Abstract
Stable, tough, and functional adhesion systems are urgently needed for a sustainable society. As a resolution, supramolecular scientists have introduced reversible and movable crosslinked materials into adhesion systems. Reversible crosslinks can repeatedly associate and dissociate. Therefore, reversible crosslinked materials show self-healing and stimuli-responsive properties. Moreover, movable crosslinks are topological crosslinks in which the polymer chains penetrate the cavities of cyclic molecules. The sliding of the movable crosslinks with deformation enabled the achievement of materials showing high toughness and self-relaxation. Adhesion systems with reversible and movable crosslinks have improved adhesion and cohesion, stability, and functionality. This novel concept for the design of adhesion systems is expected to increase the lifetime of adhesives and ameliorate environmental problems.
Similar content being viewed by others
Introduction
Adhesives are some of the most widely used tools to bond two similar or dissimilar objects together and have long been studied in chemical research. To date, various polymetric adhesives have been invented and commercialized, such as cyanoacrylate adhesives [1,2,3,4,5], silicone adhesives [6, 7], polyvinyl acetate adhesives [8, 9], acrylic adhesives [10], and epoxy adhesives [11, 12]. These conventional adhesives have been proven to exhibit good adhesion strength; however, their stability and recyclability are still challenging. It is evident from the recent global warming phenomenon that the environmental pollution caused by adhesive waste continues to be a problem for society. In response to the demand for a sustainable society, adhesives research is shifting toward long-lasting and functional adhesive systems. One promising approach involves tailoring the primary structure of adhesive polymers. For example, block copolymer-based adhesives have emerged as a powerful strategy [13], enabling the introduction of functional groups for improved strength [14,15,16], unique functionalities [17,18,19], and degradability [20, 21]. The microphase-separated structures of these materials further enhance adhesive performance [22]. Similarly, liquid crystal elastomers (LCEs), known primarily as actuators [23,24,25,26], are now being explored for their photoresponsive [27,28,29] and dynamic adhesion [30,31,32,33,34,35,36] properties. Another approach focuses on integrating secondary structures into adhesives. Natural polymers such as cellulose have been incorporated into adhesive systems [37, 38], achieving high performance [39,40,41,42,43] and reusability [44, 45]. Furthermore, interpenetrating polymer networks (IPNs) and semi-IPNs have proven particularly effective in bioadhesion [46,47,48,49], offering enhanced mechanical properties [50,51,52,53] and versatile functionalities [54,55,56,57] through secondary network integration.
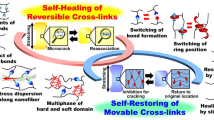
Recently, adhesion researchers have turned their attention to supramolecular science [58,59,60,61]. Supramolecular materials are expected to meet the recent demand for waste reduction, such as self-healing, stimuli responsive, self-restoring, and energy dissipation properties. To propose an adhesion system, a new strategy is to incorporate supramolecular science techniques in adhesion research to achieve recoverable, demountable, recyclable, and tough adhesives (Fig. 1).
In this review, examples of research studies based on the design of reversible or movable crosslinks in supramolecular materials are presented. Then, new functional polymer adhesion methods that combine supramolecular science and adhesion science are discussed.
Materials with reversible crosslinks
Figure 2 shows the timeline of supramolecular chemistry and material development [62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84]. The design of materials incorporating these supramolecular structures is effective for enhancing and controlling mechanical and functional properties [85,86,87].
Materials with reversible crosslinks are widely used to achieve self-healing properties. Some self-healing materials utilize noncovalent interactions, such as hydrogen bonding [82, 88,89,90,91,92,93], metal-ligand complexes [79, 94,95,96], host‒guest complexes [80, 83, 97,98,99,100,101,102,103,104,105,106], ionic bonding [78, 81, 107,108,109], and π‒π interactions [77, 110]. Other materials utilize dynamic covalent bonding, such as Diels-Alder reactions [111], olefin metathesis reactions [112], imine bonds [113], and disulfide bonds [114]. Reversible crosslinks can be reformed spontaneously or in response to stimuli such as heat, humidity, or pH to repair physical damage and restore function.
The following sections describe the material design and evaluation of materials with typical types of reversible crosslinks.
Hydrogen bonding and metal-ligand complexes
Hydrogen bonding is one of the most well-known noncovalent interactions that has been long recognized for its importance in a wide range of materials and applications. In 1989, the supramolecular materials based on hydrogen bonds were reported by Kato and Frechet [88]. In 1997, Meijer and colleagues synthesized a trifurcated polymer end-modified with 2-ureido-4-pyrimidone, which forms dimers through the robust interaction of four complementary hydrogen bonds, resulting in a high association constant (Ka > 106 M−1) (Table 1) [92]. These dimers, formed at the termini of each polymer chain, self-assemble into a polymeric network thermodynamically. Such thermoplastic behavior shows potential for use in heat-melting adhesives. Later, in 2008, Leibler et al. introduced supramolecular materials based on hydrogen bonding derived from fatty acids and urea [93]. By condensing bifunctional and trifunctional fatty acids with diethylenetriamine and subsequently reacting them with urea, they generated oligomers capable of hydrogen bonding. When dodecane was incorporated as a plasticizer, the resulting materials exhibited rubber-like elasticity and self-healing at ambient temperature. To date, the majority of reported self-healing materials have been soft and elastic. However, in 2018, Aida and colleagues developed a hard self-healing material comprised by thiourea and ethylene glycol [82]. By utilizing relatively low-molecular-weight polymers, they achieved enhanced segmental mobility along with disordered hydrogen bonding. This combination enabled the material to exhibit both self-healing capabilities and a high Young’s modulus (>1 GPa), which was achieved through the effective exchange of numerous hydrogen bonds.
Another noncovalent interaction is metal coordination bonding. The high degree of tunability is a notable characteristic of metal coordination bonding that the stem from the wide variety of possible combinations of metal species and ligands. These bonds can be tailored across a wide spectrum of binding energies, ranging from approximately 100 kJ/mol to approximately 300 kJ/mol. Lehn et al. reported supramolecular polymers based on Zn²⁺ or Ni²⁺ coordination with telechelic polydimethylsiloxanes functionalized with acyl hydrazone-pyridine or acyl hydrazone-quinoline groups [94]. When these two different supramolecular polymer films were stacked and heated at 50 °C for 24 hours, random copolymer films were formed via metal-ligand exchange.
Promotion of self-healing by external stimuli
The conditions required for self-healing—such as time and temperature—are governed by the dissociation and reassociation dynamics of the metal-ligand complexes. To facilitate rapid self-healing under mild conditions, researchers have investigated the promotion of bond exchange via external stimuli. Rowan et al. developed a supramolecular polymer with Zn²⁺ coordination to ethylene–butylene copolymer functionalized at both ends with 2,6-bis(1’-methylbenzimidazolyl)pyridine [79]. When irradiated with UV light, the metal coordination bonds produce heat through excitation, accelerating the bond association and dissociation processes and thus enhancing self-healing.
Control of viscoelastic properties
The viscoelastic behavior of these materials can be modulated by toggling the association and dissociation of the metal-ligand complexes, which facilitates self-healing. During dissociation, the fluidity of the material increases, allowing it to fill any gaps or damage, followed by reassociation to complete the repair. Waite et al. demonstrated a pH-responsive sol-gel transition material that exploits the pH-dependent changes in the coordination amount of catechol with Fe³⁺. The material, crosslinked by a combination of Fe³⁺ and terminal catechol-modified four-chain polyethylene glycol, behaves as a fluid under acidic conditions but forms a hydrogel at alkaline pH (pH > 8) [95].
Switching kinetics of metal cages
The exchange kinetics of metal-ligand complexes in metal-organic cage (MOC) structures are influenced by the material’s topology. Self-healing can thus be controlled by incorporating units that alter their binding angles in response to external stimuli. Johnson et al. reported an organogel in which the MOC structure of a small Pd₃L₆ ring was linked to polyethylene glycol [96]. The small Pd₃L₆ rings exhibited readhesion through fast ligand exchange. Upon UV irradiation, the MOC structures transformed from small Pd₃L₆ rings to Pd₂₄L₄₈ rhombicuboctahedra due to changes in the bond angles. These larger rhombicuboctahedral structures did not exhibit readhesion, as their ligand exchange kinetics were significantly slower. Upon subsequent irradiation with visible light, the structures reverted from Pd₂₄L₄₈ rhombicuboctahedra back to Pd₃L₆ rings, thereby enabling control over reattachment properties by modulating ligand exchange kinetics.
Host-guest interactions
The host-guest interaction refers to a specific type of supramolecular interaction in which a macrocyclic host molecule encapsulates a guest molecule of appropriate size and geometry, forming an inclusion complex. Macrocyclic compounds such as crown ethers, cucurbit[n]urils (CB[n]s) [115], calix[n]arenes [116], pillar[n]arenes [117], and cyclodextrins (CDs) [118] have been extensively utilized as host molecules. The stability of these inclusion complexes is governed by the association constants, which are influenced by the size, shape, and charge characteristics of the guest molecule. Material design leveraging molecular recognition is a key aspect of host-guest interactions. This molecular recognition facilitates macroscopic self-assembly and sol-gel transitions, providing inspiration for the development of self-healing materials based on host-guest chemistry. This section focuses on two different approaches to the design of self-healing materials via host-guest interactions.
Host and guest polymers
In this approach, host or guest molecules are covalently attached to polymer chains. By blending the resulting host polymers with guest polymers, inclusion complexes are formed between the host and guest residues along the polymer side chains. This leads to the creation of supramolecular materials with reversible crosslinks based on these inclusion complexes. By selecting guest molecules whose association constants change in response to external stimuli, it is possible to fabricate stimulus-responsive materials. For example, redox-responsive hydrogels were synthesized by combining β-cyclodextrin (βCD)-modified poly(acrylic acid) with ferrocene (Fc)-modified poly(acrylic acid) (Table 2) [98]. The high association constant between βCD and Fc (Ka = 1.1 × 103 M−1) facilitated the formation of inclusion complexes with reversible crosslinks. Upon cutting and rejoining, the hydrogel regained 84% of its original strength after 24 hours. Upon exposure to an oxidant (sodium hypochlorite), the hydrogel dissociated into a solution, as the oxidized form of Fc (Fc+) exhibited low affinity for βCD, leading to the breakdown of inclusion complexes. The addition of a reductant (glutathione) reduced Fc+ to Fc, reforming the inclusion complexes and restoring the hydrogel.
Unlike cyclodextrins, which are less favorable toward cationic guests, crown ethers can form inclusion complexes with cationic species. The unshared electron pairs on the oxygen atoms of crown ethers stabilize the cationic guests. pH-responsive organogels were prepared by mixing dibenzo[24]crown-8 (DB24C8)-modified poly(methyl methacrylate) with bisammonium crosslinkers [99]. These organogels exhibited reversible gel-sol and sol-gel transitions upon the addition of a base (triethylamine) and an acid (trifluoroacetic acid), as well as self-healing capabilities, with surface cracks vanishing within 4 minutes.
Pillar[n]arenes serve as host molecules for cationic or electron-deficient guest molecules. Redox-responsive organogels were prepared by mixing pillar[6]arene-modified poly(methyl acrylate) with Fc+-modified polystyrene [100]. These organogels displayed gel-sol and sol-gel transitions upon treatment with a reductant (hydrazine) and an oxidant {silver tetrakis[3,5-bis(trifluoromethyl)phenyl]borate}. As pillar[n]arenes are relatively recent macrocyclic molecules, reports on self-healing materials using them remain limited. However, their potential lies in exploiting unique properties such as self-assembly and planar chirality in host-guest systems.
In the preparation of supramolecular elastomers by drying solutions of host-guest polymers, both the chemical structure and processing techniques are crucial. Elastomers obtained by drying a solution of peracetylated βCD-modified poly(ethyl acrylate) and adamantane-modified poly(ethyl acrylate) following ball-milling treatment demonstrated higher toughness compared to the elastomers obtained by the traditional stirring and casting methods [83]. Scratches made with a hard brush on the surface of the elastomer disappeared upon heating. Ball-milling induced strong mechanical stress that disentangled the polymer chains, increasing their mobility. This enhanced mobility facilitated the reformation of inclusion complexes, improving their mechanical strength and self-healing properties.
Another family of macrocyclic molecules, CB[n]s, shows distinctive host-guest chemistry. While CB[5], CB[6], and CB[7] form inclusion complexes with single guest molecules, CB[8] can simultaneously encapsulate two guest molecules, forming 1:1:1 heteroternary complexes with different guest species. Supramolecular materials can be formed from native CB[8] without requiring complex modifications. For example, mixing CB[8] with viologen-modified poly(2-hydroxyethyl acrylate) and naphthalene-modified poly(N,N-dimethyl acrylamide) yielded supramolecular hydrogels with thermally reversible crosslinks [101]. These hydrogels exhibited gel-sol and sol-gel transitions upon heating and cooling. CB[n]s can also form homogeneous 1:2 complexes with two identical guest molecules. Following the formation of a 1:2 inclusion complex between CB[8] and guest monomers, copolymerization with acrylamide produced supramolecular networks [102]. These hydrogels exhibited high fracture strains (over 10,000%) and demonstrated complete self-healing at room temperature.
Copolymers of host and guest monomers
Efficient formation of inclusion complexes between host and guest units within the material is essential for achieving effective self-healing. A simple mixing of host polymers with guest polymers is often suboptimal because the formation of inclusion complexes may be incomplete. As inclusion complexes form, the viscosity of the system increases, leading to reduced molecular mobility before all the host and guest moieties can interact. To address this issue, an alternative method involving the polymerization of the inclusion complexes between the host and guest monomers has been developed. This approach allows insoluble guest monomers to be solubilized through complexation with host monomers, resulting in a polymer chain that incorporates both host and guest units.
For example, copolymerization between the inclusion complex of βCD monomers and adamantane monomers with acrylamide produced supramolecular hydrogels (Table 3) [80, 103]. The resulting hydrogel was cut into two pieces and rejoined, and after 24 hours, the contact surface disappeared, with the hydrogel recovering 99% of its initial mechanical strength. Polymerization of inclusion complexes significantly improves healing efficiency significantly, even though supramolecular materials with poly(acrylamide) main chains do not exhibit self-healing properties under dry conditions and require humid conditions to function effectively.
To overcome this problem, poly(methoxy triethylene glycol acrylate) (polyTEGA) was employed as the main chain polymer. With a lower glass transition temperature (Tg of polyTEGA = -50 °C compared to the Tg of polyacrylamide = 165 °C), the self-healing xerogels formed using polyTEGA demonstrated notable self-healing properties [105]. These xerogels regained 60% of their initial strength at 100 °C after standing for 24 hours. Furthermore, a high association constant between the guest unit and βCD contributed to improved healing performance.
In addition to mechanical healing, it is possible to develop materials that change color in response to stimuli by using guest molecules with different absorption spectra in the free and inclusion-complex states. For example, phenolphthalein appears purple in basic aqueous solutions and becomes colorless upon complexation with βCD. Stimulus-responsive hydrogels were created using inclusion complexes between βCD and phenolphthalein as the crosslinks [104]. These hydrogels remained colorless in basic KH₂PO₄/NaOH buffer (pH = 8), but upon heating to 87 °C, the inclusion complex dissociated, turning the gel purple. Upon cooling, the gel returned to its colorless state as the inclusion complex reformed. This hydrogel also exhibited color changes when exposed to competing guest molecules or electrical currents (Joule heating). Additionally, self-healing xerogels were produced by removing the solvent.
Bulk polymerization without solvents is another approach for the preparation of dry self-healing materials. In this method, the inclusion complex between the host and guest monomers must dissolve in the liquid main chain monomer. However, conventional CD monomers with numerous hydroxyl groups are insoluble in most hydrophobic main chain monomers [106]. To address this issue, hydrophobic CD monomers with fully acetylated hydroxyl groups were used to fabricate self-healing elastomers. The bulk copolymerization of peracetylated CD monomers with 2-ethyladamantyl acrylate and ethyl acrylate was carried out, resulting in an elastomer that, when cut into two pieces and rejoined, recovered 95% of its initial strength within 4 hours at 80 °C. Additionally, this elastomer can be recycled. The toluene-swollen sample was ground using a ball mill to form a slurry, and upon removing the toluene, a recycled sample with the same properties as the original sample was obtained.
Materials with movable crosslinks
Movable crosslinks consist of a mechanically interlocked architecture where polymer chains thread through macrocyclic molecules, forming a dynamic and flexible network. These movable crosslinked materials exhibit enhanced toughness, as stress is efficiently dispersed through the sliding motion of macrocyclic molecules along the polymer chains. This sliding motion allows the material to distribute mechanical stress more evenly, preventing localized failure. Additionally, self-restoring properties are anticipated due to the entropic elasticity of the system, which enables the movable crosslinks to return to their original positions once the stress is removed.
This section highlights typical approaches for the fabrication of movable crosslinked materials. By integrating macrocyclic compounds such as cyclodextrins, crown ethers, or cucurbiturils, the polymer network can achieve both flexibility and resilience. The dynamic nature of these movable crosslinks is crucial for applications that require materials with superior mechanical performance and self-restoring capability.
Polyrotaxane as a starting material
Polyrotaxane is a macromolecular structure in which macrocyclic molecules are threaded onto a linear polymer (referred to as the “axle”), with bulky stoppers at both ends to prevent the macrocycles from detaching [75, 119,120,121,122,123]. In 1990, α-cyclodextrins (αCDs) were first threaded onto polyethylene glycol (PEG) chains, forming poly-pseudorotaxanes. Later, in 1992, a full polyrotaxane was synthesized through an end-capping reaction of αCD-PEG bisamine complexes using 2,4-dinitrofluorobenzene. Previous reports have shown that rotaxane structures can strongly enhance the toughness of materials [124, 125].
Movable crosslinked materials can be created by using polyrotaxanes as crosslinkers. Ito et al. introduced a “topological gel” by crosslinking two αCDs in different polyrotaxanes via cyanuric chloride [76, 125] (Table 4). In contrast to the conventional covalent crosslinks that concentrate stress in localized areas, these movable crosslinks act as pulleys, distributing tension evenly along the polymer chains. This stress dispersion, often referred to as the “pulley effect,” prevents polymer chain rupture, enhancing the toughness of the material. The pulley effect has been applied in industry to scratch-healing coatings, where movable crosslinks inhibit crack formation, allowing the coating to revert to its original state due to its elasticity. Movable crosslinked materials utilizing polyrotaxanes of pillar[5]arene were also developed by Ogoshi et al. [126].
The sliding motion of polyrotaxane-based movable crosslinks further promotes the reformation of reversible crosslinks, improving self-healing properties. For example, self-healing materials were prepared by copolymerizing acrylamide with 4-vinylphenylboronic acid in the presence of polyrotaxane with hydroxypropyl-modified αCDs [127] (Table 5). The resulting hydrogel contained dynamic covalent bonds between boronic acid and the diol groups of the αCDs in the polyrotaxane. These hydrogels exhibited a significantly higher healing efficiency (~100% within 15 minutes) than the reference hydrogels with dynamic covalent bonds involving linear polysaccharides (~20% in 60 minutes). The superior healing was attributed to the enhanced mobility provided by the movable crosslinks. Additionally, self-healing hydrogels based on αCD in polyrotaxane crosslinked by inclusion complexes between βCD and adamantane have also been reported [128]. This approach leverages the reversible and dynamic nature of host-guest interactions to further enhance the self-healing of materials.
Formation of rotaxane/polyrotaxane structures in the main chain
The formation of rotaxane or polyrotaxane structures on the main polymer chain can be divided into two main methods:
Polycondensation after the formation of the pseudorotaxane/rotaxane structure
In this approach, the pseudorotaxane or rotaxane structure is formed first, followed by polycondensation. For example, photoresponsive movable crosslinked materials were fabricated using a pseudo[2]rotaxane consisting of lysine-modified αCD and diamine-modified azobenzene (Azo) [129] (Table 6). Polycondensation with succinimidyl-modified PEG produced hydrogels with movable crosslinks. Azobenzene showed photoinduced isomerization between its trans and cis forms. The trans form exhibited high affinity for αCD, while the cis form showed low affinity for αCD. When exposed to UV light (wavelength: 365 nm), isomerization from trans-Azo to cis-Azo led to the unthreading of αCD from the axle, causing the xerogels to bend toward the light source as the CD units slid along the PEG chain. The self-restoration capabilities of movable crosslinked materials can be enhanced by incorporating high-affinity guest molecules into the axle of the [2]rotaxane. Yan et al. designed materials with [2]rotaxane structures using a dibenzo-24-crown-8 ring and a dibenzylammonium salt as the axle [130]. The ring and axle were functionalized with vinyl groups, and a thiol-ene click reaction with 3,6-dioxa-1,8-octanediol was carried out to create the movable crosslinked materials. The macrocyclic rings favored the cationic guest units, leading to dissociation when mechanical stress was applied. This dissociation dissipated stress, resulting in a tougher material. After the stress was removed, host-guest interactions facilitated the reassociation of the macrocycles, allowing rapid recovery of the original shape of the material.
Copolymerization between macrocyclic monomers and main chain monomers
The second method involves the formation of movable crosslinks by copolymerizing macrocyclic monomers and main chain monomers. For example, movable crosslinked elastomers were created by bulk copolymerization of hydrophobic CD monomers with alkyl acrylates [131]. The formation of movable crosslinks depended on the cavity size of the CD and the size of the main chain monomer. When ethyl acrylate (EA) and peracetylated γCD monomers were copolymerized, the poly(EA) chains threaded through the γCD cavities, resulting in movable crosslinks. By contrast, the copolymerization of butyl acrylate with peracetylated βCD monomers resulted in fewer movable crosslinks, demonstrating the importance of the macrocyclic cavity size and the main chain monomer in achieving effective crosslinking. The resulting elastomer exhibited higher toughness compared to poly(EA) with conventional covalent crosslinks. Hydrophilic movable crosslinked materials were also prepared by the copolymerization of CD monomers with liquid acrylamide monomers such as N,N-dimethyl acrylamide (DMAAm), 4-acryloylmorpholine, and N,N-dimethylaminopropyl acrylamide [132]. While conventional methods of mixing CDs with poly(alkyl acrylate) or poly(acrylamide) derivatives in water do not yield polyrotaxane/poly-pseudorotaxane structures, these copolymerization methods enable the preparation of such structures that cannot be achieved through traditional techniques. Using similar methods, movable crosslinked materials with hydrophilic main chains and hydrophobic movable crosslinks were also prepared. The -OH groups on the CDs were converted to -OAc groups. The lack of hydrogen bonding between the CD units and the polymer main chains enhances the mobility of the movable crosslinks. Under appropriate water content, the materials exhibited enhanced toughness due to multiple relaxations of movable cross-links and reversible hydrophobic interactions [133].
Supramolecular adhesion systems
In this section, the authors introduce supramolecular adhesion systems using reversible and movable crosslinked materials. Adhesion systems have two key components: adhesives and adherends (substrates). Achieving optimal performance requires a balanced focus on two critical factors: the interaction between the adhesive and the adherends and the cohesive interactions within the adhesive itself. These combined interactions are vital for ensuring strong and reliable adhesion systems. Here, we focus on the adhesion systems on the surfaces of hard substrates, including polymer (PMMA, nylon, etc.), inorganic (glass, silicon wafers, etc.), and metal (stainless steel, copper, etc.) substrates.
Adhesion systems using reversible crosslinked materials
In the previous section, the authors discussed reversible crosslinked materials, which exhibit self-healing properties, robust mechanical performance, and multifunctional capabilities. Owing to the reversibility of the crosslinks, a variety of adhesives have been developed on the basis of mechanisms such as hydrogen bonding [134,135,136,137,138], metal-ligand complexes [139,140,141,142,143,144,145], and host-guest interactions [146].
Two primary strategies can be exploited to construct adhesion systems using reversible crosslinked materials. The first strategy involves the introduction of these crosslinks between the adherends (substrates) and the adhesives, while the second strategy involves the use of the reversible crosslinked materials directly as adhesives. The former strategy emphasizes maximizing the adhesion at the interface between the adhesives and the surfaces, whereas the latter focuses on enhancing the cohesion within the adhesive itself. Both approaches aim to achieve recoverable and stimuli-responsive adhesion, allowing the material to be rejoined or manipulated under specific conditions.
In this section, the authors outline the design principles, preparation methods, evaluation techniques, and outcomes for adhesion systems using reversible crosslinks. These systems offer significant potential for applications requiring durable yet adaptable bonding solutions.
Hydrogen bonding
Hydrogen bonding interactions are often used for microscale adhesion. However, it is challenging to achieve adhesion at the macroscale with single hydrogen bonds because of the weak binding energy. Multiple hydrogen bonding (MHB) refers to the simultaneous formation of several hydrogen bonds between two or more molecules, leading to highly specific and robust molecular interactions. The cumulative effect of MHB interactions results in increased binding strength and enhanced stability of the molecular assembly. Several studies on macroscale adhesion through MHB have been reported in the literature. Long et al. introduced nucleobase-containing units (adenine and thymine) into acylate-based adhesives (Table 7) [136]. When adenine- and thymine-functionalized copolymers were blended, they formed a thermodynamically stable complex through complementary adenine-thymine hydrogen bonding. This base pairing effectively acted as a physical cross-linking mechanism, influencing the self-assembly of the polymeric network. The resulting nucleobase-functionalized polyacrylates displayed tunable adhesion (20 N/in) and cohesion (80 N/in2). Zimmerman et al. successfully achieved macroscopic adhesion between polystyrene films and glass surfaces modified with 2,7-diamidonaphthyridine (DAN) and ureido-7-deazaguanine (DeUG) units [137]. Through the introduction of high-affinity DNA base analogs, the adhesion systems exhibited repeatable adhesion properties at the macroscale under ambient conditions. The recycling adhesion property was also confirmed. The adhesion status was maintained after three cycles. Another approach involves the functionalization of a rubbery copolymer containing thiolactone derivatives with side-chain barbiturate (Ba) and Hamilton wedge (HW) groups. The heterocomplementary interactions between Ba and HW form hydrogen-bonded supramolecular polymeric networks, significantly enhancing the integrity of the polymeric network. These interactions, coupled with the individual Ba or HW moieties, enable strong adhesion to diverse substrates, surpassing the performance of commercial glues and other adhesives.
Metal ligand complexes
According to the previous section, materials containing metal-ligand complexes exhibit tunable mechanical properties, stimuli-responsive properties, and self-healing properties. Moreover, due to the nature of metal coordination bonding, adhesion systems containing metal-ligand complexes are expected to achieve high adhesion on the surface of metal substrates. For example, Sun et al. reported a biobased supramolecular adhesive (BSA) using castor oil, melevodopa, and iron ions as building blocks (Table 8) [139]. Adhesion is reinforced through noncovalent interactions between the catechol units of melevodopa and adherends, while cohesion is enhanced by metal-ligand coordination between catechol and Fe³⁺ ions. This combination of strong adhesion and tough cohesion resulted in an exceptional adhesion strength of 14.6 MPa at ambient temperature, achieving record performance among biobased and supramolecular adhesives. Remarkably, the BSA also exhibited debond-on-demand properties under heat and near-infrared light stimuli. Additionally, the adhesive shows excellent reusability, retaining over 87% of its original strength after ten cycles of reuse. Another example of stimuli-responsive and reversible adhesion involves 2,6-bis(1’-methylbenzimidazolyl)-pyridine (Mebip) ligands (Mebip-PEB-Mebip), which can coordinate to metal ions [Zn(NTf2)2] and form a metallosupramolecular polymer [142]. The adhesives exhibited shear strengths of 1.8−2.5 MPa and could debond quickly under load when exposed to light or heat. The adhesive strength was fully restored after readhesion under the same conditions. Furthermore, metal ligand complexes can also be used as adhesion promoters. Wong et al. incorporated first-row transition metal β-diketonates, specifically Co(II) and Ni(II) hexafluoroacetylacetonate, into epoxy/anhydride resins, resulting in significant improvements in lap shear strength (over 30% before moisture aging and 50% after moisture aging) [143]. These insights provide a promising avenue for future research into functional additives aimed at the optimization of curing kinetics and enhancement of epoxy-copper joint robustness through polar group coordination strategies.
Host-guest interactions
There are two main methods to introduce host-guest interactions into adhesion systems with hard substrates:
Modified substrates with host and guest molecules
In this approach, guest molecules are decorated on the substrates in advance (guest substrates) and then attached to the host polymers or host-modified substrates (host substrates) to achieve adhesion through molecular recognition. For example, Ravoo et al. prepared azobenzene polymer brushes (PAZA-PHEA) on glass surface through surface-initiated atom transfer radical polymerization (Table 9) [147]. It was demonstrated that two surfaces coated with azobenzene brushes can be effectively glued together using a βCD polymer. This adhesive system displayed remarkable strength and could support a load of up to 700 ± 150 g/cm². They also reported supramolecular adhesion systems between two hard substrates (glass and silicon wafers) functionalized with either βCD or arylazopyrazole polymer brushes (Table 10) [148]. The adhesion performance of these systems was evaluated both in air and underwater and under UV irradiation. All the systems exhibited strong and reversible adhesion in air due to the multivalent host-guest interactions. Our group previously reported selective adhesion systems that focus on the interaction between CD-host gels and guest molecule-modified glass substrates (guest Sub) [149]. For Azo and αCD gels, adhesion is regulated through photoisomerization. When Azo molecules on a substrate (Azo Sub) are irradiated with UV light (360 nm), they switch from their trans to cis configuration, reducing their affinity for αCD and causing the gel to detach. Conversely, visible light (430 nm) restores the trans configuration, reinstating the gel’s adhesion. Similarly, redox stimuli can control adhesion between ferrocene (Fc)-modified substrates and βCD gels. When an oxidant such as FeCl₃ is applied, ferrocene is oxidized to a monovalent cation, weakening the interaction and causing the gel to detach. A reducing agent such as GSH can reverse this oxidation, restoring gel adhesion. Moreover, the adhesion of dissimilar substrates was explored based on host-guest interactions [150]. By using carbon fiber reinforced plastic (CFRP) as an Ad-modified substrate, aluminum (Al) substrates modified with βCD were found to adhere to the Ad-CFRP Sub with a strength of 1.98 MPa. After the bonded substrates were fractured, water (15 µL) was applied to the fractured surface, followed by reclipping and drying, restoring adhesion with the original rupture strength.
Adhesives with host-guest complexes
In this approach, materials containing host and guest polymers are first prepared and attached between two substrates, which is similar to conventional adhesives. For example, Scherman et al. reported CB[8]-based supramolecular hydrogel networks as dynamic adhesives [151]. These hydrogels demonstrate strong adhesion properties across a wide range of substrates (such as glass, stainless steel, aluminum, copper, and titanium) without requiring any surface pretreatment or curing agents. By curing the hydrogel around the softening temperature, a tough and healable adhesive interlayer is formed, offering flexibility in its application.
Adhesion systems using movable crosslinked materials
Materials with movable crosslinks, such as reversible crosslinked materials, have great potential for use in adhesion systems. These systems offer enhanced flexibility and adaptability due to movable crosslinks that can move under stress, leading to improved adhesion and cohesion properties. Two main strategies are employed to design adhesion systems using movable crosslinked materials. The first strategy is the direct use of movable crosslinked materials as adhesives. This approach leverages the high toughness and dynamic nature of movable crosslinked materials to ensure strong adhesion strength and excellent reusability. These materials can accommodate stress and recover their properties, making them suitable for applications requiring durability and reusability. The second strategy involves the formation of movable crosslinks between adherends via in-situ polymerization. In this method, movable crosslinks are created directly between the adherends, resulting in a unique topological structure. This strategy prioritizes achieving high cohesion strength by utilizing the dynamic yet robust nature of the crosslinked network. Several recent studies have explored the integration of rotaxane structures into adhesive formulations, demonstrating improved mechanical performance and adhesion strength [152,153,154,155,156,157,158,159,160].
In this section, the authors focus on the design, preparation, and evaluation of adhesion systems utilizing movable crosslinks. The results of these studies highlight the promising role of movable crosslinks in the development of advanced adhesives with enhanced durability, flexibility, and reusability.
Due to their high toughness and self-restoring behavior, movable crosslinked materials have been successfully employed in the development of pressure-sensitive adhesives (PSAs). PSA is a self-standing film and is widely used in industrial applications where low residue and repeated peeling are required. Kim et al. demonstrated that triacetyl βCD functionalized with 2-isocyanatoethyl acrylate (βCD−AOI) formed a threaded compound with poly(butyl acrylate) (pBA), creating a supramolecular sliding effect within the polymer matrix (Table 10) [158]. Elastomers and pressure-sensitive adhesives (PSAs) incorporating supramolecular cross-linkers exhibit remarkable stretching and adhesion properties, which are difficult to achieve with conventional cross-linkers. Our group also reported tough, recyclable PSAs by preparing self-standing films using movable crosslinks [159]. Films were formed by bulk polymerization of M-PEA-CD (0.5) copolymerized with a mixture of 99.5 mol% EA and 0.5 mol% mono-6O-acrylamidomethyl-icosaacetyl-βCD (TAcβCDAAmMe) between two detachable films. M-PEA-CD (0.5) exhibited excellent adhesive properties, showing flexibility that allows interfacial adhesion and improved mechanical properties that contribute to cohesion. While M-PEA-CD (0.5) also showed viscoelastic behavior, suggesting cross-link formation, it became a homogeneous solution when immersed in ethyl acetate which is a good solvent. At this polymerization ratio, M-PEA-CD (0.5) could be recycled at least 10 times by dissolution and reapplication/drying without a loss of adhesive properties.
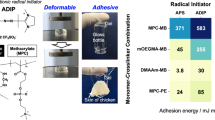
On the other hand, our group achieved adhesion systems between nylon substrates through the introduction of the single-movable cross-network (SC) materials described above [160]. The SC adhesion material [SC(DMAAm) Adh] was prepared via bulk polymerization of DMAAm monomer solutions containing photoinitiators and polymerizable acetylated γCD (TAcγCDAAmMe) between nylon substrates with silane anchors modified on the surface. In addition, adhesion systems with chemical crosslinks [CP(DMAAm) Adh] and without crosslinks [P(DMAAm) Adh] were prepared for comparison. SC(DMAAm) Adh showed high durability against deformation and stress application under various conditions, particularly when the moisture content was approximately 20%. SC(DMAAm) Adh exhibited high values for both shear stress and shear strain, with a toughness that was 1.3 ~ 1.6 times higher than those of CP(DMAAm) Adh and P(DMAAm) Adh. Furthermore, SC(DMAAm) Adh exhibited excellent stress dissipation, self-restoring behavior, and creep resistance. The movable crosslink has an interlocking structure and thus its cohesion energy is equivalent to that of a covalent bond while still exhibiting stress dissipation. This enabled enhanced durability under various conditions.
Conclusion
This review summarizes various works on supramolecular materials with reversible and movable crosslinks and their application as adhesives. By incorporating reversible or movable crosslinks into polymer networks on the basis of supramolecular science, various polymer adhesive materials have been shown to achieve unprecedented mechanical and functional properties. These designs are expected to be developed as adhesive materials with novel functions.
In recent years, global challenges such as environmental sustainability and the realization of a circular economy have also become relevant to adhesive materials. It is necessary to develop new adhesion systems that not only enable the recycling of the adhesive itself but also allow the substrates to adhere. While maintaining stability and stress relaxation, the introduction of supramolecular science techniques offers a new paradigm for adhesive technologies. Adhesion based on selective and specific molecular recognition is closely related to the formation of complexes that respond to external stimuli, which is the forte of supramolecular science.
Moving forward, we anticipate that the fusion of these adhesive and supramolecular science technologies will lead to the creation of new high-performance adhesive materials and contribute to the reuse of resources in the circular society.
References
Raja, PR. Cyanoacrylate adhesives: A critical review. Rev. Adhes. Adhesives 2016;4.
Joyner FB, Hawkins GF. Method of making alpha-cyanoacrylates. 1955. at https://patents.google.com/patent/US2721858A/en.
Leonard F, Kulkarni RK, Brandes G, Nelson J, Cameron JJ. Synthesis and degradation of poly (alkyl α‐cyanoacrylates). J Appl Polym Sci. 1966;10:259–72.
Pepper DC. Kinetics and mechanisms of zwitterionic polymerizations of Alkyl Cyanoacrylates. Polym J. 1980;12:629–37.
Petrie EM. Handbook of Adhesives and Sealants. 2004.
de Buyl F. Silicone sealants and structural adhesives. Int J Adhes Adhes. 2001;21:411–22.
Ho KY, Dodou K. Rheological studies on pressure-sensitive silicone adhesives and drug-in-adhesive layers as a means to characterise adhesive performance. Int J Pharm. 2007;333:24–33.
Gadhave RVI, Dhawale PV. State of research and trends in the development of polyvinyl acetate-based wood adhesive. Open J Polym Chem. 2022;12:13–42.
Khan U, May P, Porwal H, Nawaz K, Coleman JN. Improved adhesive strength and toughness of polyvinyl acetate glue on addition of small quantities of graphene. ACS Appl Mater Interfaces. 2013;5:1423–8.
Tobing SD, Klein A. Molecular parameters and their relation to the adhesive performance of acrylic pressure-sensitive adhesives. J Appl Polym Sci. 2001;79:2230–44.
Santiago D, Guzmán D, Padilla J, Verdugo P, De la Flor S, Serra À. Recyclable and reprocessable epoxy vitrimer adhesives. ACS Appl Polym Mater 2023;5:2006–15.
Aronovich DA. A review of modern adhesive materials operating in a wide temperature range. Epoxy Adhes Polym Sci Ser D. 2023;16:14–33.
Maji P, Naskar K. Styrenic block copolymer-based thermoplastic elastomers in smart applications: Advances in synthesis, microstructure, and structure–property relationships—A review. J Appl Polym Sci. 2022;139:e52942.
Jojibabu P, Zhang YX, Rider AN, Wang J, Wuhrer R, Prusty BG. High-performance epoxy-based adhesives modified with functionalized graphene nanoplatelets and triblock copolymers. Int J Adhes Adhes. 2020;98:102521.
Gallagher JJ, Hillmyer MA, Reineke TM. Acrylic Triblock copolymers incorporating isosorbide for pressure sensitive adhesives. ACS Sustain Chem Eng 2016;4:3379–87.
Sajjad H, Tolman WB, Reineke TM. Block copolymer pressure-sensitive adhesives derived from fatty acids and Triacetic acid lactone. ACS Appl Polym Mater 2020;2:2719–28.
Cho KG, An S, Cho DH, Kim JH, Nam J, Kim M, et al. Block copolymer-based supramolecular ionogels for accurate on-skin motion monitoring. Adv Funct Mater. 2021;31:2102386.
Zhai Y, Chen X, Yuan Z, Han X, Liu H. A mussel-inspired catecholic ABA triblock copolymer exhibits better antifouling properties compared to a diblock copolymer. Polym Chem. 2020;11:4622–9.
Ito S, Akiyama H, Sekizawa R, Mori M, Yoshida M, Kihara H. Light-induced reworkable adhesives based on ABA-type Triblock copolymers with Azopolymer Termini. ACS Appl Mater Interfaces. 2018;10:32649–58.
Kim HJ, Jin K, Shim J, Dean W, Hillmyer MA, Ellison CJ. Sustainable Triblock copolymers as tunable and degradable pressure sensitive adhesives. ACS Sustain Chem Eng 2020;8:12036–44.
Lee BP, Chao C-Y, Nunalee FN, Motan E, Shull KR, Messersmith PB. Rapid gel formation and adhesion in photocurable and biodegradable block copolymers with high DOPA content. Macromolecules. 2006;39:1740–8.
Doi T, Takagi H, Shimizu N, Igarashi N, Sakurai S. Effects of solubility difference of tackifier to respective components of block copolymers on microphase-separated structures in coated layers of pressure-sensitive adhesive prepared by solution coating process. ACS Appl Polym Mater 2020;2:4973–84.
Ohm C, Brehmer M, Zentel R. Liquid crystalline elastomers as actuators and sensors. Adv Mater. 2010;22:3366–87.
Kularatne RS, Kim H, Boothby JM, Ware TH. Liquid crystal elastomer actuators: Synthesis, alignment, and applications. J Polym Sci Part B: Polym Phys. 2017;55:395–411.
Saed MO, Gablier A, Terentjev EM. Exchangeable liquid crystalline elastomers and their applications. Chem Rev 2022;122:4927–45.
Jiang Z-C, Liu Q, Xiao Y-Y, Zhao Y. Liquid crystal elastomers for actuation: A perspective on structure-property-function relation. Prog Polym Sci. 2024;153:101829.
Ohzono T, Koyama E. Enhanced photocontrollable dynamic adhesion of nematic elastomers on rough surfaces. Polymer. 2022;260:125377.
Ohzono T, Minamikawa H, Koyama E, Norikane Y. Unlocking entropic elasticity of nematic elastomers through light and dynamic adhesion. Adv Mater Interfaces. 2021;8:2100672.
Ohzono T, Norikane Y, Saed MO, Terentjev EM. Light-driven dynamic adhesion on photosensitized nematic liquid crystalline elastomers. ACS Appl Mater Interfaces. 2020;12:31992–7.
Li L, Yin X, Zhao Y-X, Shi L-Y, Yang K-K, Wang Y-Z. High-strength thermochromic and mechanochromic liquid-crystal elastomers with responsive shape memory and dynamic adhesion. J Mater Chem A. 2024;12:18117–26.
Ohzono T, Saed MO, Terentjev EM. Enhanced dynamic adhesion in nematic liquid crystal elastomers. Adv Mater. 2019;31:1902642.
Pranda PA, Hedegaard A, Kim H, Clapper J, Nelson E, Hines L, et al. Directional adhesion of Monodomain liquid crystalline elastomers. ACS Appl Mater Interfaces. 2024;16:6394–402.
Ohzono T, Minamikawa H, Koyama E, Norikane Y. Impact of crystallites in nematic elastomers on dynamic mechanical properties and adhesion. Macromolecules. 2021;54:8987–95.
Annapooranan R, Suresh Jeyakumar S, Chambers J, Long, R R, Cai S. Ultra rate-dependent pressure sensitive adhesives enabled by soft elasticity of liquid crystal elastomers. Adv Funct Mater. 2024;34:2309123.
Guo H, Saed MO, Terentjev EM. Mechanism of pressure-sensitive adhesion in nematic elastomers. Macromolecules. 2023;56:6247–55.
Farre-Kaga HJ, Saed MO, Terentjev EM. Dynamic pressure sensitive adhesion in nematic phase of liquid crystal elastomers. Adv Funct Mater. 2022;32:2110190.
Tayeb AH, Amini E, Ghasemi S, Tajvidi M. Cellulose nanomaterials—binding properties and applications: a review. Molecules. 2018;23:2684.
Wang L, Kelly PV, Ozveren N, Zhang X, Korey M, Chen C, et al. Multifunctional polymer composite coatings and adhesives by incorporating cellulose nanomaterials. Matter. 2023;6:344–72.
Tang Z, Zhang M, Xiao H, Liu K, Li X, Du B, et al. A green Catechol-containing cellulose nanofibrils-cross-linked adhesive. ACS Biomater Sci Eng 2022;8:1096–102.
Veigel S, Müller U, Keckes J, Obersriebnig M, Gindl-Altmutter W. Cellulose nanofibrils as filler for adhesives: effect on specific fracture energy of solid wood-adhesive bonds. Cellulose. 2011;18:1227–37.
Liu S, Du G, Yang H, Su H, Ran X, Li J, et al. Developing high-performance cellulose-based wood adhesive with a cross-linked network. ACS Sustain Chem Eng. 2021;9:16849–61.
Pruksawan S, Samitsu S, Fujii Y, Torikai N, Naito M. Toughening effect of rodlike cellulose nanocrystals in epoxy adhesive. ACS Appl Polym Mater 2020;2:1234–43.
Kaboorani A, Riedl B, Blanchet P, Fellin M, Hosseinaei O, Wang S. Nanocrystalline cellulose (NCC): A renewable nano-material for polyvinyl acetate (PVA) adhesive. Eur Polym J. 2012;48:1829–37.
Cudjoe E, Herbert KM, Rowan SJ. Strong, rebondable, dynamic cross-linked cellulose nanocrystal polymer nanocomposite adhesives. ACS Appl Mater Interfaces. 2018;10:30723–31.
Tang Z, Zhao M, Li N, Xiao H, Miao Q, Zhang M, et al. Self-healing, reusable and conductive cellulose nanocrystals-containing adhesives. Colloids Surf A: Physicochem Eng Asp. 2022;643:128797.
Silverstein MS. Interpenetrating polymer networks: So happy together? Polymer. 2020;207:122929.
Dragan ES. Design and applications of interpenetrating polymer network hydrogels. A review. Chem Eng J. 2014;243:572–90.
Dragan ES. Advances in interpenetrating polymer network hydrogels and their applications. Pure Appl Chem. 2014;86:1707–21.
Farooq U, Teuwen J, Dransfeld C. Toughening of Epoxy systems with Interpenetrating Polymer Network (IPN): A review. Polymers. 2020;12:1908.
Shim G-S, Kim J-S, Kim H-J. Behavior and adhesion performance of acrylic PSAs using semi-IPN structure and UV/UV Stepwise Curing. J Ind Eng Chem. 2020;89:139–46.
Li Z, Xu W, Wang X, Jiang W, Ma X, Wang F, et al. Fabrication of PVA/PAAm IPN hydrogel with high adhesion and enhanced mechanical properties for body sensors and antibacterial activity. Eur Polym J. 2021;146:110253.
Li M, Zhang Y, Liu Y, Chen G, Zhao N, Liu C, et al. A semi-interpenetrating network acrylic pressure-sensitive adhesive for efficient transdermal application with high cohesion and adhesion. Mater Des. 2024;241:112970.
Park KH, Lee DY, Yoon SH, Kim SH, Han MS, Jeon S, et al. Adhesion improvement of solvent-free pressure-sensitive adhesives by semi-IPN using Polyurethanes and acrylic polymers. Polymers. 2022;14:3963.
Back J-H, Baek D, Park J-W, Kim H-J, Jang J-Y, Lee S-J. Shock absorption of semi-interpenetrating network acrylic pressure-sensitive adhesive for mobile display impact resistance. Int J Adhes Adhes. 2020;99:102558.
Jeong K, Lee Y, Kim Y, Mun H, Kyung K-U, Im SG. A Sub-Micron-Thick stretchable adhesive layer for the lamination of arbitrary elastomeric substrates with enhanced adhesion stability. Chem Eng J. 2022;429:132250.
Li J, Chen G, Han Y, Zhang W, Zhang S. Development of poly(vinyl acetate) adhesive with hot water resistance via constructing semi-IPN/IPN structures with hydroxymethylated lignin. J Appl Polym Sci. 2024;141:e55882.
Shen C, Li Y, Wang H, Meng Q. Mechanically strong interpenetrating network hydrogels for differential cellular adhesion. RSC Adv. 2017;7:18046–53.
Shi C-Y, Zhang Q, Tian H, Qu D-H. Supramolecular adhesive materials from small-molecule self-assembly. SmartMat. 2020;1:e1012.
Yang S, Jiang X. Nanoscale strategies for enhancing the performance of adhesive dry electrodes for the skin. ACS Nano. 2024;18:27107–25.
De Greef TFA, Smulders MMJ, Wolffs M, Schenning APHJ, Sijbesma RP, Meijer EW. Supramolecular polymerization. Chem Rev 2009;109:5687–754.
Yang L, Tan X, Wang Z, Zhang X. Supramolecular polymers: historical development, preparation, characterization, and functions. Chem Rev 2015;115:7196–239.
Crini G. Review: A history of Cyclodextrins. Chem Rev 2014;114:10940–75.
Gibbons WA. The rubber industry, 1839-939. ACS Publications 2002. https://doi.org/10.1021/ie50358a006.
Lake GJ, Thomas AG, Tabor D. The strength of highly elastic materials. Proc R Soc Lond Ser A Math Phys Sci. 1997;300:108–19.
Cram DJ, Bauer RH. Macro Rings. XX. Transannular effects in π-π-Complexes1. ACS Publications 2002. https://doi.org/10.1021/ja01531a031
Pedersen CJ, Cyclic polyethers and their complexes with metal salts. ACS Publications (2002). https://doi.org/10.1021/ja01002a035.
Ikada Y, Jamshidi K, Tsuji H, Hyon SH. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules. 1987;20:904–6.
Haraguchi K, Takehisa T. Nanocomposite hydrogels: a unique organic–inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv Mater. 2002;14:1120–4.
Gong J, Katsuyama Y, Kurokawa T, Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv Mater. 2003;15:1155–8.
Sakai T, Matsunaga T, Yamamoto Y, Ito C, Yoshida R, Suzuki S, et al. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from Tetrahedron-like macromonomers. Macromolecules. 2008;41:5379–84.
Sun J-Y, Zhao X, Illeperuma WRK, Chaudhuri O, Oh KH, Mooney DJ, et al. Highly stretchable and tough hydrogels. Nature. 2012;489:133–6.
Ducrot E, Chen Y, Bulters M, Sijbesma RP, Creton C. Toughening elastomers with sacrificial bonds and watching them break. Science. 2014;344:186–9.
Malay AD, Suzuki T, Katashima T, Kono N, Arakawa K, Numata K. Spider silk self-assembly via modular liquid-liquid phase separation and nanofibrillation. Sci Adv. 2020;6:eabb6030.
Hashimoto K, Shiwaku T, Aoki H, Yokoyama H, Mayumi K, Ito K. Strain-induced crystallization and phase separation used for fabricating a tough and stiff slide-ring solid polymer electrolyte. Sci Adv. 2023;9:eadi8505.
Harada A, Li J, Kamachi M. The molecular necklace: a rotaxane containing many threaded α-cyclodextrins. Nature. 1992;356:325–7.
Okumura Y, Ito K. The Polyrotaxane gel: a topological gel by figure-of-eight cross-links. Adv Mater. 2001;13:485–7.
Burattini, S, Colquhoun, HM, Fox, JD, Friedmann, D, Greenland, BW, Harris, PJF, et al. A self-repairing, supramolecular polymer system: healability as a consequence of donor–acceptor π–π stacking interactions. Chem Commun 2009; 6717–9 https://doi.org/10.1039/B910648K.
Wang Q, Mynar JL, Yoshida M, Lee E, Lee M, Okuro K, et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature. 2010;463:339–43.
Burnworth M, Tang L, Kumpfer JR, Duncan AJ, Beyer FL, Fiore GL, et al. Optically healable supramolecular polymers. Nature. 2011;472:334–7.
Kakuta T, Takashima Y, Nakahata M, Otsubo M, Yamaguchi H, Harada A. Preorganized hydrogel: self-healing properties of supramolecular hydrogels formed by polymerization of host–guest-monomers that contain cyclodextrins and hydrophobic guest groups. Adv Mater. 2013;25:2849–53.
Sun TL, Kurokawa T, Kuroda S, Ihsan AB, Akasaki T, Sato K, et al. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat Mater. 2013;12:932–7.
Yanagisawa Y, Nan Y, Okuro K, Aida T. Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking. Science. 2018;359:72–76.
Park J, Murayama S, Osaki M, Yamaguchi H, Harada A, Matsuba G, et al. Extremely rapid self-healable and recyclable supramolecular materials through planetary ball milling and host–guest interactions. Adv Mater. 2020;32:2002008.
Liu, J, Ikura, R, Yamaoka, K, Sugawara, A, Takahashi, Y, Kure, B, et al. Exploring enzymatic degradation, reinforcement, recycling, and upcycling of poly(ester)s-poly(urethane) with movable crosslinks. Chem 2024. https://doi.org/10.1016/j.chempr.2024.09.026.
Rosales AM, Anseth KS. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat Rev Mater. 2016;1:1–15.
Gu Y, Zhao J, Johnson JA. Polymer networks: from plastics and gels to porous frameworks. Angew Chem Int Ed. 2020;59:5022–49.
Sinawang G, Osaki M, Takashima Y, Yamaguchi H, Harada A. Biofunctional hydrogels based on host–guest interactions. Polym J. 2020;52:839–59.
Kato T, Frechet JMJ. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J Am Chem Soc 1989;111:8533–4.
Yamauchi K, Lizotte JR, Long TE. Thermoreversible Poly(alkyl acrylates) consisting of self-complementary multiple hydrogen bonding. Macromolecules. 2003;36:1083–8.
van Gemert GML, Peeters JW, Söntjens SHM, Janssen HM, Bosman AW. Self-healing supramolecular polymers in action. Macromol Chem Phys. 2012;213:234–42.
Pensec S, Nouvel N, Guilleman A, Creton C, Boué F, Bouteiller L. Self-assembly in solution of a reversible comb-shaped supramolecular polymer. Macromolecules. 2010;43:2529–34.
Sijbesma RP, Beijer FH, Brunsveld L, Folmer BJB, Hirschberg JHKK, Lange RFM, et al. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science. 1997;278:1601–4.
Cordier P, Tournilhac F, Soulié-Ziakovic C, Leibler L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature. 2008;451:977–80.
Chow C-F, Fujii S, Lehn J-M. Metallodynamers: Neutral dynamic metallosupramolecular polymers displaying transformation of mechanical and optical properties on constitutional exchange. Angew Chem Int Ed. 2007;46:5007–10.
Holten-Andersen N, Harrington MJ, Birkedal H, Lee BP, Messersmith PB, Lee KYC, et al. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc Natl Acad Sci. 2011;108:2651–5.
Gu Y, Alt EA, Wang H, Li X, Willard AP, Johnson JA. Photoswitching topology in polymer networks with metal-organic cages as crosslinks. Nature. 2018;560:65–69.
Schmidt BVKJ, Barner-Kowollik C. Dynamic macromolecular material design—the versatility of cyclodextrin-based host–guest chemistry. Angew Chem Int Ed. 2017;56:8350–69.
Nakahata M, Takashima Y, Yamaguchi H, Harada A. Redox-responsive self-healing materials formed from host–guest polymers. Nat Commun. 2011;2:511.
Zhang M, Xu D, Yan X, Chen J, Dong S, Zheng B, et al. Self-healing supramolecular gels formed by crown ether based host–guest interactions. Angew Chem Int Ed. 2012;51:7011–5.
Kardelis V, Li K, Nierengarten I, Holler M, Nierengarten J-F, Adronov A. Supramolecular organogels prepared from pillar[5]arene-functionalized conjugated polymers. Macromolecules. 2017;50:9144–50.
Appel EA, Biedermann F, Rauwald U, Jones ST, Zayed JM, Scherman OA. Supramolecular cross-linked networks via host−guest complexation with Cucurbit[8]uril. J Am Chem Soc 2010;132:14251–60.
Liu J, Tan CSY, Yu Z, Li N, Abell C, Scherman OA. Tough supramolecular polymer networks with extreme stretchability and fast room-temperature self-healing. Adv Mater. 2017;29:1605325.
Yamaoka, K, Ikura, R, Osaki, M, Shirakawa, H, Takahashi, K, Takahashi, H, et al. Viscoelastic behaviors for optimizing self-healing of gels with host-guest inclusion complexes. Polym J 2024;1–9. https://doi.org/10.1038/s41428-024-00932-7.
Takashima Y, Yonekura K, Koyanagi K, Iwaso K, Nakahata M, Yamaguchi H, et al. Multifunctional stimuli-responsive supramolecular materials with stretching, coloring, and self-healing properties functionalized via host–guest interactions. Macromolecules. 2017;50:4144–50.
Takashima Y, Sawa Y, Iwaso K, Nakahata M, Yamaguchi H, Harada A. Supramolecular materials cross-linked by host–guest inclusion complexes: the effect of side chain molecules on mechanical properties. Macromolecules. 2017;50:3254–61.
Nomimura S, Osaki M, Park J, Ikura R, Takashima Y, Yamaguchi H, et al. Self-healing Alkyl acrylate-based supramolecular elastomers cross-linked via host–guest interactions. Macromolecules. 2019;52:2659–68.
Filippidi E, Cristiani TR, Eisenbach CD, Waite JH, Israelachvili JN, Ahn BK, et al. Toughening elastomers using mussel-inspired iron-catechol complexes. Science. 2017;358:502–5.
Lai J-C, Li L, Wang D-P, Zhang M-H, Mo S-R, Wang X, et al. A rigid and healable polymer cross-linked by weak but abundant Zn(II)-carboxylate interactions. Nat Commun. 2018;9:2725.
Miwa Y, Taira K, Kurachi J, Udagawa T, Kutsumizu S. A gas-plastic elastomer that quickly self-heals damage with the aid of CO2 gas. Nat Commun. 2019;10:1828.
Burattini S, Greenland BW, Merino DH, Weng W, Seppala J, Colquhoun HM, et al. A healable supramolecular polymer blend based on aromatic pi-pi stacking and hydrogen-bonding interactions. J Am Chem Soc. 2010;132:12051–8.
Pratama PA, Sharifi M, Peterson AM, Palmese GR. Room temperature self-healing thermoset based on the Diels–Alder reaction. ACS Appl Mater Interfaces. 2013;5:12425–31.
Capelot M, Montarnal D, Tournilhac F, Leibler L. Metal-catalyzed transesterification for healing and assembling of thermosets. J Am Chem Soc 2012;134:7664–7.
Glink PT, Oliva AI, Stoddart JF, White AJP, Williams DJ. Template-directed synthesis of a [2]Rotaxane by the clipping under thermodynamic control of a Crown Ether like macrocycle around a Dialkylammonium ion. Angew Chem Int Ed. 2001;40:1870–5.
Otsuka H, Nagano S, Kobashi Y, Maeda T, Takahara A. A dynamic covalent polymer driven by disulfide metathesis under photoirradiation. Chem Commun 2010;46:1150–2.
Freeman WA, Mock WL, Shih N Y. Cucurbituril. J Am Chem Soc 1981;103:7367–8.
Gutsche CD, Dhawan B, No KH, Muthukrishnan R. Calixarenes. 4. The synthesis, characterization, and properties of the calixarenes from p-tert-butylphenol. J Am Chem Soc. 1981;103:3782–92.
Ogoshi T, Kanai S, Fujinami S, Yamagishi T, Nakamoto Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J Am Chem Soc 2008;130:5022–3.
Bender ML, Komiyama M. Cyclodextrin Chemistry. (Springer Science & Business Media, 2012).
Yang W, Li Y, Liu H, Chi L, Li Y. Design and Assembly of Rotaxane-Based Molecular Switches and Machines. Small. 2012;8:504–16.
Harada A, Hashidzume A, Yamaguchi H, Takashima Y. Polymeric Rotaxanes. Chem Rev 2009;109:5974–6023.
Zhou H-Y, Zong Q-S, Han Y, Chen C-F. Recent advances in higher order rotaxane architectures. Chem Commun. 2020;56:9916–36.
Okada M, Kamachi M, Harada A. Preparation and Characterization of Inclusion Complexes of Poly(propylene glycol) with Methylated Cyclodextrins. J Phys Chem B. 1999;103:2607–13.
Harrison I. Thomas. & Harrison, Shuyen. Synthesis of a stable complex of a macrocycle and a threaded chain. J Am Chem Soc 1967;89:5723–4.
Liu C, Morimoto N, Jiang L, Kawahara S, Noritomi T, Yokoyama H, et al. Tough hydrogels with rapid self-reinforcement. Science. 2021;372:1078–81.
Noda Y, Hayashi Y, Ito K. From topological gels to slide-ring materials. Journal of Applied Polymer Science 2014:131.
Ogoshi T, Aoki T, Ueda S, Tamura Y, Yamagishi T. Pillar[5]arene-based nonionic polyrotaxanes and a topological gel prepared from cyclic host liquids. Chem Commun 2014;50:6607–9.
Nakahata M, Mori S, Takashima Y, Yamaguchi H, Harada A. Self-Healing Materials Formed by Cross-Linked Polyrotaxanes with Reversible Bonds. Chem. 2016;1:766–75.
Kobayashi Y, Zheng Y, Takashima Y, Yamaguchi H, Harada A. Physical and Adhesion Properties of Supramolecular Hydrogels Cross-linked by Movable Cross-linking Molecule and Host-guest Interactions. Chem Lett. 2018;47:1387–90.
Takashima Y, Hayashi Y, Osaki M, Kaneko F, Yamaguchi H, Harada A. A Photoresponsive Polymeric Actuator Topologically Cross-Linked by Movable Units Based on a [2]. Rotaxane Macromolecules. 2018;51:4688–93.
Zhao D, Zhang Z, Zhao J, Liu K, Liu Y, Li G, et al. A Mortise-and-Tenon Joint Inspired Mechanically Interlocked Network. Angew Chem Int Ed. 2021;60:16224–9.
Ikura R, Park J, Osaki M, Yamaguchi H, Harada A, Takashima Y. Supramolecular Elastomers with Movable Cross-Linkers Showing High Fracture Energy Based on Stress Dispersion. Macromolecules. 2019;52:6953–62.
Ikura R, Ikemoto Y, Osaki M, Yamaguchi H, Harada A, Takashima Y. Preparation of hydrophilic polymeric materials with movable cross-linkers and their mechanical property. Polymer. 2020;196:122465.
Nishida K, Ikura R, Yamaoka K, Urakawa O, Konishi T, Inoue T, et al. Relation between the Water Content and Mechanical Properties of Hydrogels with Movable Cross-Links. Macromolecules. 2024;57:7745–54.
Wilson GO, Caruso MM, Schelkopf SR, Sottos NR, White SR, Moore JS. Adhesion Promotion via Noncovalent Interactions in Self-Healing Polymers. ACS Appl Mater Interfaces. 2011;3:3072–7.
Courtois J, Baroudi I, Nouvel N, Degrandi E, Pensec S, Ducouret G, et al. Supramolecular Soft Adhesive Materials. Adv Funct Mater. 2010;20:1803–11.
Cheng S, Zhang M, Dixit N, Moore RB, Long TE. Nucleobase Self-Assembly in Supramolecular Adhesives. Macromolecules. 2012;45:805–12.
Anderson CA, Jones AR, Briggs EM, Novitsky EJ, Kuykendall DW, Sottos NR, et al. High-Affinity DNA Base Analogs as Supramolecular, Nanoscale Promoters of Macroscopic Adhesion. J Am Chem Soc 2013;135:7288–95.
Chen S, Li Z, Wu Y, Mahmood N, Lortie F, Bernard J, et al. Hydrogen-Bonded Supramolecular Polymer Adhesives: Straightforward Synthesis and Strong Substrate Interaction. Angew Chem. 2022;134:e202203876.
Sun P, Mei S, Xu J-F, Zhang X. A Bio-Based Supramolecular Adhesive: Ultra-High Adhesion Strengths at both Ambient and Cryogenic Temperatures and Excellent Multi-Reusability. Adv Sci. 2022;9:2203182.
Nakamura T, Takashima Y, Hashidzume A, Yamaguchi H, Harada A. A metal–ion-responsive adhesive material via switching of molecular recognition properties. Nat Commun. 2014;5:4622.
Darby DR, Lai E, Holten-Andersen N, Pham JT. Interfacial Adhesion of Fully Transient, Mussel-Inspired Hydrogels with Different Network Crosslink Modalities. Adv Mater Interfaces. 2021;8:2100319.
Heinzmann C, Coulibaly S, Roulin A, Fiore GL, Weder C. Light-Induced Bonding and Debonding with Supramolecular Adhesives. ACS Appl Mater Interfaces. 2014;6:4713–9.
Li J, Cheung D, Wilson J, Sun Z, Yu F, Kim D, et al. Transition Metal β-Diketonate Adhesion Promoters in Epoxy-Anhydride Resin. Macromol Rapid Commun. 2023;44:2200973.
Liu J, Huang Y-S, Liu Y, Zhang D, Koynov K, Butt H-J, et al. Reconfiguring hydrogel assemblies using a photocontrolled metallopolymer adhesive for multiple customized functions. Nat Chem 2024;16:1024–33.
Yarullin AF, Abzaldinov KhS, Kuznetsova LE, Yarullina AF, Stoyanov OV. Structure and Properties of Coatings Based on Acrylic-Containing Coordination Metal Complexes. Polym Sci Ser D. 2020;13:146–50.
Osaki M, Sekine T, Yamaguchi H, Takashima Y, Harada A. Material Adhesion through Direct Covalent Bond Formation Assisted by Noncovalent Interactions. ACS Appl Polym Mater 2021;3:2189–96.
Roling O, Stricker L, Voskuhl J, Lamping S, Jan Ravoo B. Supramolecular surface adhesion mediated by azobenzene polymer brushes. Chem Commun. 2016;52:1964–6.
Lamping S, Stricker L, Ravoo BJ. Responsive surface adhesion based on host–guest interaction of polymer brushes with cyclodextrins and arylazopyrazoles. Polym Chem 2019;10:683–90.
Takashima Y, Sahara T, Sekine T, Kakuta T, Nakahata M, Otsubo M, et al. Supramolecular Adhesives to Hard Surfaces: Adhesion Between Host Hydrogels and Guest Glass Substrates Through Molecular Recognition. Macromol Rapid Commun. 2014;35:1646–52.
Takashima Y, Shojima Y, Sekine T, Osaki M, Kobayashi Y, Yamaguchi H, et al. Adhesion of Dissimilar Materials through Host-Guest Interactions and Its Re-adhesion Properties. Chem Lett 2018;47:1255–7.
Liu J, Scherman OA. Cucurbit[n]uril Supramolecular Hydrogel Networks as Tough and Healable Adhesives. Adv Funct Mater. 2018;28:1800848.
Watanabe J, Ooya T, Nitta K-H, Park KD, Kim YH, Yui N. Fibroblast adhesion and proliferation on poly(ethylene glycol) hydrogels crosslinked by hydrolyzable polyrotaxane. Biomaterials. 2002;23:4041–8.
Pruksawan S, Samitsu S, Yokoyama H, Naito M. Homogeneously Dispersed Polyrotaxane in Epoxy Adhesive and Its Improvement in the Fracture Toughness. Macromolecules. 2019;52:2464–75.
Xiong X, Chen Y, Wang Z, Liu H, Le M, Lin C, et al. Polymerizable rotaxane hydrogels for three-dimensional printing fabrication of wearable sensors. Nat Commun. 2023;14:1331.
Gao Y, Tian X, Xiong X, Wang Y, Huang W, Shi X, et al. Polymerizable rotaxane of cucurbituril protecting dopamine based adhesive hydrogels. Int J Biol Macromolecules. 2024;265:130680.
Dikshit KV, Visal AM, Janssen F, Larsen A, Bruns CJ. Pressure-Sensitive Supramolecular Adhesives Based on Lipoic Acid and Biofriendly Dynamic Cyclodextrin and Polyrotaxane Cross-Linkers. ACS Appl Mater Interfaces. 2023;15:17256–67.
Yi M-B, Lee T-H, Lee S-J, Kim J-S, Kim H-J. Topologically designed cross-linking network for stretchable and recoverable pressure-sensitive adhesives with exceptional softness. Mater Today Chem. 2022;26:101141.
Yi M-B, Lee T-H, Han G-Y, Kim H, Kim H-J, Kim Y, et al. Movable Cross-linking in Adhesives: Superior Stretching and Adhesion Properties via a Supramolecular Sliding Effect. ACS Appl Polym Mater 2021;3:2678–86.
Kosaba S, Ikura R, Yamaoka K, Arai T, Takashima Y. Recyclable Tough Adhesive Sheets with Movable Cross-Links for Sustainable Use. ACS Appl Mater Interfaces. 2024;16:25393–403.
Qian Y, Ikura R, Kawai Y, Park J, Yamaoka K, Takashima Y. Improvement in Cohesive Properties of Adhesion Systems Using Movable Cross-Linked Materials with Stress Relaxation Properties. ACS Appl Mater Interfaces. 2024;16:3935–43.
Acknowledgements
This research was funded by Scientific Research on Innovative Areas JP19H05714 and JP19H05721 from the MEXT of Japan; the Core Research for Evolutional Science and Technology (CREST) program JPMJCR22L4 from the JST; the COI-NEXT program JPMJPF2218 from the JST; the JSPS Core-to-Core Program JPJSCCA20220006; the Asahi Glass Foundation; the Yazaki Memorial Foundation for Science; the International Polyurethane Technology Foundation; the Toyota Riken Scholar Program; the Iketani Science and Technology Foundation, 0351026-A and 0361034-A; and the Suzuki Foundation.
Funding
Open Access funding provided by Osaka University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qian, Y., Kosaba, S., Ikura, R. et al. Design of functional and stable adhesion systems using reversible and movable crosslinked materials. Polym J 57, 491–512 (2025). https://doi.org/10.1038/s41428-024-01011-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-024-01011-7