Abstract
Implantable neural probes capable of monitoring deep brain activities with single-neuron resolution have contributed substantially to our understanding of brain function, treatment of neurological diseases, and application in brain-machine interface. However, conventional probes comprised primarily of rigid inorganic materials with large feature sizes face several limitations when being chronically implanted, including chronic recording instability, elevated immune responses within the brain, and deleterious neuron death. Driven by the strong desire for long-term stable brain interfaces and innovations in biomaterials and probe designs, novel neural probes are emerging and being rapidly adopted in academic and clinical settings. Here, we first review the historical progression of conventional implantable neural probes. We then discuss their limitations in long-term brain interfaces and the underlying biological mechanisms. Next, we summarize recent progress in next-generation chronically stable probe technologies enabled by materials innovations and structural engineering. Last, we highlight several outstanding challenges and future opportunities of the field. We argue that advancements in biomaterial engineering integrated with innovations in probe design and manufacturing will not only play an increasingly critical role in neuroscience and therapeutics but also offer a general approach to achieve long-term stable tissue monitoring by blurring the distinction between man-made devices and natural-born organisms.
Similar content being viewed by others
Introduction
The brain’s extraordinary computational capabilities arise from the intricate networks of massive numbers of interconnected neurons1. Neurons—the functional units of the nervous system—integrate, process, and transmit information via changes in electrical potentials, including low frequency (<250 Hz) local field potential (LFP) oscillations originated from ensemble neuron dynamics and high frequency (~1 kHz) action potentials (APs) of individual neurons1,2,3. Stable long-term extracellular monitoring of large neuronal populations across multiple brain regions, from both superficial and deep structures, with single-neuron resolution holds the promise to significantly advance fundamental neuroscience research (e.g., deciphering the neural representations behind specific behaviors), treatment of neurological diseases (e.g., Parkinson’s and Alzheimer’s), and brain-machine interface (BMI)2,4,5,6,7.
The intricate, dynamic, and delicate nature of the brain poses the following requirements for an ideal neural probe (Fig. 1). First, it should be capable of mapping the dynamics from a massive number of neurons at single-neuron resolution as growing evidence suggests that spatially distributed neuronal ensembles represent the functional motifs of the mammalian brain7. Second, it should be capable of tracking individual neurons’ APs at millisecond temporal resolution and stably over chronic timescales because many crucial brain processes, including learning, memory, and aging, occur over months to years. Finally, it should introduce minimal disturbance to the brain’s neural and glial networks to enable true physiological monitoring of the brain in its native state.
Vertical and horizontal axes represent the spatial and temporal scales, respectively. The shaded rectangles indicate the spatiotemporal span of conventional and ideal probes. Schematic illustrations highlight the spatiotemporal scales of a single-neuron spike, a neural population trajectory, and human aging.
Current surface neural recording technologies, including electroencephalography (EEG)8,9 and electrocorticography (ECoG)10, offer minimally-invasive solutions to capture neural activity across large areas. However, they fall short in achieving high spatial resolution due to the low-pass filter properties of the scalp, skull, and brain tissue. Recent progress in ECoG technologies has achieved single-neuron resolution, but they are still restricted to probing the surface brain regions, unable to study activities in deeper regions that contribute to a broad range of brain functions10,11. This limitation calls for implantable neural probes that can penetrate into the brain and record from intracortical and subcortical areas.
Over the past century, various types of implantable neural probes were invented and have achieved highly multiplexed recordings from both superficial and deep brain regions at single-neuron resolution12. These technological advances have led to a significant leap in our understanding of the brain, including several Nobel-winning discoveries13,14,15. However, their capability to sustain stable chronic recording is severely limited.
In this review, we focus on discussing recent innovations in materials engineering and structural designs that led to substantial improvement in the long-term recording stability of implantable neural probes. We begin with reviewing the historical development of implantable neural probes and their achievements. Then, we introduce the challenges associated with their long-term recording performance and examine the underlying biological mechanisms. Next, we summarize recent developments in materials engineering and structural designs that enabled long-term stable recordings at the single-neuron level. On the other hand, these developments also brought new challenges regarding probe implantation. We will compare different strategies to overcome these challenges. We end the review with a discussion on remaining challenges and future directions. As it is impossible to cover all the exciting progress in this rapidly evolving field, we refer interested readers to other comprehensive reviews for additional information16,17,18,19,20,21,22,23.
Brief overview of implantable neural probes
To capture the APs from individual neurons in the extracellular space, an electrically conducting device, such as a metal wire or electrode, needs to be placed in the vicinity, typically within 100 μm, of the target neuron24. Microwire probes are conventional recording probes that typically consist of a conductive metal wire encapsulated by an insulation layer while leaving the tip of the wire exposed (Fig. 2a, b). A milestone in the development of microwire probes is the invention of tungsten probes in 195725. Prior to this, neural probes such as glass-insulated electrodes were often too brittle to access deep brain regions and were difficult to miniaturize. Tungsten probes, with tips diameter sharpened to the sub-micron scale, enabled high-precision extracellular recordings from individual neurons in mammalian brains25,26. This technological breakthrough has laid the foundation of a series of seminal discoveries made by David Hubel and Torsten Wiesel that greatly expanded our knowledge of sensory information processing mechanism of the brain14. Besides tungsten, other metallic materials, including insulated steel, gold (Au), and platinum (Pt), have also been adopted. A key issue of microwires is their limited scalability for spatial multiplexing, which undermines both their capability to sort out APs generated by different neurons and the total number of neurons that can be recorded simultaneously24. To sort out APs from different neurons, stereotrodes27 and tetrodes28, which consist of two and four closely bundled microwires, respectively, were invented: each microwire in the bundle records slightly different signals from nearby neurons. By comparing recorded traces across different wires, the identity of individual APs can be assigned. Nevertheless, the total number of neurons that can be recorded remained low.
a Milestones of conventional implantable probes for in vivo neural recordings. b Milestones of microwire type probes, including tungsten probes (left), stereotrodes (middle), and tetrodes (right) (Reproduced with permission from refs. 25,27,122). c Milestones of silicon probes, including Utah arrays (left), Michigan probe (middle), and Neuropixels (right) (Reproduced with permission from refs. 30,123,124).
By the 1980s, advancements in semiconductor microfabrication had led to the invention of silicon-based multielectrode arrays (Fig. 2a, c), where densely packed and spatially distributed electrode arrays were lithographically defined. Among them, the most widely adopted ones are the Utah array and the Michigan probe. Utah array is a three-dimensional (3D) silicon-based array consisting of ~100 intracortical electrodes29. Each electrode is composed of a 1.5 mm long polyimide insulated silicon needle with its tip coated with Pt. The electrode arrays with a typical spacing of 0.4 mm are held together by a thin silicon substrate. During recording, the electrodes penetrate into the cortex while the silicon substrate sits on the cortical surface. Thanks to their surface area and high electrode count, Utah arrays have become the go-to technology for studying cortical circuits, particularly in non-human primate models, and BMI applications in the motor and visual cortex. The major limitations of Utah arrays are their restricted penetration depths, inaccessibility to subcortical areas, and low multiplexing along the dorsal-ventral axis.
To overcome these limitations, an alternative architecture was developed in the 1980s named Michigan probe: a 2D array of microelectrodes is lithographically defined on a silicon substrate that is often referred to as a shank. Michigan probe offers precise multi-site recordings at targeted subcortical regions. To further scale up the total number of neurons that can be recorded, several strategies have been implemented, including improving the interfacial impedance of the microelectrodes to shrink the electrodes’ size for higher packing density, as well as integrating and packaging multiple shanks to a single probe. A recent breakthrough, named Neuropixel (Fig. 2c), utilizes complementary metal-oxide-semiconductor (CMOS) technology to integrate signal amplifiers into the shank and achieves high-density, high-resolution recordings over 1000 recording sites on a single shank12,30. By further improving the probe insertion process, reducing electrical noise, and minimizing motion-induced artifacts, Neuropixels have enabled large-scale intraoperative recordings from humans5.
Despite the successes of conventional implantable neural probes in facilitating discoveries in neuroscience and applications in BMI, they face a key challenge—being incapable of tracking the same neuron over long term—that limits their use in chronic studies. Although approaches such as microdrive have been attempted to reposition the probes to compensate for the issue, these approaches often lead to further brain damage and preclude monitoring of the initially targeted neurons18. Addressing this challenge asks for an understanding of the fundamental mechanisms behind this long-term instability and the development of next-generation implantable neural probes based on new materials and probe designs.
Contributing factors to the long-term recording instability
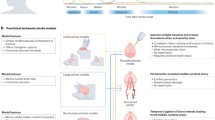
The long-term instability of neural probe recording, manifested as a gradual degradation of signal quality over time following implantation (Fig. 3a), is primarily caused by gliosis and neuronal death (Fig. 3b)20. Gliosis refers to the formation of a dense encapsulation layer—glial scar—around the neural probe induced by chronic foreign body reaction (FBR)31. Glial scar is mainly composed of reactive astrocytes, which are the activated phenotype of astrocytes that are characterized by an upregulation of the intermediate filaments of polymerized glial fibrillary acid protein (GFAP) signal in immunohistochemical staining (Fig. 3b)31. The formation of the glial scar enlarges the distance between the recording electrodes and the targeted neurons, increases the interfacial impedance of the electrodes, and, as a result, leads to a decay in signal-to-noise-ratio (SNR) of recorded neuronal activities31. In addition to gliosis, neuronal death near the probe is suggested to be another major cause of signal decline31. Since the probe typically records from neurons within 100 μm, the death of neurons in the vicinity of the probe can result in the loss of recorded signals31.
a Neural signals recorded from conventional implantable neural probes degraded typically over a few days after implantation (left, reproduced with permission from ref. 37) with signal instability across days (right, reproduced with permission from ref. 38). b ED1 (microglia), GFAP (active astrocytes), NeuN (neurons), and NF (neurofilament) signals around chronically implanted silicon probe (Reproduced with permission from ref. 125).
One common cause of gliosis and neuronal death is chronic inflammation, which is characterized by the upregulation of pro-inflammatory cytokines and an increase in oxidative stress from free radicals, surrounding the neural probes32,33. These pro-inflammatory cytokines and free radicals can activate astrocytes and transform them into the reactive phenotype that forms glial scars34. In addition, the neurotoxicity caused by pro-inflammatory cytokines and free radicals also leads to neuronal death32,35.
Activated microglia and chronic blood-brain barrier (BBB) disruption are considered among the key contributors to chronic inflammation surrounding the probes31,33. Microglia are the primary immune cells in the central nervous system. When activated, they release pro-inflammatory cytokines, including IL-1, TNF-α, and IL-6, and trigger an inflammatory response31. Additionally, BBB disruption leads to the leakage of neurotoxic serum proteins, red blood cells, and pro-inflammatory factors, further increasing the levels of free radicals and pro-inflammatory cytokines, which exacerbates the inflammation around the probes36.
Mechanical mismatch between the probes and the brain tissue, along with the brain tissue’s micromotion, are found to contribute to the activation of microglia and chronic BBB disruption37,38,39. The elastic modulus of metal and silicon neural probes typically exceeds 100 GPa. In contrast, brain tissue’s elastic modulus is only a few kPa. This significant difference in elastic moduli, together with the brain tissue’s micromotion that is driven by respiration, vascular pulses, and rotational acceleration, induces mechanical strain in the brain tissues surrounding the probes40. In vitro experiments reveal that even low-magnitude strain can induce the upregulation of gliosis markers and anti-inflammatory cytokine responses40. In addition to the induced mechanical strain, differences in elastic moduli alone can also trigger chronic immune responses. For example, studies have shown that glial cells are more likely to be activated on stiffer surfaces41. Furthermore, probe-tissue mechanical mismatch and brain tissue’s micromotion have also been hypothesized to be associated with long-term BBB damage36.
It should be noted that despite significant efforts to unravel the mechanisms behind recording failure, a full understanding is still beyond reach due to the large variability of diverse neural probes and insufficient knowledge of the brain’s immune system.
Strategies to improve long-term recording capability
Various strategies have been proposed to extend the longevity of implantable neural probes, targeting different potential biological causes mentioned above. These strategies include functional coatings to directly mitigate neuroinflammation and improve brain-probe integration, replacing conventional rigid probe substrates with soft polymer substrates, and engineering novel probe structures with reduced dimensions to minimize the mechanical mismatch between the probe and the brain tissues.
Functional coatings to improve long-term stability
Anti-inflammatory coatings
Dexamethasone (DEX) is a widely used anti-inflammatory agent that mitigates inflammation associated with chronic implants42,43,44,45. To circumvent the side effects brought by systemic DEX injection, including myopathy and diabetes, surface coatings of DEX have been explored42. For example, evaporating a mixture of DEX and nitrocellulose solution on a silicon probe has been shown to reduce neuronal death at both 1 week and 4 weeks post-implantation42. For sustained drug release, DEX was stored in polypyrrole/carbon nanotube composite film or poly(3,4-ethylenedioxythiophene) (PEDOT) coating43,44, allowing for controlled release upon electrical stimulation. Besides DEX, minocycline hydrochloride and α-melanocyte stimulating hormone have also been used as anti-inflammatory coatings46,47,48.
In addition to anti-inflammatory drugs, nanozymes with antioxidative and biocatalytic properties are reported to reduce activated molecules at the electrode-brain interface and effectively alleviate neuroinflammation49. Histological analysis 2 months post-implantation showed that nanozyme-coated probes exhibited a significant reduction in glial scar volume, microglia activities, and neuronal death. Besides, signals recorded from nanozyme-coated probes remained stable, in contrast to a 58% amplitude reduction in uncoated ones49.
Biomimetic coatings
Biomechanical analysis predicts that improved tissue-probe integration via surface coating can reduce micromotion-induced strains to the surrounding brain tissue, thereby improving the probes’ chronic stability39. Experimental studies have been attempted.
Laminin, a key component of the extracellular matrix (ECM), serves as an effective biomimetic coating material that promotes tissue-probe integration50. Silicon probes coated with laminin-1 showed reduced gliosis and lower pro-inflammatory cytokine production 4 weeks post-implantation in rat brains, indicating mitigated long-term immune reactions51. Similarly, researchers demonstrated that ECM coatings derived from primary rat astrocytes on a planar silicon microelectrode array can effectively reduce astrocyte activation 8 weeks after implantation in adult rat cortex52. In another study, researchers covalently linked neuron-adhesive protein L1 to the surfaces of Michigan probes and showed improvement in recording quality, enhancement of neuronal and axonal density, as well as reduction in microglial activation 16 weeks after implantation in mouse brains53.
Although functional coatings have been proven to mitigate FBR, they do not address the fundamental issue of mechanical mismatch between the implantable neural probes and the brain tissues. Strategies that overcome the mechanical mismatch (Fig. 4a) hold the promise to fundamentally solve the long-term recording instability of implantable neural probes.
a Elastic modulus of brain tissues and commonly used materials for implantable neural probes. b Neural probes with commonly used plastic polymer substrates, including polyimide, Parylene C, and SU-8 (Reproduced with permission from refs. 68,126,127). c Neural probes with elastomer-based substrates, including PDMS, Ecoflex, and PFPE-DMA (Reproduced with permission from refs. 74,75,78,79). d Neural probes with hydrogel-based substrates (Reproduced with permission from ref. 83). e Neural probes with liquid metal electrodes (Reproduced with permission from ref. 94). f Neural probes with conductive polymer electrodes (Reproduced with permission from ref. 85).
Soft materials to improve long-term stability
Both in vitro and in vivo studies indicate that matching the mechanical properties of the neural probe to those of the surrounding tissues can significantly mitigate FBR41. Therefore, a straightforward strategy to enhance the long-term recording stability of implantable neural probes is to construct their components with soft materials, including the substrate that offers mechanical support and electrodes that provide electrical sensing capability.
Soft substrate materials
Plastic polymers have been used as substrate materials for microfabricated flexible probes, serving as alternative options to the rigid silicon substrate (Fig. 4b). The elastic moduli of polymers are typically on the order of a few GPa, which is two orders of magnitude lower than silicon54. Among the diverse groups of plastic polymers, polyimide, Parylene C, and SU-8 are the most commonly used ones55,56,57,58,59,60,61,62,63. Polyimide is a commercially available polymer that has been used for microelectronics and biomedical applications for over 40 years64. It features excellent electrical insulation as well as robust chemical and thermal properties64,65. Polyimide’s application as the substrate material for implantable neural probes can be traced back to the early 2000s56. Parylene C, another polymer material known for its outstanding biocompatibility, has been broadly applied in biomedical devices66. Parylene C can be deposited by chemical vapor deposition at low temperatures, allowing it to conform to most surfaces67. SU-8, an epoxy-based photoresist, can be directly patterned into high aspect ratio structures via photolithography, making it a versatile option in various applications. The use of plastic polymer substrates has been proven to successfully reduce FBR68.
While the fabrication methods of these plastic polymer materials are well-established, their elastic moduli are still around 1 million times greater than that of the brain. Therefore, researchers have also been exploring softer and stretchable elastomers as the substrate materials (Fig. 4c). Poly(dimethylsiloxane) (PDMS), a transparent silicone-based material, usually consists of a prepolymer and a cross-linker with tunable mechanical properties by adjusting their ratio. It has an elastic modulus on the order of hundreds of kPa69, and has been used as substrate material for surface neural probes, including ECoG and devices interfacing with peripheral nerves and spinal cord70,71,72,73. However, PDMS is only occasionally adopted in penetrating neural probes74,75,76,77. Ecoflex is another type of biocompatible silicone elastomer that can serve as the substrate material. It has been used to fabricate mechanically matched brain implants78. Compared to silicon probes of the same dimensions, mechanically matched brain implants elicited a lower FBR with reduced levels of activated microglia, reactive astrocytes, and neuronal death at 3 and 9 weeks post-implantation78. The fabrication of Ecoflex substrate usually requires casting the material into a separate master mold, which limits the achievable resolution and structural complexity. Recently, researchers have employed a photo-patternable fluorinated elastomer as the substrate of neural probes. The perfluoropolyether (PFPE)-based material can be fabricated with micrometer resolution79,80.
Hydrogels represent another group of soft materials, with mechanical and chemical properties closely resembling those of the brain tissues (Fig. 4d)81. They typically have elastic modulus on the order of a few kPa. To test their ability to reduce mechanical mismatch and associated FBR, researchers coated 25–100 μm polyethylene glycol dimethacrylate (PEG-DMA) hydrogel layers on implantable neural probes and compared their performance with uncoated ones82. In vitro micromotion simulations in a 0.6% agarose brain phantom showed a significant reduction in the local strain field surrounding the hydrogel-coated probes under both axial and perpendicular displacements82. In vivo studies showed that hydrogel-coated probes exhibited significantly reduced GFAP reactivity within 50 μm of the probes at 1, 4, and 8 weeks post-implantation82.
However, applying hydrogel as a coating material does not fundamentally address the mechanical mismatch issue because the overall mechanical property of the coated probes remains dominated by the stiffness of the probe itself83. To address this limitation, researchers directly utilized hydrogel as a substrate material83. Individual functional fibers were aligned into an assembly that is dip-coated with a hydrogel pre-gel solution. The assembly is then cured by ultraviolet light to form a 25 μm thick hydrogel substrate83. A key issue of hydrogel-based probes is that hydrogels are generally fragile and prone to damage during implantation. One approach that partly solves this issue is to dehydrate hydrogels before implantation. Once inside the brains, hydrogels absorb water and return to a hydrated state82,83,84.
Soft electrode materials
As probes transition from rigid to soft substrates, the stiffness of the recording electrodes, once insignificant, begins to dominate. To maintain flexibility, the electrodes must also be ‘softened’.
Conductive polymers are a class of soft conductive materials that have been widely explored, particularly as electrode coatings to reduce interfacial impedance85. For example, coating recording electrodes with PEDOT can greatly decrease recording electrodes’ impedance, which leads to increased SNR of recorded neural signals86,87. Although conductive polymers are not typically used as primary electrode materials, there have been efforts investigating their potential in this capacity with promising outcomes. For example, studies reported 3D printing of PEDOT: polystyrene sulfonate (PEDOT: PSS) to construct soft electrodes77,88. In another study, researchers designed a neural electrode array based on a biocompatible supramolecular network with both high electrical conductivity and mechanical robustness (Fig. 4f)85. When placed between cerebellum and brainstem, this neural electrode array leads to reduced tissue damage and inflammatory responses85. Recently, in contrast to the regular approach of fabricating the neural probes first followed by implantation, an innovative approach uses in vivo polymerization and directly creates electrodes within brain tissues89. In this experiment, a precursor solution containing conductive polymer monomers and enzymes was injected into biological tissue. Endogenous metabolites, such as glucose or lactate, reacted with the enzymes to generate oxidizing species, which subsequently triggered polymerization of the monomers89.
In addition to intrinsic conductive polymers, soft electrodes can also be constructed using conductive polymer composites, which are blends of non-conductive polymer and conductive materials. For example, a conductive polyethylene (CPE) polymer composite was used as the recording electrode material for fabricating an all-polymer multifunctional neural probe90. To enhance the conductivity of CPE, researchers blended it with 5% graphite91. Similarly, impregnating 2 vol% carbon nanofiber into CPE also effectively enhances the conductivity92.
Although conductive polymers and conductive polymer composites showed great potential, they are limited due to their low conductivity. Liquid metals whose electrical conductivity is approximately 30 times higher than that of intrinsic conductive polymers make it possible to form soft electrodes with high electrical conductivity (Fig. 4e)93. Liquid metals have been used in fabricating devices to interface with peripheral nerve, brain surface, and retina. Researchers utilized the near-body melting point properties of liquid metals to both conduct electrical interconnection and probe stiffening for implantation aid75. For example, pressurized liquid gallium (Ga) was introduced into PDMS microfluidic channels and then frozen to a stiff state before implantation. After implantation, the solidified Ga was melted by body temperature and removed to reduce the stiffness of the implantable probe75. In another study, researchers used high-resolution printing to fabricate eutectic gallium-indium alloy (EGaIn) liquid metal wires94. The EGaIn wire, which is structurally and mechanically similar to neurons, can recover after physical disconnections94. Tissue slices from mice 8 weeks post-implantation showed no significant neuronal death or glial activities94.
Structural engineering to improve long-term stability
Improving the long-term stability of implantable neural probes solely with soft materials faces challenges in several aspects, including poor mechanical durability, insufficient conductivity, and limited compatibility with microfabrication techniques. A quick examination of the bending stiffness of a neural probe shows that it not only depends on the elastic modulus, but also on the area moment of inertia that is determined by the size and geometry of the probe95. For an implantable neural probe with a beam-like geometry and a rectangular cross-section, its bending stiffness, \(K\), can be approximated as:
where\(E\) is the elastic modulus of the substrate materials, \(I\) is the area moment of inertia that is proportional to the probe’s width and the cubic power of its thickness. By rationally designing the structures and minimizing the dimensions of the probe, its bending stiffness can be substantially decreased.
The influence of probe dimension on brain FBR has been experimentally studied by systematically examining tissue responses to neural implants with different sizes82. Researchers implanted glass microcapillaries with diameters ranging from 150 µm to 400 µm into rodent brains. Immunohistochemical analysis at 8 weeks post-implantation revealed a clear size-dependent effect on chronic gliosis, with the 400 µm capillary showing the most severe gliosis. Larger capillaries were also found to be associated with increased BBB permeability and elevated macrophage activation82.
Different types of implantable neural probes with reduced dimensions and carefully engineered structures have been developed to reduce bending stiffness. For example, fiber-like probes were developed as miniaturized analogs to conventional microwire probes (Fig. 5a). Researchers fabricated ultrasmall Parylene-N coated carbon-fiber probes with diameters of approximately 8.5 μm, which are comparable to the size of a neuron96. This ultrasmall carbon-fiber probe achieved stable recording capabilities in rat brains for up to 5 weeks post-implantation96. To further integrate fibers with multifunctionalities, a thermal drawing process was applied to fabricate multifunctional neural probes90.
a Fiber-like neural probes (Reproduced with permission from refs. 90,96,128). b Thread-like neural probes (Reproduced with permission from refs. 99,100,101). c Planar flexible neural probes (Reproduced with permission from refs. 97,98,102). d Mesh electronics (Reproduced with permission from refs. 61,105,108).
Similarly, researchers have developed thin-film planar neural probes as analogs to the Michigan-style probes (Fig. 5c)97,98. Researchers have designed a Michigan-style probe with a linear array of microelectrodes on a 50 μm wide and 1 μm thick SU-8 substrate97. With significantly reduced dimensions, the probe exhibited a dramatically decreased bending stiffness and greatly enhanced biocompatibility compared to conventional Michigan probes. Further reductions in probe width led to the development of thread-like neural probes (Fig. 5b), which can be ‘sewn’ into the brain tissue with stiff ‘needles’97,99,100,101. Recently, researchers developed an innovative way to roll a flexible polyimide film probe into a cylindrical probe (Fig. 5c). This rolled cylindrical probe, named ‘Neuroscroll’ probe, houses a large number of densely packed recording electrodes on the cylinder surface with the interconnects embedded inside the scroll. The probe has been proven to be capable of recording from large neural populations up to 105 weeks in rats102.
A unique design distinct from conventional probe structures is the mesh electronics (Fig. 5d)103,104. Mesh electronics eliminate substrate areas that are not covered by either recording electrodes or interconnects and create a 3D macroporous structure that resembles the neural network for enhanced chemical diffusion and tissue integration. The recording electrodes and interconnects of mesh electronics can be fabricated with dimensions similar to those of neuronal soma and axons, respectively105. Mesh electronics have been demonstrated to achieve stable long-term monitoring and modulation of the same neuron and neural circuit activities over 8 months103. In addition, the unique structure of mesh electronics also enables interface with tissues that are inaccessible to conventional neural probes, including retina106, spinal cord107, and vasculatures inside the brain108. Mesh electronics can also act as tissue scaffolds to promote cell migration within the brain105,109.
Delivery methods for flexible implantable neural probes
While a combination of materials and structural engineering significantly enhances the neural probes’ long-term recording stability, their flexibility—the very characteristic that leads to the probes’ long-term advantages—also brings a new implantation challenge: probe buckling95. The critical force beyond which buckling happens can be described by the following equation:
where \(I\) is the area moment of inertia, \(E\) is the elastic modulus of the probe, \(L\) is the length of the probe, and \(\kappa\) is the effective length factor95. As can be seen from the equation, probes with small bending stiffness (a small product of \(I\) and \(E\)) also have a small critical buckling force and are, therefore, easily deformed during implantation. Alternative delivery strategies are necessary to ensure successful stereotaxic implantation while maintaining the structural and functional integrity of the probes.
Shuttle-facilitated delivery
Monolithically bonding a flexible probe to a stiff shuttle is a commonly used method to facilitate probe delivery (Fig. 6a). Prior to implantation, the flexible probe is attached to a stiff shuttle using an adhesive but dissolvable material. This shuttle provides the necessary mechanical support for tissue penetration. Once the probe reaches the desired depth, the adhesive material dissolves, allowing the probe and the shuttle to separate. The shuttle is then extracted, leaving the flexible probe in place.
a Shuttle-facilitated delivery (Reproduced with permission from ref. 97,98,99). b Mechanically adaptive implantation (Reproduced with permission from ref. 83,116,117) c Syringe injection of mesh electronics (Reproduced with permission from ref. 61) d Bio-inspired implantation (Reproduced with permission from ref. 121).
Silicon is a commonly used shuttle material. For example, researchers used a silicon shuttle with a carboxyl terminal self-assembled monolayer (SAM) modified surface to deliver a polymer probe110. SAM modified surface allows the temporary attachment of the probe to the shuttle. When the assembly was inside the brain, a drop of artificial cerebrospinal fluid (CSF) was applied to separate the probe from the shuttle110. Besides silicon, tungsten was also used for shuttles111. Tungsten’s high mechanical stiffness and strength allow for minimizing the size of the shuttle to reduce tissue damage during insertion.
In addition to rigid-materials-based shuttles, people also fabricated shuttles out of plastic polymers. For example, a 250 µm SU-8 shuttle was designed to deliver a soft polyimide probe by attaching them together with bio-dissolvable silk98. After the probe was inserted into the brain, artificial CSF was added to dissolve the silk followed by shuttle extraction98. Besides the shuttles that require removal after implantation, researchers have developed shuttles that dissolve within the brain following probe delivery112,113. One limitation of using an adhesive layer to bond the probe and shuttle together is that it increases the footprint of acute implantation damage. Moreover, this method suffers from the risk of unintentional probe-shuttle separation because the adhesive layer gets continuously dissolved during the implantation process114.
An alternative delivery method is to use mechanically tethered shuttles57. In this method, the shuttle is designed to hook onto an insertion loop located on the tip of the probe. For example, a tungsten-rhenium wire is used to hook onto a thread-like polyimide probe and is used to ‘stitch’ individual threads into brain tissues. The rapid retraction acceleration (up to 30,000 mm/s2) causes the separation of the probe from the shuttle99. Another similar example used a carbon fiber as a shuttle. The tip of the carbon fiber was micromilled down to a 2 µm diameter and 5 µm length micropost to engage the fiber to the microholes at the end of the probe97. During insertion, the shuttle dragged the probe to the desired depth, after which the shuttle was disengaged with the probe and retracted. The small footprint of the shuttle allows high-density probe implantation101. In a recent study, a tungsten shuttle was used to insert fiber bundles114. The probe fibers were self-assembled into bundles and bonded with biodegradable glue. The shuttle was mechanically coupled to the bundle by looping it through the tip of just one fiber in each bundle114.
Mechanically adaptive methods
Mechanically adaptive materials have been employed to temporarily enhance the performance of neural probes during implantation (Fig. 6b)115. For example, researchers enhanced the stiffness of hydrogel hybrid probes by drying them prior to implantation. After implantation, the probes rehydrated by absorbing water from surrounding tissues to restore their softness and low bending stiffness83.
In addition to changing the mechanical properties of the probe itself, mechanically adaptive coatings have been utilized to increase the overall stiffness of the probe momentarily. For example, by freezing a mesh electronics probe taken out from an aqueous solution with liquid nitrogen, the frozen probe gains sufficient rigidity to penetrate the brain116. After 150 freeze/thaw cycles, 86% of the sensors on the probe remained connected, demonstrating the reliability of this approach116. In another study, an elastocapillary self-assembled neural probe was withdrawn from molten PEG at 120 °C, causing PEG to quickly solidify in ambient air117. The surface tension and capillary forces bound the high-aspect ratio filaments together, forming a straight, thin, and stiff fiber assembly rigid enough to penetrate the mouse brain117.
Syringe injection
Syringe injection represents a distinct class of delivery method (Fig. 6c)61,118. This method involves loading the flexible mesh electronics into a glass needle using a syringe, stereotaxically inserting the needle into targeted positions of the tissues, and injecting the mesh electronics while simultaneously retracting the needle61,119. This approach allows for the implantation of devices with needle diameters as small as 100 µm120. The flexibility of syringe injection allows precise delivery of mesh electronics into other tissues, including retina, spinal cord, and vasculatures of the brain106,107,108. For example, a recent study demonstrated the delivery of mesh electronics into the rat’s brain through the neck blood vessels108. A micro-catheter loaded with the mesh electronics probe was connected to a syringe and inserted through the rat’s neck blood vessels to reach the bifurcation of the middle cerebral artery and anterior cerebral artery108.
Bio-inspired implantation
Inspired by mosquitoes, researchers used laser cutting to fabricate a 1 mm thick transparent polymethyl methacrylate (PMMA) insertion guide with a 250 µm slit to guide the flexible probe implantation (Fig. 6d). This bio-inspired method successfully reduced the probe’s tendency to buckle during implantation by providing additional lateral support121.
Conclusion and outlook
By combining soft materials engineering and structure innovations, researchers over the past decade have developed a new generation of implantable neural probes that resemble the mechanical, chemical, and topological properties of the brain. Importantly, these new generation probes overcome the long-term recording instability of conventional rigid probes, offering new opportunities to neuroscience, therapeutics, and BMI.
Looking forward, further conceptual innovations and technological advances are urgently needed. First, with chronic recording stability now surpassing the scale of years103, accelerated life testing that faithfully reflects the physiological microenvironments of the brain but uncovers potential modes of failure of the probes in a short amount of time needs to be developed for fast iteration and development of novel substrate materials and probe architectures. Second, while human brains contain 86 billion neurons, current flexible neural probes can only record from ~1000 channels due to the flexible probes’ incompatibility with CMOS technology. The development of CMOS-compatible manufacturing process for these next-generation neural probes is in high demand. Third, the density of recording electrodes hit a bottleneck as further reduction of the electrode’s size will lead to an interfacial electrical impedance that is too high to detect single-neuron APs with sufficient SNR. Innovations in electrode materials, surface morphology, surface coating, fundamentally new sensing mechanisms (e.g., field-effect transistor based device), and 3D device integration are urgently needed to further increase the recording density. Last but not least, new paradigms to allow for cell-type-specific recordings and stimulations will bring the much-needed capability that is missing from existing electrophysiology technologies.
In addition to further developments of the neural probes, innovations in delivery methods are also indispensable and urgent. First, besides chronic recording challenges, acute damage caused by probe implantation is a critical but often overlooked issue. Acute damage from probe implantation leads to tissue removal, BBB breaching, and local mechanical stress, which can result in disruption of the brain physiology over both short and long term33. Delivery methods that minimize this acute damage are crucial for preserving tissue integrity and ensuring sensing that faithfully reflects the brain’s dynamics in its native state. Second, current delivery methods only allow for a straight insertion trajectory into the brain, which can cause unnecessary tissue injury outside the targeted regions. Besides, the lack of controllability of the insertion path can result in breaches of major blood vessels and, therefore, hemorrhage. Minimally invasive delivery of these next-generation neural probes with controlled 3D trajectories that conform to the target brain regions’ complex 3D structures while avoiding major blood vessels can lead to high throughput monitoring inside the brain with minimum disruption to the brain’s functions, and bring a fundamentally new mapping capability of neural probes.
In summary, progress in materials engineering and probe designs over the past decades has led to a revolution in implantable neural probes that fundamentally overcome the chronic instability limitation of conventional probes and achieved stable long-term monitoring of the brain at single-neuron level. With further innovations in both device engineering and delivery methods, these next-generation flexible implantable neural probes will unlock the possibilities for exploring and controlling long-term changes of neural circuits with unprecedented resolution and stability, offering insights that were previously beyond reach.
References
Kandel, E. R., Schwartz, J. H., Jessell, T. M., Siegelbaum, S. A. & Hudspeth, A. J. Principles of Neural Science 5th edn (McGraw-Hill, 2013).
Cunningham, J. P. & Yu, B. M. Dimensionality reduction for large-scale neural recordings. Nat. Neurosci. 17, 1500–1509 (2014).
Buzsáki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Stevenson, I. H. & Kording, K. P. How advances in neural recording affect data analysis. Nat. Neurosci. 14, 139–142 (2011).
Lee, A. T., Chang, E. F., Paredes, M. F. & Nowakowski, T. J. Large-scale neurophysiology and single-cell profiling in human neuroscience. Nature 630, 587–595 (2024).
Gilja, V. et al. Challenges and opportunities for next-generation intracortically based neural prostheses. IEEE Trans. Biomed. Eng. 58, 1891–1899 (2011).
Nicolelis, M. A. & Lebedev, M. A. Principles of neural ensemble physiology underlying the operation of brain–machine interfaces. Nat. Rev. Neurosci. 10, 530–540 (2009).
Nunez, P. L. & Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG 2nd edn (Oxford University Press, 2006).
Niedermeyer, E. & Lopes da Silva, F. H. (eds) Electroencephalography: Basic Principles, Clinical Applications, and Related Fields 5th edn (Lippincott Williams & Wilkins, 2005).
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315 (2015).
Lee, J. M. et al. The ultra-thin, minimally invasive surface electrode array NeuroWeb for probing neural activity. Nat. Commun. 14, 7088 (2023).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Hubel, D. H. & Wiesel, T. N. Receptive fields of single neurones in the cat’s striate cortex. J. Physiol. 148, 574–591 (1959).
Hubel, D. H. & Wiesel, T. N. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 160, 106 (1962).
O’Keefe, J. & Dostrovsky, J. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971).
Hong, G. & Lieber, C. M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 20, 330–345 (2019).
Won, S. M., Song, E., Reeder, J. T. & Rogers, J. A. Emerging modalities and implantable technologies for neuromodulation. Cell 181, 115–135 (2020).
Rivnay, J., Wang, H., Fenno, L., Deisseroth, K. & Malliaras, G. G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 3, e1601649 (2017).
Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 1, 1–14 (2016).
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 1–16 (2017).
Chortos, A., Liu, J. & Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 15, 937–950 (2016).
Luan, L. et al. Recent advances in electrical neural interface engineering: minimal invasiveness, longevity, and scalability. Neuron 108, 302–321 (2020).
Luan, L., Yin, R., Zhu, H. & Xie, C. Emerging penetrating neural electrodes: in pursuit of large scale and longevity. Annu. Rev. Biomed. Eng. 25, 185–205 (2023).
Buzsáki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 7, 446–451 (2004).
Hubel, D. H. Tungsten microelectrode for recording from single units. Science 125, 549–550 (1957).
Dowben, R. M. & Rose, J. E. A metal-filled microelectrode. Science 118, 22–24 (1953).
McNaughton, B. L., O’Keefe, J. & Barnes, C. A. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J. Neurosci. Methods 8, 391–397 (1983).
Gray, C. M., Maldonado, P. E., Wilson, M. & McNaughton, B. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J. Neurosci. Methods 63, 43–54 (1995).
Campbell, P. K., Jones, K. E., Huber, R. J., Horch, K. W. & Normann, R. A. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 38, 758–768 (1991).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Polikov, V. S., Tresco, P. A. & Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
McConnell, G. C. et al. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 6, 056003 (2009).
Kozai, T. D., Jaquins-Gerstl, A. S., Vazquez, A. L., Michael, A. C. & Cui, X. T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 6, 48–67 (2015).
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010).
Salatino, J. W., Ludwig, K. A., Kozai, T. D. & Purcell, E. K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 1, 862–877 (2017).
Saxena, T. et al. The impact of chronic blood–brain barrier breach on intracortical electrode function. Biomaterials 34, 4703–4713 (2013).
Jackson, A. & Fetz, E. E. Compact movable microwire array for long-term chronic unit recording in cerebral cortex of primates. J. Neurophysiol. 98, 3109–3118 (2007).
Chestek, C. A. et al. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J. Neural Eng. 8, 045005 (2011).
Lee, H., Bellamkonda, R. V., Sun, W. & Levenston, M. E. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J. Neural Eng. 2, 81 (2005).
Karumbaiah, L. et al. The upregulation of specific interleukin (IL) receptor antagonists and paradoxical enhancement of neuronal apoptosis due to electrode induced strain and brain micromotion. Biomaterials 33, 5983–5996 (2012).
Moshayedi, P. et al. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials 35, 3919–3925 (2014).
Zhong, Y. & Bellamkonda, R. V. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 1148, 15–27 (2007).
Böhler, C. et al. Actively controlled release of Dexamethasone from neural microelectrodes in a chronic in vivo study. Biomaterials 129, 176–187 (2017).
Luo, X., Matranga, C., Tan, S., Alba, N. & Cui, X. T. Carbon nanotube nanoreservior for controlled release of anti-inflammatory dexamethasone. Biomaterials 32, 6316–6323 (2011).
Kim, D.-H. & Martin, D. C. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials 27, 3031–3037 (2006).
Zhang, Z., Nong, J. & Zhong, Y. Antibacterial, anti-inflammatory and neuroprotective layer-by-layer coatings for neural implants. J. Neural Eng. 12, 046015 (2015).
Zhong, Y. & Bellamkonda, R. V. Controlled release of anti-inflammatory agent α-MSH from neural implants. J. Control Release 106, 309–318 (2005).
He, W., McConnell, G. C., Schneider, T. M. & Bellamkonda, R. V. A novel anti-inflammatory surface for neural electrodes. Adv. Mater. 19, 3529–3533 (2007).
Liu, S. et al. A nanozyme-based electrode for high-performance neural recording. Adv. Mater. 36, 2304297 (2024).
Jeong, J. et al. Biomimetic design of biocompatible neural probes for deep brain signal monitoring and stimulation: super static interface for immune response-enhanced contact. Adv. Funct. Mater. 35, 2417727 (2025).
He, W., McConnell, G. C. & Bellamkonda, R. V. Nanoscale laminin coating modulates cortical scarring response around implanted silicon microelectrode arrays. J. Neural Eng. 3, 316 (2006).
Oakes, R. S., Polei, M. D., Skousen, J. L. & Tresco, P. A. An astrocyte derived extracellular matrix coating reduces astrogliosis surrounding chronically implanted microelectrode arrays in rat cortex. Biomaterials 154, 1–11 (2018).
Golabchi, A., Woeppel, K. M., Li, X., Lagenaur, C. F. & Cui, X. T. Neuroadhesive protein coating improves the chronic performance of neuroelectronics in mouse brain. Biosens. Bioelectron. 155, 112096 (2020).
Kolarcik, C. L. et al. Elastomeric and soft conducting microwires for implantable neural interfaces. Soft Matter 11, 4847–4861 (2015).
Scholten, K. & Meng, E. Materials for microfabricated implantable devices: a review. Lab Chip 15, 4256–4272 (2015).
Rousche, P. J. et al. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 48, 361–371 (2001).
Zhang, S. et al. A removable insertion shuttle for ultraflexible neural probe implantation with stable chronic brain electrophysiological recording. Adv. Mater. Interfaces 7, 1901775 (2020).
Lv, S. et al. Tentacle microelectrode arrays uncover soft boundary neurons in hippocampal CA1. Adv. Sci. 11, 2401670 (2024).
Malekoshoaraie, M. H. et al. Fully flexible implantable neural probes for electrophysiology recording and controlled neurochemical modulation. Microsyst. Nanoeng. 10, 91 (2024).
Cointe, C. et al. Scalable batch fabrication of ultrathin flexible neural probes using a bioresorbable silk layer. Microsyst. Nanoeng. 8, 21 (2022).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
He, F., Lycke, R., Ganji, M., Xie, C. & Luan, L. Ultraflexible neural electrodes for long-lasting intracortical recording.iScience 23, 101387 (2020).
Wang, X. et al. Ultraflexible neural electrodes enabled synchronized long-term dopamine detection and wideband chronic recording deep in brain. ACS Nano 18, 34272–34287 (2024).
Hassler, C., Boretius, T. & Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 49, 18–33 (2011).
Bettinger, C. J. et al. Recent advances in neural interfaces—materials chemistry to clinical translation. MRS Bull. 45, 655–668 (2020).
Kim, B. J. & Meng, E. Micromachining of parylene C for bioMEMS. Polym. Adv. Technol. 27, 564–576 (2016).
Gablech, I. & Głowacki, E. D. State-of-the-art electronic materials for thin films in bioelectronics. Adv. Electron. Mater. 9, 2300258 (2023).
Mercanzini, A. et al. Demonstration of cortical recording using novel flexible polymer neural probes. Sens. Actuators A Phys. 143, 90–96 (2008).
Weltman, A., Yoo, J. & Meng, E. Flexible, penetrating brain probes enabled by advances in polymer microfabrication. Micromachines 7, 180 (2016).
Park, S. I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 33, 1280–1286 (2015).
Tybrandt, K. et al. High-density stretchable electrode grids for chronic neural recording. Adv. Mater. 30, 1706520 (2018).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Zhang, Y. et al. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Sci. Adv. 5, eaaw5296 (2019).
Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Wen, X. et al. Flexible, multifunctional neural probe with liquid metal enabled, ultra-large tunable stiffness for deep-brain chemical sensing and agent delivery. Biosens. Bioelectron. 131, 37–45 (2019).
Rezaei, S., Xu, Y. & Pang, S. Control of neural probe shank flexibility by fluidic pressure in embedded microchannel using PDMS/PI hybrid substrate. PLoS ONE 14, e0220258 (2019).
Momin, M. 3D-printed flexible neural probes for recordings at single-neuron level. Device 2, 100519 (2024).
Zhang, E. N. et al. Mechanically matched silicone brain implants reduce brain foreign body response. Adv. Mater. Technol. 6, 2000909 (2021).
Le Floch, P. et al. 3D spatiotemporally scalable in vivo neural probes based on fluorinated elastomers. Nat. Nanotechnol. 19, 319–329 (2024).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Liang, Q. et al. Highly stretchable hydrogels as wearable and implantable sensors for recording physiological and brain neural signals. Adv. Sci. 9, 2201059 (2022).
Spencer, K. C. et al. Characterization of mechanically matched hydrogel coatings to improve the biocompatibility of neural implants. Sci. Rep. 7, 1952 (2017).
Park, S. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat. Commun. 12, 3435 (2021).
Ren, X. et al. Uniaxial extending neural probes for bleeding-absent implantation. npj Flex. Electron 8, 36 (2024).
Jiang, Y. et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 375, 1411–1417 (2022).
Castagnola, V. et al. Parylene-based flexible neural probes with PEDOT coated surface for brain stimulation and recording. Biosens. Bioelectron. 67, 450–457 (2015).
Ludwig, K. A., Uram, J. D., Yang, J., Martin, D. C. & Kipke, D. R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly (3, 4-ethylenedioxythiophene)(PEDOT) film. J. Neural Eng. 3, 59 (2006).
Yuk, H. et al. 3D printing of conducting polymers. Nat. Commun. 11, 1604 (2020).
Strakosas, X. et al. Metabolite-induced in vivo fabrication of substrate-free organic bioelectronics. Science 379, 795–802 (2023).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Park, S. et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 20, 612–619 (2017).
Guo, Y. et al. Polymer composite with carbon nanofibers aligned during thermal drawing as a microelectrode for chronic neural interfaces. ACS Nano 11, 6574–6585 (2017).
Qi, J. et al. Liquid metal–polymer conductor-based conformal cyborg devices. Chem. Rev. 124, 2081–2137 (2024).
Park, Y.-G. et al. In-vivo integration of soft neural probes through high-resolution printing of liquid electronics on the cranium. Nat. Commun. 15, 1772 (2024).
Apollo, N. V. et al. Gels, jets, mosquitoes, and magnets: a review of implantation strategies for soft neural probes. J. Neural Eng. 17, 041002 (2020).
Kozai, T. D. Y. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 11, 1065–1073 (2012).
Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 3, e1601966 (2017).
Kim, T.-I. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 21, e16194 (2019).
Hanson, T. L., Diaz-Botia, C. A., Kharazia, V., Maharbiz, M. M. & Sabes, P. N. The “sewing machine” for minimally invasive neural recording. Preprint at bioRxiv https://doi.org/10.1101/578542 (2019).
Wei, X. et al. Nanofabricated ultraflexible electrode arrays for high-density intracortical recording. Adv. Sci. 5, 1700625 (2018).
Liu, Y. et al. A high-density 1,024-channel probe for brain-wide recordings in non-human primates. Nat. Neurosci 27, 1–12 (2024).
Fu, T.-M. et al. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882 (2016).
Fu, T.-M., Hong, G., Viveros, R. D., Zhou, T. & Lieber, C. M. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc. Natl. Acad. Sci. USA 114, E10046–E10055 (2017).
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
Hong, G. et al. A method for single-neuron chronic recording from the retina in awake mice. Science 360, 1447–1451 (2018).
Lin, D., Lee, J. M., Wang, C., Park, H.-G. & Lieber, C. M. Injectable ventral spinal stimulator evokes programmable and biomimetic hindlimb motion. Nano Lett. 23, 6184–6192 (2023).
Zhang, A. et al. Ultraflexible endovascular probes for brain recording through micrometer-scale vasculature. Science 381, 306–312 (2023).
Yang, X. et al. Laminin-coated electronic scaffolds with vascular topography for tracking and promoting the migration of brain cells after injury. Nat. Biomed. Eng. 7, 1282–1292 (2023).
Kozai, T. D. Y. & Kipke, D. R. Insertion shuttle with carboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain. J. Neurosci. Methods 184, 199–205 (2009).
Zhao, Z. et al. Parallel, minimally-invasive implantation of ultra-flexible neural electrode arrays. J. Neural Eng. 16, 035001 (2019).
Oh, S. et al. A stealthy neural recorder for the study of behaviour in primates. Nat. Biomed. Eng. 9, 882-895 (2025).
Roh, Y. et al. Transient shuttle for a widespread neural probe with minimal perturbation. npj Flex. Electron 8, 40 (2024).
Yasar, T. B. et al. Months-long tracking of neuronal ensembles spanning multiple brain areas with ultra-flexible tentacle electrodes. Nat. Commun. 15, 4822 (2024).
Wang, S. et al. Mechanically adaptive and deployable intracortical probes enable long-term neural electrophysiological recordings. Proc. Natl. Acad. Sci. USA 121, e2403380121 (2024).
Xie, C. et al. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 14, 1286–1292 (2015).
Guan, S. et al. Elastocapillary self-assembled neurotassels for stable neural activity recordings. Sci. Adv. 5, eaav2842 (2019).
Thielen, B. & Meng, E. A comparison of insertion methods for surgical placement of penetrating neural interfaces. J. Neural Eng. 18, 041003 (2021).
Hong, G. et al. Syringe injectable electronics: precise targeted delivery with quantitative input/output connectivity. Nano Lett. 15, 6979–6984 (2015).
Viveros, R. D. et al. Advanced one-and two-dimensional mesh designs for injectable electronics. Nano Lett. 19, 4180–4187 (2019).
Shoffstall, A. J. et al. A mosquito inspired strategy to implant microprobes into the brain. Sci. Rep. 8, 122 (2018).
Ferguson, J. E., Boldt, C. & Redish, A. D. Creating low-impedance tetrodes by electroplating with additives. Sens. Actuators A Phys. 156, 388–393 (2009).
Kim, S.-J., Manyam, S. C., Warren, D. J. & Normann, R. A. Electrophysiological mapping of cat primary auditory cortex with multielectrode arrays. Ann. Biomed. Eng. 34, 300–309 (2006).
Vetter, R. J., Williams, J. C., Hetke, J. F., Nunamaker, E. A. & Kipke, D. R. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans. Biomed. Eng. 51, 896–904 (2004).
Biran, R., Martin, D. C. & Tresco, P. A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 195, 115–126 (2005).
Lotfi Marchoubeh, M. et al. Miniaturized probe on polymer SU-8 with array of individually addressable microelectrodes for electrochemical analysis in neural and other biological tissues. Anal. Bioanal. Chem. 413, 6777–6791 (2021).
Lecomte, A. et al. Silk and PEG as means to stiffen a parylene probe for insertion in the brain: toward a double time-scale tool for local drug delivery. J. Micromech. Microeng. 25, 125003 (2015).
Vitale, F., Summerson, S. R., Aazhang, B., Kemere, C. & Pasquali, M. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano 9, 4465–4474 (2015).
Acknowledgements
We thank Sara Kacmoli, Christopher Warren, Mengyang Li, Yanke Yuan, and Yuqing Qiu for stimulating discussions. We gratefully acknowledge funding from Princeton Alliance for Collaborative Research and Innovation, Princeton Catalysis Initiative, School of Engineering and Applied Science, and departmental start-up funds via the Department of Electrical and Computer Engineering and the Omenn–Darling Bioengineering Institute at Princeton University.
Author information
Authors and Affiliations
Contributions
F.L. conceived the study, collected the literature, and wrote the manuscript; T.-M.F. conceived the study, reviewed and edited the manuscript, and supervised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, F., Fu, TM. Material and design strategies for chronically-implantable neural probes. NPG Asia Mater 17, 32 (2025). https://doi.org/10.1038/s41427-025-00613-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41427-025-00613-8