer unique advantages in the study of gynecologic tumors. By constructing patient-derived organoids (PDOs), not only can the development of tumors and their influencing factors be studied, but also drug sensitivity tests can be carried out to guide clinical medication and achieve personalized medicine. This review explores the application of organoids in various diseases of the female reproductive system, with a focus on gynecologic tumors. It also highlights the limitations of current research, aiming to provide a reference for the future development and application of female reproductive tract tumor organoids.
Cultivation methods and application scenarios of PDOs
Organoids are generated by culturing stem cells with specific growth factors, extracellular matrix (ECM) components, signaling molecules, and nutrients, allowing them to self-organize into miniature, three-dimensional structures resembling organs [6, 7]. During the formation of the female reproductive system and its tumor organoids, different stem cell sources and media components that favor the formation of various organoids are involved in.
PDOs derived from ASCs or PSCs
Stem cell sources for organoids including pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), as well as tissue-specific adult stem cells (ASCs). The methods by which ASCs and PSCs form organoids are distinct. For ASCs-derived organoids, primary tissues from patients are dissociated into functional units containing adult stem cells, or tumor cells are isolated from tumor tissues. These cells are then enriched and cultured in specific three-dimensional media, forming organoids and tumoroids. In contrast, PSCs-derived organoids involve the directed differentiation of embryonic stem cells from human embryonic tissues or induced pluripotent stem cells from adult tissues. This process generates floating cell aggregates, known as spheroids, which are subsequently transferred to an extracellular matrix in specific media to form organoids.
By adding growth factors to the culture medium, mimic the stem cell niche that supports the homeostasis of ASCs, and promotes the division of stem cells while appropriately inhibiting differentiation, ASCs-derived organoids can be constructed. For cancer research, organoids developed from cancer stem cells from patient tumor tissues fall into this category, which are relatively simpler and capable of including various tumor cell subtypes, presenting a more complex genetic background and capturing the patient’s cancer genome to preserve tumor heterogeneity [8]. Consequently, ASCs-derived organoids are extensively used in studies of tumor heterogeneity and personalized drug evaluation due to their ability to reproduce adult tissues well, and have been preserved in the form of biobanks. Besides the establishment of various tumor organoids, including cervical cancer, ovarian cancer, and endometrial cancer, organoids that represent precancerous lesions, such as endometriosis, endometrial hyperplasia [9], and cervical squamous metaplasia [10, 11], have also been developed from patients’ precancerous lesion tissues.
In contrast, organoids derived from ESCs or iPSCs initially possess a more consistent genetic background. Through stepwise induced differentiation, these organoids develop into structures that include more complex cell types, potentially encompassing mesenchymal cells, epithelial cells, and even endothelial cells [12]. However, this process can also lead to the inclusion of cell types that the tissue itself would not normally possess [13]. ESCs involve the use of early embryos, which raises ethical concerns. In 2006, the Yamanaka team first discovered that the introduction of transcription factors OCT4, SOX2, KLF4, c-MYC (supplemented with factors NANOG and LIN28) can reprogram mature somatic cells in mice, termed induced pluripotent stem cells, which have the potential to differentiate into various somatic cells [14,15,16]. Organoids derived from iPSCs provide a widely applicable model for studying tissue development or disease progression. In the process of generating fallopian tube organoids, iPSCs are directed to differentiate into intermediate mesoderm (IM), the origin of the fallopian tube, by adding CHIR99021, activin A, and BMP4. Further, by modulating Wnt signaling, IM is driven to develop into the Müllerian duct and female reproductive tract rather than the kidneys, and then to promote differentiation into fallopian tube-like precursor cells by using pre-Müllerian growth factor, which eventually develop into fallopian tube organoids with luminal structures over time [17]. In addition to establishing normal fallopian tube organoids using iPSC lines, iPSCs derived from ovarian cancer patients can generate abnormal fallopian tube organoids that carry the same BRCA1 mutations as patients and exhibit cancerous characteristics [18].
ECM and growth factors help the formation of female reproductive system organoids
The formation and stable culture of organoids require the support of two key exogenous factors: the extracellular matrix and the culture medium. By being embedded into an extracellular-matrix-mimicking scaffold, such as the commonly used Matrigel, which is derived from a viscous protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells, stem cells can form complex three-dimensional structures using the matrix as a carrier [19]. In addition to using matrix gel as support, organoids can also be cultured using hydrogel matrices [20, 21], chip cultures [22] and even directly in suspension [23]. Given the dense and difficult-to-digest nature of gynecological tumors, a new dual-layer Matrigel organoid culture method can significantly improve success rates and robustly propagate organoids from different stages and subtypes of gynecological tumors [24].
In addition to a few organoids that only need endogenous signals for formation [25], most organoids require exogenous growth factors throughout [26, 27] or at least in the initial phase [28] of the culture process to help cells to self-organize and gradually divide and differentiate along developmental cues similar to organogenesis. In the culture of female reproductive system organoids, commonly added formulations include: ① growth factors that stimulate cell division and differentiation, such as Wnt pathway activators that maintain stemness—Wnt3a and R-spondin1, Noggin that inhibits bone morphogenetic protein (BMP)-driven differentiation, small molecule inhibitors of activin receptor-like kinase-4,5,7 such as A83-01 and p38 inhibitor SB202190; ② hormones such as 17β-estradiol (E2); ③ cytokines such as EGF, FGFs, HGF that promote cell proliferation and N2 supplement that supports neuron growth; ④ other nutritional supplements such as B27 supplement, N-Acetyl-L-cysteine, niacinamide, insulin-transferrin-selenium and so on. In the initial stages of culture, a ROCK inhibitor (Y27632) is often added to prevent stem cell apoptosis. Table 1 lists representative growth factors added to different gynecologic tumor organoid media cocktails, we selected studies that reported a success rate of ≥50% and/or had at least 10 successful reports.
Depending on the organ and whether it’s healthy or diseased, the growth factors that organoids rely on differ. Continuous growth of most female reproductive system organoids requires Wnt paracrine signaling to maintain stem cell stemness [25]. Moreover, it also needs the members of the leucine-rich repeat-containing G protein-coupled receptor (Lgr) family Lgr4, Lgr5 and Lgr6 [29,30,31], with the R-spondin protein family acting as Lgr receptor agonists [32]. In the culture of fallopian tube organoids, adding Wnt3a and R-spondin1 activates the Wnt signaling pathway for amplification, while Notch signaling maintains cell stemness [33]. Conversely, fallopian tube organoids with triple knockdown of p53, PTEN and RB which are major known tumor drivers to model high-grade serous ovarian cancer (HGSOC) development require a low Wnt environment for long-term growth [34], indicating that the signaling pathways relied upon by normal epithelium and tumor organoids may be different. Compared to healthy fallopian tube organoids that rely on BMP inhibition by Noggin, organoids from HGSOC originating from the fallopian tubes almost always require active BMP signaling [34]. Altering the types and ratios of factors in the culture medium for tumor organoids can also prevent contamination from normal cells [35, 36].
Application scenarios of PDOs
Since organoids are derived from stem cells, the verification of organoid formation can also be used to identify stem cells, thereby assisting in the study of organ development. The endometrium continuously undergoes cycles of proliferation, secretion and shedding during the menstrual cycle. Understanding its regeneration process is of great significance for research in gynecology and regenerative biology. In the endometrium, EPCAM+GFPhighLgr5 cells [37] or long-lived bipotent epithelial progenitors expressing the Wnt reporter gene Axin2 have been shown to grow into complete endometrial organoids [38].
As PDOs can capture patient heterogeneity, they can be applied to disease modeling, etiological and heterogeneity studies among other fields. For example, gene knockout in fallopian tube organoids can effectively characterize the origin and progression of ovarian cancer [39]. Single-cell transcriptomic analysis results once again demonstrate that key cell subgroups of the patient’s tissues can be preserved and amplified during culture, retaining heterogeneity [40].
In terms of drug testing and personalized treatment, female reproductive system tumor organoids help to deepen the understanding of tumor pathogenesis and biological features, thereby discovering new therapeutic targets and strategies. Combining high-throughput drug screening with personalized medicine technology accelerates new drug development. By establishing patient-specific organoid models, the best treatment plan can be tailored for the patient based on individual pathological characteristics and drug responsiveness. Organoids from ovarian cancer patients summarized the response to carboplatin/paclitaxel combination therapy and identified sensitive drug for 88% of patients, proving their value in drug screening [41]. Sensitivity or resistance exhibited by ovarian cancer PDOs with homologous recombination repair (HRR) deficiency to PARPi not only reflected the variability in patient drug responses but also promoted research into potential resistance mechanisms related to replication fork protection and restoration of HRR functions [42]. Moreover, for drugs with complex anti-tumor mechanisms, such as traditional Chinese medicines [43,44,45,46], organoids may offer significant advantages in studying their anti-tumor mechanisms, as they can partially recapitulate the tumor microenvironment.
The role of organoids in female reproductive system organs and tumors
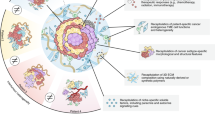
At present, organoids including cervix, endometrium, ovary and fallopian tube and their related cancers have been established and applied to drug high-throughput screening, drug response prediction, disease target screening and so on. Table 2 shows the application details of different organoid models derived from female reproductive tract organs and cancers. Different organoid models related to the female reproductive tract are also showed in Fig. 1.
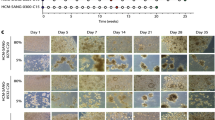
Isolated or reprogrammed stem cells were cultured in an ECM surrounded by a culture medium supplemented with niche factors specific to organoids. These stem cells proliferate in the culture medium and self-organize into functional 3D structures. The brightfield images shows the morphology of cervical adenocarcinoma organoids on day 0, day 3 and day 6. Cervical adenocarcinoma organoids show a more dense structure and marked vacuolation than normal cystic endocervical organoids. The organoid structure of cervical squamous cell carcinoma is less defined, with stratified loss and poor cell polarity. Endometrial carcinoma organoids usually appear as glandular-like morphology with a well-defined to moderately defined lumen, but with higher cancer stages the structure appears dense without lumen. Ovarian cancer organoids show extensive morphological differences among different histological subgroups. Most ovarian cancer organoids have a dense structure and contain multiple lumens. The structures of these organoids were mapped based on HE staining features.
Cervix and cervical cancer
The cervix is a narrow channel that connects the uterus and the vagina, performing functions such as secreting mucus and protecting the upper reproductive tract. It is primarily composed of the endocervix with its secretory columnar epithelium and the ectocervix with its multilayered squamous epithelial cells. The transformation zone between these two, where the squamous and columnar epithelia meet, is the most common area for cervical cancer to arise [47]. The epithelial structures of the cervix and the organoids derived from different epithelial origin using normal or tumor tissue are shown in Fig. 2. As a gateway to the upper reproductive tract, the cervix encounters a multitude of pathogens. Although the cervix can clear most pathogenic microorganisms and prevent ascending infections, some viruses and bacteria can evade innate immunity, leading to infections and lesions such as cervicitis, polyps, warts and cervical cancer. Cervical cancer is primarily caused by high-risk types of HPV and Chlamydia trachomatis infections. Despite the containment of cervical cancer incidence rates with the advancement of HPV testing and vaccination, GLOBOCAN2020 statistics indicated that it remained the fourth highest cause of cancer incidence and mortality among women worldwide [48].
The cervix is composed of three parts from the position near the uterus downwards: the endocervix consisting of a single layer of columnar epithelium, the transformation zone where the columnar and squamous epithelia meet, and the ectocervix with multiple layers of squamous cells. Cervical cancer mainly includes cervical adenocarcinoma (25%) or squamous cell carcinoma (70%), while the rest are rare types such as adenosquamous carcinoma. Adenocarcinoma is characterized by mutations in the PIK3CA, ERBB2, FBXW7 and FAT1 genes. The mutations in TP53, RB1, PIK3CA and PTEN genes are common in squamous cell carcinoma. Organoids derived from the squamocolumnar junction usually appear round in shape, with dense and cystic characteristics, but grow irregularly after several passes, with multiple budding or chain-like structures.
Originally, patient-derived cervical tissue organoids were cultivated from cervical keratinocytes isolated from biopsied tissue using skin 3D organotypic rafts, but the success rate was low [49]. In 2021, the team of Hans Clevers successfully established organoids from clinically sourced healthy ecto- and endocervical epithelia. Furthermore, they constructed squamous and adenocarcinoma organoids of the cervix using small samples from pap tests and investigated the effects of HPV virus on cervical carcinogenesis. They demonstrated that cervical organoids could be cultured long-term and consistently represented the genetic mutation characteristics and tumor marker features of clinical tissues [50].
The cervical transformation zone containing the squamocolumnar junction (SCJ) is the prevalent site for HPV infection and cervical cancer [51]. The reason for the predisposition of transformation zone to malignant transformation remains unclear, but some potential hypotheses include localized immune suppression [52, 53], accelerated cell proliferation and unstable differentiation [54], increased estrogen and progesterone receptors [55], etc. Considering the differentiation observed in organotypic culture, HPV16-immortalized cells from the transformation zone or endocervix are more dysplastic, express higher levels of Ki-67 and p-AKT, and more frequently invade the collagen rafts composed of cervical stromal cells while expressing MMP-1—potentially facilitating tumor development [56]. Organoids from the cervical SCJ [11] and may offer new insights into the cytological characteristics of the cervical transformation zone and the development of cervical cancer.
Human and murine cervix organoids can be used to simulate HPV infection or Chlamydia trachomatis infection by transducing HPV oncogenes to cervical stem cells [57]. Studies on patient-derived ectocervical organoids simulating solitary and concurrent infections of HPV and Chlamydia trachomatis showed that HPV interfered with the growth of Chlamydia trachomatis leading to persistent infection. Chlamydia trachomatis infection disrupted some of the cellular protective mechanisms induced during HPV infection, including mismatch repair, increasing the likelihood of mutations and more severe cytopathic changes, thus raising the cancer development risk [58]. Besides HPV oncogenes E6 and E7, introduction of c-MYC and HRAS genes can collaboratively induce cervical tumor models [59]. Stimulating the expression of putative differentiation factors and/or reducing the SMAD4 gene can simulate the carcinogenic process of cervical adenocarcinoma in organoids. FOXA2 was found to potentially play a significant role in regulating the histopathology and formation of cervical cancer organoids [60]. High-grade squamous intraepithelial lesions (HSIL) caused by persistent high-risk HPV infection are considered precancerous conditions of the cervix. If left untreated, HSIL can progress to cervical cancer [61, 62]. Organoids derived from patients’ HSIL can accurately represent the histological and genomic characteristics of the patient’s precancerous lesions, including HPV viral transcripts. Additionally, these organoids exhibit relative high sensitivity to standard chemotherapeutic drugs [63].
Cervical cancer PDOs could be used for drug screening and radiosensitivity testing, and serve as a platform for personalized medicine. Subjecting organoids from four different cervical cancer subtypes to screening with over 170 drugs revealed differential sensitivity in the organoids to seven of these treatments. [64]. For cervical squamous cell carcinoma, PDOs can demonstrate patient-specific drug responses to four standard chemotherapeutic compounds, including cisplatin, carboplatin, gemcitabine, and olaparib. For instance, PDOs exhibiting the highest resistance to platinum-based drugs correspondingly show consistent low sensitivity treatment outcomes in their originating patients [63].
Investigating the infiltration and cytotoxicity of immune cells towards cervical cancer cells in three-dimensional in vitro models has seen preliminary progress with spheroid models [65,66,67]. Recently, the development of more valuable cervical cancer organoid-immune cell co-culture models has gained attention. When co-cultured with γδ T cells, cervical cancer PDOs and HPV E6E7-transformed cervical organoids exhibited greater sensitivity to cytotoxicity compared to healthy cervical organoids. This co-culture system can be combined with RNA-seq to further explore potential immune effector pathways [68], suggesting that organoid-immune cell co-culture systems hold promise for research in immunotherapy and immune mechanisms. A recent study supports this outlook: by co-culturing PDOs with peripheral blood immune cells (PBMCs) activated by HPV associated antigenic peptides from healthy donors, immune cell-mediated structural disruption and cytotoxicity towards PDOs were observed. Moreover, PDOs identified different antigenic peptides and reacted accordingly [63]. When the immune cells were selected as tumor-infiltrating lymphocytes (TILs) matched to the patient, the PDO-TIL co-culture system showed distinct TIL-mediated tumor killing and immune responses. This co-culture model could potentially serve as a platform for simulating personalized responses to immunotherapies, such as adoptive T cell therapy [69].
Moreover, organoids of specific cervical pathological types have been successfully constructed. An organoid model of cervical clear cell carcinoma (cCCC) was successfully developed from a patient biopsy sample and xenograft models can be established from these organoids at early culture stages. This organoid closely mirrored the patient tumor in terms of histology, pathology, and genomic characteristics [70]. Organoids of small cell neuroendocrine carcinoma [71] and small cell carcinoma of the uterine cervix of clinical origin [72] have been used to evaluate sensitivity to clinical chemotherapy drugs. When combined with whole exome sequencing and RNA-seq analyses, they can assist in identifying treatment targets for specific tumor types.
Endometrium and endometrial cancer
The endometrium is the mucosal lining of the uterus, playing a crucial role in maintaining normal female reproductive functions. The endometrium can be divided into the superficial functionalis and the deeper basalis [73]. The superficial layer is influenced by estrogen and progesterone, undergoing cyclical changes and periodic shedding during the menstrual cycle. Endometrial epithelial stem cells in the basalis drive the regeneration of the endometrium [74,75,76]. Dysfunction of the endometrium can lead to several common gynecological diseases, including abnormal uterine bleeding, infertility, miscarriage, pre-eclampsia, endometriosis and endometrial cancer.
Tissue from endometrial biopsies, shed menstrual endometrium and even cryopreserved tissue can be used for organoid culture [77]. In addition to histological and genetic consistency, organoids summarize the functions of endometrial tissue by responding to the administration of sex hormones, prolactin, and placental hormones and by having secretory functions. It can be said that hormonal interventions can aid organoids in recapitulating the menstrual cycle at morphological and molecular levels [78]. Endometrial organoids have demonstrated clonogenic capacity and can differentiate towards secretory glandular and ciliated phenotypes [79]. Single-cell and spatial transcriptomics have been used to compare the cell composition and gene expression of endometrial organoids with in vivo endometrium, identifying Wnt and Notch signaling as key factors regulating secretory and ciliated cell differentiation [25]. Within the organoids, Wnt-related genes FOXJ1 and LGR5 are highly expressed in luminal parts, while transcription factors induced by Wnt inhibition and Notch activation are highly expressed in glandular regions [25]. The varied organoid phenotypes, either cystic or compact, can be induced by different intensities of Wnt signaling [78]. Experiments indicated that estrogen induction and Notch signaling elicited differentiation of ciliated cells in endometrial organoids [80]. Intervention with Wnt and Notch signals in organoids confirmed that ciliated differentiation strongly depended on Wnt signals, while conditions with Wnt inhibition and Notch activation facilitated secretory differentiation, in which estrogen and progesterone also play crucial regulatory roles [25].
The regeneration of the endometrium after its cyclic shedding has long been a focus of stem cell and gynecological research. The Axin2 gene was identified in organoid models as a marker of long-lived bipotent epithelial progenitor cells in the endometrial glands. Eliminating Axin2 cells severely impacted endometrial homeostasis and regenerative capacity and might even lead to tumorigenesis [38].
Endometrial cancer is a malignant tumor whose incidence and mortality rates are rising annually [81], causing significant harm to patients’ reproductive and overall health. Patient-derived endometrial organoids have been established from conditions including endometriosis, endometrial cancer, endometrial hyperplasia, and Lynch syndrome [9]. Organoids from endometrial cancer are typically derived from tissue obtained from hysterectomy patients, suspended in expansion medium after enzymatic digestion [79, 82]. After verifying histology, mutation spectrum and tumor heterogeneity, endometrial cancer organoids consistently maintained characteristics of the originating tumors [24] and showed differential responses to various anti-tumor compounds and hormone inhibitors [82]. Combining organoids with proteomics can provide new insights into the heterogeneity of endometrial cancer [83]. Like other patient-derived tumor organoids, endometrial cancer organoids can also be used for testing potential treatment methods and for screening patient drug sensitivities [24, 82, 84, 85]. A study involving 43 endometrial cancer and ovarian cancer PDOs has demonstrated that this drug sensitivity screening can predict patient resistance to some extent [86]. Additionally, endometrial cancer organoids can be utilized to identify new therapeutic targets for endometrial cancer [87], study mechanisms of platinum-based drug resistance [88], and investigate sensitivity to death receptor ligand TRAIL therapy [89]. Single-cell sequencing of endometrial organoids and corresponding tumors revealed that ciliated cell markers (DYDC2, CTH, FOXJ1, and p73) and secretory cell markers MPST are expressed in endometrial tumors and positively correlated with disease-specific survival and overall survival rates in patients with endometrial cancer [90]. FOXA2 was also identified as a significant tumor suppressor in endometrial cancer through validation in organoid-based models, with a synergistic interaction with the PI3K signaling pathway [91]. Considering the potential of inhibition of the PI3K pathway in anti-inflammatory and anti-tumor, it would be useful to develop its inhibitors for endometrial cancer treatment [92, 93].
While various stages and grades of endometrial cancer organoids have been established, the efficiency of creating organoids from malignant tumors remains low [78]. Co-culturing cancer-associated fibroblasts isolated from endometrial cancer with endometrial cancer organoids can improve the slow growth and limited proliferation of these organoids [94]. Katcher et al. managed to establish organoids representing all histological subtypes of endometrial cancer that could be cultured long-term after refining the cultivation methods [95]. In addition to endometrial cancer, exposing a novel multicellular, scaffold-free endometrial organoid to high levels of androgens simulates the impact of elevated androgen levels on the endometrium as seen in polycystic ovary syndrome [96]. Endometriosis can also be modeled in vitro through the generation of spheroids [97]. Recently, decellularized hydrogels as novel organoid scaffolds offer new possibilities for the application of endometrial and tumoral organoids. Organoids grown in endometrial hydrogels are more similar in proteomics to native tissue compared to those cultured in Matrigel [98].
Fallopian tube, ovary and ovarian cancer
The ovary is a female gonadal organ that produces ova and sex hormones. Ovarian cancer is a highly heterogeneous group of tumors with a lack of early symptoms and signs, often leading to its diagnosis at late stages [99]. It exhibits high drug resistance and recurrence rates, and carries a high mortality rate [100]. In 2019, the group led by Hans Clevers [39] established 56 ovarian cancer organoid lines from 32 patients, representing all subtypes of ovarian cancer, maintaining their heterogeneity. After drug screening and xenografting, it has been proved that these organoids can be used to test drug sensitivity in vitro and in vivo, and response to chemotherapy and drug resistance across different tumor subtypes. This extensive and long-term ovarian cancer organoid platform construction provided guidance for subsequent in vitro and in vivo expansion and analysis of ovarian cancer organoids. Additionally, 23 whole-genome characterized PDOs from 36 ovarian cancer patients preserved the genomic features of the original tumors and provided an overview of patient response to neoadjuvant carboplatin/paclitaxel combination therapy [41]. Compared to single-layer cells, organoids display more diverse drug responses and can reveal the correlation between drug sensitivity and DNA repair defects. Clinically determined treatment choices made based on drug sensitivity testing from organoids have brought significant clinical turning points for patients [101]. Furthermore, constructing a normal ovarian organoid alongside the cancerous organoid from the same patient allows for testing the non-specific cytotoxicity of drugs on normal cells at the tumor-killing dosages, providing more compelling evidence for evaluating drug safety [86]. As for drug resistance mechanism research, organoids have significant advantages over cell and animal models because they are closer to the in vivo environment, simulate tumor heterogeneity, and provide more realistic drug penetration and metabolism models.
HGSOC is one of the deadliest types of ovarian epithelial cancers whose origin is still uncertain. Currently, it is mainly believed that HGSOC originates from ovarian surface epithelium (OSE) and fallopian tube epithelium (FTE) [102,103,104,105]. OSE and FTE organoids from genetically engineered mice demonstrated that both can cause HGSOC with different latency and metastasis properties, possibly representing two different subtypes, and exhibiting varying chemotherapeutic drug sensitivity [106]. Human FTE organoids provide a valuable model for studying the origins and pathogenesis of HGSOC. A large number of stem cells have been found in the FTE cells, which can effectively form spheroids and organoids in a Wnt environment [107, 108], and organoids can respond to progesterone and estradiol [33]. The fallopian tube is a conduit for oocyte, it is a muscular tube lined by simple columnar epithelium containing secretory and ciliated cells, which produce tubular fluid and facilitate transport of gametes, respectively [109, 110]. Different Wnt and Notch signals can stimulate cilia and secretory cell differentiation in fallopian tube organoids [33], which is significant for understanding fallopian tube lesions and carcinogenesis. The secretory cells of the fallopian tube epithelium are the origin cells of serous tubal intraepithelial carcinoma (STIC), which is considered a precursor to HGSOC [111, 112]. In recent years, abnormal proliferation of secretory cells, often referred to as early serous proliferations (ESPs), has been suspected to be a direct precursor to HGSOC [113, 114], and this process is often associated with TP53 mutations [115]. HR deficiency caused by BRCA1/2 mutations is considered another important risk factor for the occurrence of HGSOC [116]. Fallopian tube organoids derived from iPSCs of ovarian cancer patients carrying BRCA1 mutations showed cell abnormalities consistent with tumor development [18]. Similarly derived from iPSCs, FTE organoids from healthy donors are established through differentiation steps, such as Mullerian duct and fallopian tube epithelial precursors. These organoids encompass cell types representing the mature differentiation of the FTE lineage and can self-organize into lumen-forming structures. These iPSC-derived FTE organoids provide a powerful in vitro model for studying FTE cell transformation and the early development of HGSOC. Furthermore, using Chlamydia trachomatis to infect fallopian tube organoids enhanced cell stemness and accelerated DNA aging. This suggests that chronic infection of FTE by Chlamydia trachomatis may be a potential factor in fallopian tube lesions and the development of HGSOC [117].
Since mouse-source tumor tissue is easily obtainable and shows similarity to the human HGSOC model in genetic dependence surrounding the tumor microenvironment and drug response [118], it has applied advantages. Constructing HGSOC of homologous recombination (HR)-proficient (Trp53−/−; Ccne1OE; Akt2OE; KrasOE), HR-deficient (Trp53−/−; Brca1−/−; MycOE), and unclassified (Trp53−/−; Pten−/−; Nf1−/−) organoids from mice with genetic engineering source provided fast evaluation platforms for developing effective treatment methods, resolving the difficulty in treating the CCNE1 subtype that is highly drug-resistant. These models reveal genotype-specific immune microenvironments and chemotherapeutic sensitivities, demonstrating durable T-cell dependent responses in HR-proficient genotypes and none in others, underscoring the significance of immune context in therapeutic development [118].
Organoids established from clinical tumor samples better represent tumor heterogeneity. After bridging species differences, they could be used more accurately for drug screening, including targeted drugs and immune therapy. Based on a fast-growing and high success rate HGSOC organoid platform, it proved that organoids’ sensitivity to PARPi was only related to HR dysfunction but not DNA repair gene mutation status, while the sensitivity to paraplatin, CHK1, and ATR inhibitors related to replication fork protection deficiency [119]. Since the launch of PARPi for the treatment of ovarian cancer, it has revolutionized the treatment of the disease by targeting HR deficiency, which accounts for approximately 41–50% of ovarian cancer [120, 121]. HR deficiency caused by BRCA1/2 mutations is considered another important risk factor for the occurrence of HGSOC. Similar to other genetic factors, HR deficiency is associated with cancer susceptibility and prognosis [122, 123]. In drug testing facing 23 ovarian cancer PDOs, all PDOs showed resistance to olaparib, rucaparib, and niraparib, consistent with HR deficiency classification based on whole-genome sequencing data [41]. The PARPi exhibit synthetic lethality to selectively kill tumor cells with HR deficiency, with BRCA1/2 mutations being the most significant pathogenic mutations in HRR related-genes [122, 124]. This was confirmed in organoids carried BRCA1 mutations, which exhibited a higher sensitivity to the PARPi olaparib and platinum-based drugs [125]. However, exceptions exist where resistance to PARPi occurs despite BRCA2 deletions [126]. Combining with patients’ clinical drug responses, HGSOC PDOs derived from debulking surgeries (two neoadjuvant-carboplatin-exposed and four chemo-naïve) have been demonstrated to be predictive of carboplatin resistance [127]. This platinum drug resistance may be associated with RAD51 expression levels, which has been observed and validated in both HGSOC organoids and patient cohorts. A low RAD51 score predicts sensitivity to platinum-based drugs, as well as better progression-free survival and overall survival [128]. Moreover, by testing the effects of inhibitors and analyzing transcriptomic changes, patient-derived HGSOC organoids can also be used to identify potential therapeutic vulnerabilities [129].
The interaction between the tumor immune microenvironment and the tumor itself is often difficult for organoids to replicate, yet it is crucial. Tumor-derived UBR5 has been shown to promote the recruitment and activation of tumor-associated macrophages, leading to ovarian cancer progression. Additionally, UBR5 promotes organoid formation by controlling p53 protein levels through β-catenin-mediated signaling [130]. By incorporating immune cells into the organoid culture system, the co-culture model of ovarian cancer organoids and immune cells has provided valuable insights for developing ovarian cancer immunotherapy and identifying new immune therapeutic targets. Testing the effects and cellular states following bispecific anti-PD-1/PD-L1 antibody treatment revealed that enhanced immunotherapy efficacy depends on the activation and cytotoxic activity of NK and CD8 T cell subsets. These cellular state changes are partially regulated by downregulation of the bromodomain-containing protein BRD1 [131], which contributes to understanding the epigenetic regulation of resistance to PD-1/PD-L1 blockade cancer immunotherapy [132]. Furthermore, 3D organotypic models of ovarian cancer containing primary human fibroblasts and mesothelial cells can be used for high-throughput screening of a wide range of antitumor drugs [133]. In the future, organoid co-culture models that include a greater variety of cell types and more closely mimic the complex tumor microenvironment will become more powerful preclinical models.
Building HGSOC organoids with higher success rates, including organoid establishment from cryopreserved tissues [134], and developing more high-throughput drug screening platforms, are some areas of exploration to expand the application of ovarian cancer organoids. Phan et al. developed a platform for the high-throughput identification of drug sensitivity for HGSOC and ovarian sarcoma, demonstrating the feasibility of using 240 kinase inhibitors for testing and achieving an excellent fit with clinical decision times in just 1 week [135].
Vaginal cancer and vulva cancer
The vaginal epithelium can be divided into three layers based on the degree of cell differentiation: the basal layer, composed of basal cells; the intermediate layer, consisting of multiple layers of polygonal or prickle cells; and the superficial layer, made up of keratinized or nearly keratinized squamous epithelial cells [136]. The basal cells, which have a high proliferative capacity, are the proliferating region of the vaginal epithelial cells and thus have a strong ability to form organoids [137]. Notably, Axin2-labeled basal cells can self-renew without relying on hormones [137]. Similar to other female reproductive system organoids, the proliferation and differentiation of vaginal organoids are also regulated by the key Wnt signaling pathway. Even after ovarian removal, Axin2-expressing vaginal epithelial cells can respond to Wnt signaling and significantly promote vaginal epithelial regeneration following estradiol administration [137]. In addition to organoids, the human vaginal mucosa organ chip (vagina-on-a-chip) provides a valuable preclinical model for understanding interactions between the vaginal microbiome and host tissues [138]. Another commercially available in vitro 3D tissue model, MatTek EpiVaginal, can be used for studying vaginal drug delivery, pharmacokinetics, and other applications [139, 140]. Vaginal cancer and vulvar cancer are relatively rare types of tumors, accounting for about 4% of gynecological tumors [4]. Similar to cervical cancer, the main cause of vaginal and vulvar cancer is also high-risk HPV infection. However, there is currently a lack of established vaginal and vulvar cancer organoids, in the future, the use of organoid technology will better bridge the gap in preclinical research on vaginal and vulvar cancer.
Challenges and prospects of organoids in female reproductive tract tumors
Despite the increasing maturity and widespread application of organoid technology, there are still limitations as a novel model. Tumor organoids, which largely arise from epithelial cells, often lack the blood vessels, nerves, immune cells, and fibroblasts needed to reproduce the complex tumor microenvironment. This limits their ability to model cell-cell interactions, cell-ECM interactions, and cell-medium interactions. Although significant progress has been made in co-culturing gynecological tumor organoids with immune cells, the establishment of more complex co-culture models that include other cell types is still quite limited in gynecological tumor organoid research. For example, co-culture of tumor organoids with endothelial cells can mimic angiocrine crosstalk in real tumors and can be a critical model for understanding interactions between angiogenesis and the immune environment [141]. At the same time, by integrating multiple omics data (such as genomics, transcriptomics, proteomics and metabolomics, etc.), a more comprehensive and precise understanding of the composition and changes of tumor microenvironment can be obtained.
Furthermore, co-culturing organoids with microbiota is a powerful model for studying their interactions in female reproductive tract cancer, especially in relation to endometrial and ovarian cancers [142, 143]. Currently, co-culture of organoids with gut microbiota has seen many explorations, including dispersed single-cell co-culture with bacteria [144], microinjection co-culture [145] and anaerobic bacterial co-culture [146]. If more complex organoid models including cell-microbial interactions can be constructed in the field of female reproductive tract cancer, it will provide a great boost to the accurate modeling of female reproductive tract cancer.
Gene editing techniques such as CRISPR-Cas9 have shown great potential in life science research [147, 148]. CRISPR-Cas9 gene editing has been used in various organoid models to study organ development, gene function and disease modeling [149,150,151,152,153]. Selectively activating or inhibiting gene expression, epigenetic regulation at specific sites, or whole-genome screening through gene editing techniques can help reveal key genes and pathways involved in tumor development and verify their function. For example, CRISPR-Cas9 introduction of Trp53, Brca1, Nf1, and Pten mutations in OSE and FTE organoids were used to confirm the origin of HGSOC [154], and TP53 and RB1 knockout was used to study their impact in patient-derived ovarian cancer organoids [39]. A specialized study investigating the application of base editors in human adult stem cell-derived cancer organoids has been conducted. Using a C > T base editor-mediated CRISPR-stop, targeted stop codons were introduced in endometrial cancer-relevant genes. By combining different cytosine base editors, endometrial tumor organoids for the study of early tumorigenesis can be generated. Additionally, simultaneously introducing oncogene activation and tumor suppressor inactivation mutations can further enhance the development of tumor models [155]. In addition to CRISPR-Cas9, a novel gene labeling method called In-trans paired nicking has been developed that prompts fluorescent gene labeling of organoids without the need for double-stranded breakage [156]. Nanoblade may also be a new organoid-appropriate gene-editing technology to replace CRISPR-Cas9 engineering [157].
In addition, ECM and growth factors added during organoid culture may affect drug screening [158], and established culture conditions may be optimal for specific cancer cell subtypes. The commonly used ECM from EHS mouse sarcoma lacks identification of its components and has potential immunogenicity, which obstructs more precise disease modeling. Currently, decellularized extracellular matrix (dECM) [159, 160] and polyethylene glycol hydrogel [20] are being developed as new organoid culture matrices. Human or pig ECM-derived hydrogels have been used to support human-derived organoid culture [159, 161]. Compared to Matrigel, dECM provides the accurate microenvironment characteristics suitable for different tissue types with its special physical properties, allowing it to be used in minimally invasive delivery[162]. Polyethylene glycol hydrogel, as a synthetic matrix with clear physical and chemical parameters, has been used for the expansion of intestinal stem cells and the culture of intestinal organoids [163]. Replacing them with soft fibrin matrices also supports the culture of intestinal epithelial organoids [164]. In endometrial organoids, this fully defined synthetic polyethylene glycol hydrogel matrix can be used to study epithelial-matrix crosstalk in the face of endometrial inflammation [165]. Overall, the development of increasingly specific and clear composition new matrices will provide new support for the further applications of organoids.
The organ-on-a-chip technology, based on a microfluidic platform, enables more complex and dynamic simulations of tissues and diseases using small-scale cultures. Recently, organ-on-a-chip models have been applied to the female reproductive system and even gynecological tumors (cancer-on-a-chip), encompassing nearly all major female reproductive organs as well as gynecological cancers, including cervical cancer, endometrial cancer, and ovarian cancer [166]. By culturing primary human vaginal epithelial cells on the upper surface and primary human uterine fibroblasts on the lower surface of an extracellular matrix-coated porous membrane within a microfluidic system, a human vagina chip can be constructed. Further co-culturing this model with microorganisms allows for the identification of interactions between different vaginal microbial consortia and host tissues [138]. In recent years, the advent of microfluidic technology has offered valuable models for enhancing the management of gynecological tumors. For instance, microfluidic chips can regulate oxygen gradients and nutrients in ovarian cancer, effectively simulating cell growth, migration, invasion, apoptosis, and drug response [167, 168]. These chips can be used in ovarian cancer diagnostics to screen for new biomarkers [169, 170] or to detect tumors using exosome detection techniques that utilize nanomaterials [171, 172], as well as in therapy development [173]. For cervical cancer, microfluidic platform-based detection methods [174, 175] and drug research [176] have also been developed. These cancer-on-a-chip models, due to their precise control of fluid dynamics and chemical gradients, offer promising approaches for early cancer diagnosis and have potential for evaluating therapeutic strategies. Multi-organ chips integrate multiple different organoid types on one chip, changing the current isolated use of a single tissue type in mainstream organoid research and enabling drug compound screening [177] and evaluation of prodrug metabolism and downstream toxicity of drugs [178]. The use of the polysaccharide-based synthetic hydrogel VitroGel-ORGANOID-3 to integrate dendritic cells-gastric organoids to the gut organoid flow chip (GOFlowChip) enables faster and more accurate study of immune cell-organoid interactions [179]. In the future, combining organoids of female reproductive system tumors with microfluidic platforms may enable more refined and controllable in vitro tumor simulations.
Moreover, improving organoid generation efficiency, reducing costs, and developing methods suitable for high-throughput drug and immune therapy screening can shorten the preclinical screening cycle and dock with the treatment window. Currently, organoid-based high-throughput screening platforms have been developed, including Z-stack imaging and fluorescent labeling to evaluate survival rate [180], miniaturization of organoids in high-density wells to suit high-throughput screening [181], and even fully automated liquid handling robots for high-throughput screening [182]. By applying microfluidics and biomaterials to organoid culture and utilizing MicroFlu-IDIC technology for precise control, dynamic physical conditions can be provided to uniformly cultivate a large number of organoids while maintaining a complex microenvironment [183]. Combined with biotechnology techniques such as tissue engineering and 3D printing, it is possible to construct more complex and realistic organoid structures in a stable and rapid manner, further improving the biological similarity and reliability of organoid models. The future development of female reproductive tract organoids is shown in Fig. 3.
In terms of culture methods, the large-scale controllable production of organoids and new culture methods can be achieved by combining bioengineering, customized synthetic hydrogels and microfluidic organoid chips. Various cells and microbes may be co-cultured in vitro to simulate the body microenvironment. At the same time, the application of organoids will be expanded by combination with multi-omics, new gene editing methods and other technologies, and it will be used in high throughput drug screening.
Conclusion
The female reproductive system is crucial to women’s health and reproductive function, and its diseases and even cancer pose challenges for many women worldwide. The lack of representative models hinders research on gynecologic tumors. Organoid technology, especially tumor organ models, has been increasingly applied in the study of female reproductive system tumors due to their advantages of high heterogeneity, easy accessibility and cultivation. They play a significant role in understanding the origin and causes of tumors, drug screening and personalized treatment. In the future, the combination of female reproductive system tumor organ models and various new technologies will help further understand the occurrence of female reproductive system tumors, develop new treatment methods, and open up new possibilities for personalized and precision medicine. This will contribute to improving treatment outcomes and survival rates for female reproductive system tumor patients, providing better protection for women’s health and reproductive function.
Data availability
The materials in this study are available to provide by corresponding authors on reasonable request.
References
Morand S, Devanaboyina M, Staats H, Stanbery L, Nemunaitis J. Ovarian cancer immunotherapy and personalized medicine. Int J Mol Sci. 2021;22:6532
Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69:258–79.
Huang Y, Liu Y, Zheng C, Shen C. Investigation of cross-contamination and misidentification of 278 widely used tumor cell lines. PLoS ONE. 2017;12:e0170384.
Lõhmussaar K, Boretto M, Clevers H. Human-derived model systems in gynecological cancer research. Trends Cancer. 2020;6:1031–43.
Tang C, Liu C, Maffei B, Niragire B, Cohen H, Kane A, et al. Primary ectocervical epithelial cells display lower permissivity to Chlamydia trachomatis than HeLa cells and a globally higher pro-inflammatory profile. Sci Rep. 2021;11:5848.
Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–97.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125.
Yan HHN, Chan AS, Lai FP, Leung SY. Organoid cultures for cancer modeling. Cell Stem Cell. 2023;30:917–37.
Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21:1041–51.
Chumduri C, Gurumurthy RK, Berger H, Dietrich O, Kumar N, Koster S, et al. Opposing Wnt signals regulate cervical squamocolumnar homeostasis and emergence of metaplasia. Nat Cell Biol. 2021;23:184–97.
Maru Y, Kawata A, Taguchi A, Ishii Y, Baba S, Mori M. et al. Establishment and molecular phenotyping of organoids from the squamocolumnar junction region of the uterine cervix. Cancers. 2020;12:694.
Azar J, Bahmad HF, Daher D, Moubarak MM, Hadadeh O, Monzer A. et al. The use of stem cell-derived organoids in disease modeling: an update. Int J Mol Sci. 2021;22:7667.
Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 2018;23:869–81.e8.
Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–84.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20.
Yucer N, Holzapfel M, Jenkins Vogel T, Lenaeus L, Ornelas L, Laury A, et al. Directed differentiation of human induced pluripotent stem cells into fallopian tube epithelium. Sci Rep. 2017;7:10741.
Yucer N, Ahdoot R, Workman MJ, Laperle AH, Recouvreux MS, Kurowski K, et al. Human iPSC-derived fallopian tube organoids with BRCA1 mutation recapitulate early-stage carcinogenesis. Cell Rep. 2021;37:110146.
Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145:204–20.
Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–4.
Lindborg BA, Brekke JH, Vegoe AL, Ulrich CB, Haider KT, Subramaniam S, et al. Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl Med. 2016;5:970–9.
Hu Y, Sui X, Song F, Li Y, Li K, Chen Z, et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat Commun. 2021;12:2581.
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–6.
Maru Y, Tanaka N, Itami M, Hippo Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol Oncol. 2019;154:189–98.
Garcia-Alonso L, Handfield LF, Roberts K, Nikolakopoulou K, Fernando RC, Gardner L, et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet. 2021;53:1698–711.
McCracken KW, Aihara E, Martin B, Crawford CM, Broda T, Treguier J, et al. Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182–7.
McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–4.
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118–26.
Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–96.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7.
Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–9.
de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–7.
Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6:8989.
Hoffmann K, Berger H, Kulbe H, Thillainadarasan S, Mollenkopf HJ, Zemojtel T, et al. Stable expansion of high-grade serous ovarian cancer organoids requires a low-Wnt environment. EMBO J. 2020;39:e104013.
Guo L, Li C, Gong W. Toward reproducible tumor organoid culture: focusing on primary liver cancer. Front Immunol. 2024;15:1290504.
Parseh B, Khosravi A, Fazel A, Ai J, Ebrahimi-Barough S, Verdi J, et al. 3-Dimensional model to study apoptosis induction of activated natural killer cells conditioned medium using patient-derived colorectal cancer organoids. Front Cell Dev Biol. 2022;10:895284.
Seishima R, Leung C, Yada S, Murad KBA, Tan LT, Hajamohideen A, et al. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat Commun. 2019;10:5378.
Syed SM, Kumar M, Ghosh A, Tomasetig F, Ali A, Whan RM, et al. Endometrial Axin2(+) cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell. 2020;26:64–80.e13.
Kopper O, de Witte CJ, Lõhmussaar K, Valle-Inclan JE, Hami N, Kester L, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25:838–49.
Velletri T, Villa CE, Cilli D, Barzaghi B, Lo Riso P, Lupia M, et al. Single cell-derived spheroids capture the self-renewing subpopulations of metastatic ovarian cancer. Cell Death Differ. 2022;29:614–26.
de Witte CJ, Espejo Valle-Inclan J, Hami N, Lõhmussaar K, Kopper O, Vreuls CPH, et al. Patient-derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Rep. 2020;31:107762.
Tao M, Sun F, Wang J, Wang Y, Zhu H, Chen M, et al. Developing patient-derived organoids to predict PARP inhibitor response and explore resistance overcoming strategies in ovarian cancer. Pharm Res. 2022;179:106232.
Kong F, Wang C, Zhao L, Liao D, Wang X, Sun B, et al. Traditional Chinese medicines for non-small cell lung cancer: therapies and mechanisms. Chin Herb Med. 2023;15:509–15.
Liu T, Li Q, Xu X, Li G, Tian C, Zhang T. Molecular mechanisms of anti-cancer bioactivities of seaweed polysaccharides. Chin Herb Med. 2022;14:528–34.
Tian Y, Ma B, Yu S, Li Y, Pei H, Tian S, et al. Clinical antitumor application and pharmacological mechanisms of Dahuang Zhechong Pill. Chin Herb Med. 2023;15:169–80.
Zhai J, Song Z, Chang H, Wang Y, Han N, Liu Z, et al. He-Wei Granule enhances anti-tumor activity of cyclophosphamide by changing tumor microenvironment. Chin Herb Med. 2022;14:79–89.
Chumduri C, Turco MY. Organoids of the female reproductive tract. J Mol Med. 2021;99:531–53.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Villa PL, Jackson R, Eade S, Escott N, Zehbe I. Isolation of biopsy-derived, human cervical keratinocytes propagated as monolayer and organoid cultures. Sci Rep. 2018;8:17869.
Lõhmussaar K, Oka R, Espejo Valle-Inclan J, Smits MHH, Wardak H, Korving J, et al. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell. 2021;28:1380–96.e6.
Reich O, Regauer S, McCluggage WG, Bergeron C, Redman C. Defining the cervical transformation zone and squamocolumnar junction: can we reach a common colposcopic and histologic definition? Int J Gynecol Pathol. 2017;36:517–22.
Giannini SL, Hubert P, Doyen J, Boniver J, Delvenne P. Influence of the mucosal epithelium microenvironment on Langerhans cells: implications for the development of squamous intraepithelial lesions of the cervix. Int J Cancer. 2002;97:654–9.
Jacobs N, Renard I, Al-Saleh W, Hubert P, Doyen J, Kedzia W, et al. Distinct T cell subsets and cytokine production in cultures derived from transformation zone and squamous intraepithelial lesion biopsies of the uterine cervix. Am J Reprod Immunol. 2003;49:6–13.
Martens JE, Smedts FM, Ploeger D, Helmerhorst TJ, Ramaekers FC, Arends JW, et al. Distribution pattern and marker profile show two subpopulations of reserve cells in the endocervical canal. Int J Gynecol Pathol. 2009;28:381–8.
Remoue F, Jacobs N, Miot V, Boniver J, Delvenne P. High intraepithelial expression of estrogen and progesterone receptors in the transformation zone of the uterine cervix. Am J Obstet Gynecol. 2003;189:1660–5.
Deng H, Hillpot E, Mondal S, Khurana KK, Woodworth CD. HPV16-immortalized cells from human transformation zone and endocervix are more dysplastic than ectocervical cells in organotypic culture. Sci Rep. 2018;8:15402.
Gurumurthy RK, Koster S, Kumar N, Meyer TF, Chumduri C. Patient-derived and mouse endo-ectocervical organoid generation, genetic manipulation and applications to model infection. Nat Protoc. 2022;17:1658–90.
Koster S, Gurumurthy RK, Kumar N, Prakash PG, Dhanraj J, Bayer S, et al. Modelling Chlamydia and HPV co-infection in patient-derived ectocervix organoids reveals distinct cellular reprogramming. Nat Commun. 2022;13:1030.
Narisawa-Saito M, Yoshimatsu Y, Ohno S, Yugawa T, Egawa N, Fujita M, et al. An in vitro multistep carcinogenesis model for human cervical cancer. Cancer Res. 2008;68:5699–705.
Zhang M, Kiyono T, Aoki K, Goshima N, Kobayashi S, Hiranuma K, et al. Development of an in vitro carcinogenesis model of human papillomavirus-induced cervical adenocarcinoma. Cancer Sci. 2022;113:904–15.
Perkins RB, Wentzensen N, Guido RS, Schiffman M. Cervical cancer screening: a review. JAMA. 2023;330:547–58.
Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Santos M, Miyamura RA, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106–14.
Hu B, Wang R, Wu D, Long R, Fan J, Hu Z, et al. A promising new model: establishment of patient-derived organoid models covering HPV-related cervical pre-cancerous lesions and their cancers. Adv Sci. 2024;11:e2302340.
Seol HS, Oh JH, Choi E, Kim S, Kim H, Nam EJ. Preclinical investigation of patient-derived cervical cancer organoids for precision medicine. J Gynecol Oncol. 2023;34:e35.
Giannattasio A, Weil S, Kloess S, Ansari N, Stelzer EH, Cerwenka A, et al. Cytotoxicity and infiltration of human NK cells in in vivo-like tumor spheroids. BMC Cancer. 2015;15:351.
Park D, Son K, Hwang Y, Ko J, Lee Y, Doh J, et al. High-throughput microfluidic 3D cytotoxicity assay for cancer immunotherapy (CACI-IMPACT platform). Front Immunol. 2019;10:1133.
Yuti P, Wutti-In Y, Sawasdee N, Kongkhla K, Phanthaphol N, Choomee K, et al. Anti-CD19 chimeric antigen receptor T cells secreting anti-PD-L1 single-chain variable fragment attenuate PD-L1 mediated T cell inhibition. Int Immunopharmacol. 2022;113:109442.
Dong J, Holthaus D, Peters C, Koster S, Ehsani M, Quevedo-Olmos A, et al. γδ T cell-mediated cytotoxicity against patient-derived healthy and cancer cervical organoids. Front Immunol. 2023;14:1281646.
Huang H, Pan Y, Huang J, Zhang C, Liao Y, Du Q, et al. Patient-derived organoids as personalized avatars and a potential immunotherapy model in cervical cancer. iScience. 2023;26:108198.
Maru Y, Tanaka N, Ebisawa K, Odaka A, Sugiyama T, Itami M, et al. Establishment and characterization of patient-derived organoids from a young patient with cervical clear cell carcinoma. Cancer Sci. 2019;110:2992–3005.
Tanaka M, Kondo J, Kaneko K, Endo H, Onuma K, Coppo R, et al. Heterogenous chemosensitivity of a panel of organoid lines derived from small cell neuroendocrine carcinoma of the uterine cervix. Hum Cell. 2021;34:889–900.
Kusakabe M, Taguchi A, Tanikawa M, Hoshi D, Tsuchimochi S, Qian X, et al. Application of organoid culture from HPV18-positive small cell carcinoma of the uterine cervix for precision medicine. Cancer Med. 2023;12:8476–89.
Ferenczy A, Bergeron C. Histology of the human endometrium: from birth to senescence. Ann N Y Acad Sci. 1991;622:6–27.
Tempest N, Hill CJ, Maclean A, Marston K, Powell SG, Al-Lamee H, et al. Novel microarchitecture of human endometrial glands: implications in endometrial regeneration and pathologies. Hum Reprod Update. 2022;28:153–71.
Tempest N, Jansen M, Baker AM, Hill CJ, Hale M, Magee D, et al. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J Pathol. 2020;251:440–51.
Yamaguchi M, Yoshihara K, Suda K, Nakaoka H, Yachida N, Ueda H, et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience. 2021;24:102258.
Bui BN, Boretto M, Kobayashi H, van Hoesel M, Steba GS, van Hoogenhuijze N, et al. Organoids can be established reliably from cryopreserved biopsy catheter-derived endometrial tissue of infertile women. Reprod Biomed Online. 2020;41:465–73.
Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144:1775–86.
Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19:568–77.
Haider S, Gamperl M, Burkard TR, Kunihs V, Kaindl U, Junttila S, et al. Estrogen signaling drives ciliogenesis in human endometrial organoids. Endocrinology. 2019;160:2282–97.
Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2020;126:2225–49.
Girda E, Huang EC, Leiserowitz GS, Smith LH. The use of endometrial cancer patient-derived organoid culture for drug sensitivity testing is feasible. Int J Gynecol Cancer. 2017;27:1701–7.
Jamaluddin MFB, Ko YA, Ghosh A, Syed SM, Ius Y, O’Sullivan R, et al. Proteomic and functional characterization of intra-tumor heterogeneity in human endometrial cancer. Cell Rep Med. 2022;3:100738.
Chen J, Zhao L, Peng H, Dai S, Quan Y, Wang M, et al. An organoid-based drug screening identified a menin-MLL inhibitor for endometrial cancer through regulating the HIF pathway. Cancer Gene Ther. 2021;28:112–25.
Dasari VR, Mazack V, Feng W, Nash J, Carey DJ, Gogoi R. Verteporfin exhibits YAP-independent anti-proliferative and cytotoxic effects in endometrial cancer cells. Oncotarget. 2017;8:28628–40.
Bi J, Newtson AM, Zhang Y, Devor EJ, Samuelson MI, Thiel KW. et al. Successful patient-derived organoid culture of gynecologic cancers for disease modeling and drug sensitivity testing. Cancers. 2021;13:2901.
Chen X, Liu X, Li QH, Lu BF, Xie BM, Ji YM, et al. A patient-derived organoid-based study identified an ASO targeting SNORD14E for endometrial cancer through reducing aberrant FOXM1 Expression and β-catenin nuclear accumulation. J Exp Clin Cancer Res. 2023;42:230.
Su P, Mao X, Ma J, Huang L, Yu L, Tang S, et al. ERRα promotes glycolytic metabolism and targets the NLRP3/caspase-1/GSDMD pathway to regulate pyroptosis in endometrial cancer. J Exp Clin Cancer Res. 2023;42:274.
Espinosa-Gil S, Ivanova S, Alari-Pahissa E, Denizli M, Villafranca-Magdalena B, Viñas-Casas M, et al. MAP kinase ERK5 modulates cancer cell sensitivity to extrinsic apoptosis induced by death-receptor agonists. Cell Death Dis. 2023;14:715.
Cochrane DR, Campbell KR, Greening K, Ho GC, Hopkins J, Bui M, et al. Single cell transcriptomes of normal endometrial derived organoids uncover novel cell type markers and cryptic differentiation of primary tumours. J Pathol. 2020;252:201–14.
Sahoo SS, Ramanand SG, Gao Y, Abbas A, Kumar A, Cuevas IC. et al. FOXA2 suppresses endometrial carcinogenesis and epithelial-mesenchymal transition by regulating enhancer activity. J Clin Invest. 2022;132:e157574.
Qiao Z, Xia C, Shen S, Corwin FD, Liu M, Guan R, et al. Suppression of the PI3K pathway in vivo reduces cystitis-induced bladder hypertrophy and restores bladder capacity examined by magnetic resonance imaging. PLoS ONE. 2014;9:e114536.
Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18:5856–64.
Wu YL, Li JQ, Sulaiman Z, Liu Q, Wang CY, Liu SP, et al. Optimization of endometrial cancer organoids establishment by cancer-associated fibroblasts. Neoplasma. 2022;69:877–85.
Katcher A, Yueh B, Ozler K, Nizam A, Kredentser A, Chung C, et al. Establishing patient-derived organoids from human endometrial cancer and normal endometrium. Front Endocrinol. 2023;14:1059228.
Wiwatpanit T, Murphy AR, Lu Z, Urbanek M, Burdette JE, Woodruff TK, et al. Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2020;105:769–80.
Song Y, Burns GW, Joshi NR, Arora R, Kim JJ, Fazleabas AT. Spheroids as a model for endometriotic lesions. JCI Insight. 2023;8:e160815.
Jamaluddin MFB, Ghosh A, Ingle A, Mohammed R, Ali A, Bahrami M, et al. Bovine and human endometrium-derived hydrogels support organoid culture from healthy and cancerous tissues. Proc Natl Acad Sci USA. 2022;119:e2208040119.
Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17:65–74.
Liu HD, Xia BR, Jin MZ, Lou G. Organoid of ovarian cancer: genomic analysis and drug screening. Clin Transl Oncol. 2020;22:1240–51.
Gray HJ, Chatterjee P, Rosati R, Appleyard LR, Durenberger GJ, Diaz RL, et al. Extraordinary clinical response to ibrutinib in low-grade ovarian cancer guided by organoid drug testing. NPJ Precis Oncol. 2023;7:45.
Auersperg N. Ovarian surface epithelium as a source of ovarian cancers: unwarranted speculation or evidence-based hypothesis? Gynecol Oncol. 2013;130:246–51.
Klotz DM, Wimberger P. Cells of origin of ovarian cancer: ovarian surface epithelium or fallopian tube? Arch Gynecol Obstet. 2017;296:1055–62.
Lo Riso P, Villa CE, Gasparoni G, Vingiani A, Luongo R, Manfredi A, et al. A cell-of-origin epigenetic tracer reveals clinically distinct subtypes of high-grade serous ovarian cancer. Genome Med. 2020;12:94.
Zweemer RP, van Diest PJ, Verheijen RH, Ryan A, Gille JJ, Sijmons RH, et al. Molecular evidence linking primary cancer of the fallopian tube to BRCA1 germline mutations. Gynecol Oncol. 2000;76:45–50.
Zhang S, Dolgalev I, Zhang T, Ran H, Levine DA, Neel BG. Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma. Nat Commun. 2019;10:5367.
Chang YH, Chu TY, Ding DC. Human fallopian tube epithelial cells exhibit stemness features, self-renewal capacity, and Wnt-related organoid formation. J Biomed Sci. 2020;27:32.
Lawrenson K, Notaridou M, Lee N, Benjamin E, Jacobs IJ, Jones C, et al. In vitro three-dimensional modeling of fallopian tube secretory epithelial cells. BMC Cell Biol. 2013;14:43.
Ferenczy A, Richart RM, Agate FJ Jr., Purkerson ML, Dempsey EW. Scanning electron microscopy of the human fallopian tube. Science. 1972;175:783–4.
Jansen RP. Endocrine response in the fallopian tube. Endocr Rev. 1984;5:525–51.
Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med. 2009;6:e1000114.
Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–26.
Chen EY, Mehra K, Mehrad M, Ning G, Miron A, Mutter GL, et al. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J Pathol. 2010;222:110–6.
Soong TR, Howitt BE, Horowitz N, Nucci MR, Crum CP. The fallopian tube, “precursor escape” and narrowing the knowledge gap to the origins of high-grade serous carcinoma. Gynecol Oncol. 2019;152:426–33.
Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35.
Launonen IM, Lyytikäinen N, Casado J, Anttila EA, Szabó A, Haltia UM, et al. Single-cell tumor-immune microenvironment of BRCA1/2 mutated high-grade serous ovarian cancer. Nat Commun. 2022;13:835.
Kessler M, Hoffmann K, Fritsche K, Brinkmann V, Mollenkopf HJ, Thieck O, et al. Chronic Chlamydia infection in human organoids increases stemness and promotes age-dependent CpG methylation. Nat Commun. 2019;10:1194.
Zhang S, Iyer S, Ran H, Dolgalev I, Gu S, Wei W, et al. Genetically defined, syngeneic organoid platform for developing combination therapies for ovarian cancer. Cancer Discov. 2021;11:362–83.
Hill SJ, Decker B, Roberts EA, Horowitz NS, Muto MG, Worley MJ Jr., et al. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 2018;8:1404–21.
Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer. 2016;60:49–58.
Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–8.
Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137–54.
Suzuki M, Liu M, Kurosaki T, Suzuki M, Arai T, Sawabe M, et al. Association of rs6983561 polymorphism at 8q24 with prostate cancer mortality in a Japanese population. Clin Genitourin Cancer. 2011;9:46–52.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Nanki Y, Chiyoda T, Hirasawa A, Ookubo A, Itoh M, Ueno M, et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci Rep. 2020;10:12581.
Jabs J, Zickgraf FM, Park J, Wagner S, Jiang X, Jechow K, et al. Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol Syst Biol. 2017;13:955.
Gorski JW, Zhang Z, McCorkle JR, DeJohn JM, Wang C, Miller RW. et al. Utilizing patient-derived epithelial ovarian cancer tumor organoids to predict carboplatin resistance. Biomedicines. 2021;9:1021.
Compadre AJ, van Biljon LN, Valentine MC, Llop-Guevara A, Graham E, Fashemi B, et al. RAD51 foci as a biomarker predictive of platinum chemotherapy response in ovarian cancer. Clin Cancer Res. 2023;29:2466–79.
Cesari E, Ciucci A, Pieraccioli M, Caggiano C, Nero C, Bonvissuto D, et al. Dual inhibition of CDK12 and CDK13 uncovers actionable vulnerabilities in patient-derived ovarian cancer organoids. J Exp Clin Cancer Res. 2023;42:126.
Song M, Yeku OO, Rafiq S, Purdon T, Dong X, Zhu L, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat Commun. 2020;11:6298.
Wan C, Keany MP, Dong H, Al-Alem LF, Pandya UM, Lazo S, et al. Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint blockade in high-grade serous ovarian cancer. Cancer Res. 2021;81:158–73.
Dai M, Liu M, Yang H, Küçük C, You H. New insights into epigenetic regulation of resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms and therapeutic opportunities. Exp Hematol Oncol. 2022;11:101.
Kenny HA, Lal-Nag M, Shen M, Kara B, Nahotko DA, Wroblewski K, et al. Quantitative high-throughput screening using an organotypic model identifies compounds that inhibit ovarian cancer metastasis. Mol Cancer Ther. 2020;19:52–62.
Senkowski W, Gall-Mas L, Falco MM, Li Y, Lavikka K, Kriegbaum MC, et al. A platform for efficient establishment and drug-response profiling of high-grade serous ovarian cancer organoids. Dev Cell. 2023;58:1106–21.e7.
Phan N, Hong JJ, Tofig B, Mapua M, Elashoff D, Moatamed NA, et al. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun Biol. 2019;2:78.
D’Amati G, di Gioia CR, Proietti Pannunzi L, Pistilli D, Carosa E, Lenzi A, et al. Functional anatomy of the human vagina. J Endocrinol Invest. 2003;26:92–6.
Ali A, Syed SM, Jamaluddin MFB, Colino-Sanguino Y, Gallego-Ortega D, Tanwar PS. Cell lineage tracing identifies hormone-regulated and Wnt-responsive vaginal epithelial stem cells. Cell Rep. 2020;30:1463–77.e7.
Mahajan G, Doherty E, To T, Sutherland A, Grant J, Junaid A, et al. Vaginal microbiome-host interactions modeled in a human vagina-on-a-chip. Microbiome. 2022;10:201.
Kaur M, Gupta KM, Poursaid AE, Karra P, Mahalingam A, Aliyar HA, et al. Engineering a degradable polyurethane intravaginal ring for sustained delivery of dapivirine. Drug Deliv Transl Res. 2011;1:223–37.
Shapiro RL, DeLong K, Zulfiqar F, Carter D, Better M, Ensign LM. In vitro and ex vivo models for evaluating vaginal drug delivery systems. Adv Drug Deliv Rev. 2022;191:114543.
Lim JTC, Kwang LG, Ho NCW, Toh CCM, Too NSH, Hooi L, et al. Hepatocellular carcinoma organoid co-cultures mimic angiocrine crosstalk to generate inflammatory tumor microenvironment. Biomaterials. 2022;284:121527.
Walther-António MR, Chen J, Multinu F, Hokenstad A, Distad TJ, Cheek EH, et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016;8:122.
Nené NR, Reisel D, Leimbach A, Franchi D, Jones A, Evans I, et al. Association between the cervicovaginal microbiome, BRCA1 mutation status, and risk of ovarian cancer: a case-control study. Lancet Oncol. 2019;20:1171–82.
Zhang J, Hernandez-Gordillo V, Trapecar M, Wright C, Taketani M, Schneider K, et al. Coculture of primary human colon monolayer with human gut bacteria. Nat Protoc. 2021;16:3874–900.
Puschhof J, Pleguezuelos-Manzano C, Martinez-Silgado A, Akkerman N, Saftien A, Boot C, et al. Intestinal organoid cocultures with microbes. Nat Protoc. 2021;16:4633–49.
Puschhof J, Pleguezuelos-Manzano C, Clevers H. Organoids and organs-on-chips: insights into human gut-microbe interactions. Cell Host Microbe. 2021;29:867–78.
Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–8.
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7.
Artegiani B, Hendriks D, Beumer J, Kok R, Zheng X, Joore I, et al. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR-Cas9 precision genome editing. Nat Cell Biol. 2020;22:321–31.
Drost J, van Boxtel R, Blokzijl F, Mizutani T, Sasaki N, Sasselli V, et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science. 2017;358:234–8.
Hendriks D, Artegiani B, Hu H, Chuva de Sousa Lopes S, Clevers H. Establishment of human fetal hepatocyte organoids and CRISPR-Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat Protoc. 2021;16:182–217.
Lo YH, Kolahi KS, Du Y, Chang CY, Krokhotin A, Nair A, et al. A CRISPR/Cas9-engineered ARID1A-deficient human gastric cancer organoid model reveals essential and nonessential modes of oncogenic transformation. Cancer Discov. 2021;11:1562–81.
Xiaoshuai L, Qiushi W, Rui W. Advantages of CRISPR-Cas9 combined organoid model in the study of congenital nervous system malformations. Front Bioeng Biotechnol. 2022;10:932936.
Lõhmussaar K, Kopper O, Korving J, Begthel H, Vreuls CPH, van Es JH, et al. Assessing the origin of high-grade serous ovarian cancer using CRISPR-modification of mouse organoids. Nat Commun. 2020;11:2660.
Geurts MH, Gandhi S, Boretto MG, Akkerman N, Derks LLM, van Son G, et al. One-step generation of tumor models by base editor multiplexing in adult stem cell-derived organoids. Nat Commun. 2023;14:4998.
Bollen Y, Hageman JH, van Leenen P, Derks LLM, Ponsioen B, Buissant des Amorie JR, et al. Efficient and error-free fluorescent gene tagging in human organoids without double-strand DNA cleavage. PLoS Biol. 2022;20:e3001527.
Tiroille V, Krug A, Bokobza E, Kahi M, Bulcaen M, Ensinck MM, et al. Nanoblades allow high-level genome editing in murine and human organoids. Mol Ther Nucleic Acids. 2023;33:57–74.
Mazzocchi A, Devarasetty M, Herberg S, Petty WJ, Marini F, Miller L, et al. Pleural effusion aspirate for use in 3D Lung cancer modeling and chemotherapy screening. ACS Biomater Sci Eng. 2019;5:1937–43.
Giobbe GG, Crowley C, Luni C, Campinoti S, Khedr M, Kretzschmar K, et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun. 2019;10:5658.
Jin Y, Lee JS, Kim J, Min S, Wi S, Yu JH, et al. Three-dimensional brain-like microenvironments facilitate the direct reprogramming of fibroblasts into therapeutic neurons. Nat Biomed Eng. 2018;2:522–39.
Willemse J, van Tienderen G, van Hengel E, Schurink I, van der Ven D, Kan Y, et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials. 2022;284:121473.
Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 2017;49:1–15.
Gjorevski N, Lutolf MP. Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nat Protoc. 2017;12:2263–74.
Broguiere N, Isenmann L, Hirt C, Ringel T, Placzek S, Cavalli E, et al. Growth of epithelial organoids in a defined hydrogel. Adv Mater. 2018;30:e1801621.
Gnecco JS, Brown A, Buttrey K, Ives C, Goods BA, Baugh L, et al. Organoid co-culture model of the human endometrium in a fully synthetic extracellular matrix enables the study of epithelial-stromal crosstalk. Med. 2023;4:554–79.e9.
Yan J, Wu T, Zhang J, Gao Y, Wu JM, Wang S. Revolutionizing the female reproductive system research using microfluidic chip platform. J Nanobiotechnol. 2023;21:490.
Ibrahim LI, Hajal C, Offeddu GS, Gillrie MR, Kamm RD. Omentum-on-a-chip: a multicellular, vascularized microfluidic model of the human peritoneum for the study of ovarian cancer metastases. Biomaterials. 2022;288:121728.
Rizvi I, Gurkan UA, Tasoglu S, Alagic N, Celli JP, Mensah LB, et al. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc Natl Acad Sci USA. 2013;110:E1974–83.
Vandghanooni S, Sanaat Z, Barar J, Adibkia K, Eskandani M, Omidi Y. Recent advances in aptamer-based nanosystems and microfluidics devices for the detection of ovarian cancer biomarkers. Trac Trends Anal Chem. 2021;143:116343.
Wu Y, Wang C, Wang P, Wang C, Zhang Y, Han L. A high-performance microfluidic detection platform to conduct a novel multiple-biomarker panel for ovarian cancer screening. RSC Adv. 2021;11:8124–33.
Zhang P, Zhou X, He M, Shang Y, Tetlow AL, Godwin AK, et al. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nat Biomed Eng. 2019;3:438–51.
Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16:489–96.
Dorayappan KDP, Gardner ML, Hisey CL, Zingarelli RA, Smith BQ, Lightfoot MDS, et al. A microfluidic chip enables isolation of exosomes and establishment of their protein profiles and associated signaling pathways in ovarian cancer. Cancer Res. 2019;79:3503–13.
Gu Y, Li Z, Ge S, Mao Y, Gu Y, Cao X, et al. A microfluidic chip using Au@SiO(2) array-based highly SERS-active substrates for ultrasensitive detection of dual cervical cancer-related biomarkers. Anal Bioanal Chem. 2022;414:7659–73.
Inan H, Wang S, Inci F, Baday M, Zangar R, Kesiraju S, et al. Isolation, detection, and quantification of cancer biomarkers in HPV-associated malignancies. Sci Rep. 2017;7:3322.
Wang N, Wang J, Meng X, Bao Y, Wang S, Li T. 3D microfluidic in vitro model and bioinformatics integration to study the effects of Spatholobi Caulis tannin in cervical cancer. Sci Rep. 2018;8:12285.
Skardal A, Aleman J, Forsythe S, Rajan S, Murphy S, Devarasetty M, et al. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication. 2020;12:025017.
Rajan SAP, Aleman J, Wan M, Pourhabibi Zarandi N, Nzou G, Murphy S, et al. Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform. Acta Biomater. 2020;106:124–35.
Cherne MD, Sidar B, Sebrell TA, Sanchez HS, Heaton K, Kassama FJ, et al. A synthetic hydrogel, VitroGel(®) ORGANOID-3, improves immune cell-epithelial interactions in a tissue chip co-culture model of human gastric organoids and dendritic cells. Front Pharm. 2021;12:707891.
Li X, Fu G, Zhang L, Guan R, Tang P, Zhang J, et al. Assay establishment and validation of a high-throughput organoid-based drug screening platform. Stem Cell Res Ther. 2022;13:219.
Du Y, Li X, Niu Q, Mo X, Qui M, Ma T, et al. Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening. J Mol Cell Biol. 2020;12:630–43.
Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, et al. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell. 2018;22:929–40.e4.
Brandenberg N, Hoehnel S, Kuttler F, Homicsko K, Ceroni C, Ringel T, et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat Biomed Eng. 2020;4:863–74.
Maenhoudt N, Defraye C, Boretto M, Jan Z, Heremans R, Boeckx B, et al. Developing organoids from ovarian cancer as experimental and preclinical models. Stem Cell Rep. 2020;14:717–29.
Funding
This work was supported by the Key R&D Project of Sichuan Provincial Department of Science and Technology, No. 2021YFS0409; Sichuan Provincial Administration of Traditional Chinese Medicine Major Science and Technology Project, No. 2021XYCZ001.
Author information
Authors and Affiliations
Contributions
RT and RL were responsible for the overall conception and design of the article. JL and MZ completed the literature search, data collection, and initial drafting of the manuscript. JX and JC prepared the pictures and tables. MY, CY, and SC discussed and revised the manuscript. RL and ML were responsible for reviewing, critically analyzing, and revising the article. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Zhou, M., Xie, J. et al. Organoid modeling meets cancers of female reproductive tract. Cell Death Discov. 10, 410 (2024). https://doi.org/10.1038/s41420-024-02186-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41420-024-02186-x
This article is cited by
-
Organoids as powerful models of endometrium epithelium in transcriptomic, cellular and functional mimicry
Cellular and Molecular Life Sciences (2025)