Abstract
Assessing the digestive and absorptive capacity of the gastro-intestinal tract (GIT) using minimally- or non-invasive methods, particularly in children, has been difficult owing to the complex physiology and variability in functional measurements. However, measuring GIT function is increasingly important with the emerging relevance of childhood environmental enteropathy (EE) as a mediating factor in linear growth faltering, severe acute malnutrition, poor oral vaccine uptake and impaired cognition. In EE, sub-optimal nutrient digestion and absorption (malabsorption) forms the critical link to the conditions mentioned above. The present narrative review discusses probable mechanisms that can cause malabsorption of macronutrients, along with mechanistic and experimental evidence, in children (if not, in adults) with EE. The strengths and limitations of the human experimental studies are examined in relation to a battery of existing and potential tests that are used to measure malabsorption. From the available studies conducted in children, lactose and fat malabsorption are more likely to occur in EE. Breath tests (non-invasive) measuring carbohydrate (13C-starch/sucrose/lactose), fat (13C-mixed triglyceride) and dipeptide (benzoyl-L-tyrosyl-L-1-13C-alanine) malabsorption with modifications to the existing protocols seem suitable for use in children with EE. Future research should focus on understanding the degree of macronutrient malabsorption using these tests, in different settings, and link them to functional outcomes (such as growth, muscle strength, cognition).
Similar content being viewed by others
Introduction
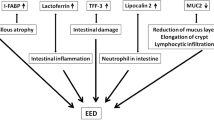
Environmental enteropathy (EE, pathology) or environmental enteric dysfunction (EED, functional consequence) is an acquired subclinical condition affecting the gut, which is thought to result from a complex interaction between poor nutrient intake, heavy intestinal pathogen burden and impaired host intestinal barrier function. It has been implicated as an important factor in growth faltering, poor cognitive development, and sub-optimal vaccine response in children [1]. In support for the role of EE in growth faltering, children from Zambia, Pakistan and Bangladesh classified at baseline as stunted or at risk for stunting and who were non-responsive to nutritional supplementation (2–6 months), showed evidence of EE (partial villous atrophy with/without intraepithelial lymphocytic infiltration) in varying degrees, on duodenal biopsies [2,3,4]. However, when associations between EE histopathological score and length or height-for age z-score (LAZ or HAZ) were explored in children from Pakistan and Bangladesh, the only significant association was between the intra-epithelial lymphocyte density and HAZ (ß = −1.1, 95% CI −1.8 to −0.5) in the Pakistani cohort [3]. This lack of association between the degree of EE and growth, suggests that a biopsy read-out of EE may not fully reflect the functional consequences that negatively influence growth, or simply the multi-factorial nature of growth faltering. The key dysfunction postulated in growth faltering, in EE, is sub-optimal digestion/absorption (malabsorption) of nutrients and/or systemic inflammation [1, 5]. Therefore, there is a need first to first establish the nature and degree of malabsorption in children with EE.
Macronutrients are crucial for child growth, given their role as the main source of energy, and protein in particular as a linear growth regulator [6, 7]. Requirements for protein may be increased in the presence of infection and inflammation (as in EE), which can limit its availability for growth [7]. Malabsorption due to EE, can further compromise this nutrient availability. Thus, an understanding of the type and extent of macronutrient malabsorption, in children with EE, is urgently required. The evaluation of EE has been difficult when using only proxy biomarkers that are non- or minimally invasive measures, while histopathological diagnosis on intestinal biopsy is the accepted “gold” standard [8]. The present narrative review, therefore, aims to examine available evidence of malabsorption in EE, preferably when confirmed by biopsy, or by using other proxy measures. The review attempts to answer the following questions with focus on macronutrients; (1) What are the possible mechanisms that impair digestion and/or absorption of carbohydrate, protein, and fat, in EE? (2) Is there experimental evidence for reduced digestion and absorption of these macronutrients in EE? Whether EE-related malabsorption can explain linear growth faltering in children is difficult to establish, as other biological mechanisms (like growth signaling) related to EE but potentially distinct from macronutrient malabsorption can also influence growth [7] and this question is therefore not discussed in this review. The strengths and limitations of the human experimental studies are examined in relation to a battery of tests used to measure digestive and absorptive functions of the gut. Finally, research gaps related to mechanisms and experimental evidence, and directions for future studies using functional measures of the gut are provided.
Search strategy and selection criteria
References for this review were identified through searches of PubMed and Google Scholar. Search terms were specifically strung together for the questions related to mechanism, evidence, and the tests used to measure macronutrient malabsorption, in children or adults, and animals where necessary. The following search terms were used, “adults” or “children” or “animal”, “absorption” or “digestion” or “malabsorption”, “lipids” or “fat”, “carbohydrates”, “protein” or “amino acids” or “peptides”, “enteropathy” or “environmental enteropathy”, “exocrine pancreatic insufficiency”, “tests” or “breath tests” or “pancreatic tests”, “severe acute malnutrition”, without using a year filter. Boolean operators ‘AND’ and ‘OR’ were included to ensure comprehensiveness. This was complemented with additional searching of cross-references in the eligible articles and “similar articles” on PubMed. Only papers published in English were reviewed. For the question on experimental evidence, studies were eligible if their primary focus was on measuring digestion or absorption of macronutrients, in children or adults with either a biopsy confirmation or by using a proxy measure of EE. The final reference list was generated on the basis of originality and relevance to the broad scope of this review. A narrative approach was adopted as the review dealt with connecting questions, wherein mostly theoretical deductions were made for the section on mechanism that needed supporting literature, followed by evidence, which was further complemented by tests to measure malabsorption.
How can digestive and absorptive functions of the gut be measured in children?
The tests used for estimating digestive or absorptive capacity of the pancreas or the intestinal brush border membrane (BBM) are described in Table 1. Other relevant articles, which consists of tests that assess clinical pathology of gastric acid secretion, pancreatic exocrine (secretory) function (direct-invasive and non-invasive, and indirect tests), small intestinal malabsorption, and breath tests, along with their interpretation and disadvantages, have been reviewed in the past [9, 10]. Inclusive of the tests mentioned in both the past reviews, Table 1 provides details of up-to date, in current use, established and potential in vivo tests for macronutrient digestion and absorption, with focus on their relevance to children with EE. The table comprises information on the function probed by each test, standardization (against a gold standard) and validation of test protocols, the degree of insufficiency/impairment the test detects, normal values in children, and their disadvantages.
The following characteristics are preferable in tests that measure intestinal function in young children with EE, (1) non-invasive or minimally invasive, (2) detect sub-clinical or mild to moderate degree of derangement and (3) ensure participant compliance through short duration testing, less frequent biological sampling, acceptable and standardized test meals, and experiment pre-requisites such as short duration of fasting (in children <2 years). Of the tests discussed in Table 1, the direct pancreatic stimulation test could detect mild-moderate deficiency in pancreatic enzyme output but is invasive and is limited by compliance. The carbohydrate, fat and dipeptide breath tests using 13C-starch/sucrose/lactose/mixed triglyceride and benzoyl-L-tyrosyl-L-1-13C-alanine respectively, meet the criteria of being non-invasive and are relatively easy to perform, with test time varying between 1.5–6 h. A number of these tests, however, lack standardization/validation in the pediatric population, with no clarity on the degree of derangement they detect. Additionally, the normative cut-offs have not been established, and differ from lab to lab. Nevertheless, if these limitations are addressed, there is potential for improvisation of the existing tests for their use in children with EE.
Macronutrient malabsorption in EE
To understand the impact of EE on macronutrient digestion and assimilation, knowledge of the site, extent and timing of EE is necessary for the following reasons; (1) there is a proximal to distal intestinal gradient of brush-border membrane (BBM) enzymes and nutrient transporters [11], (2) intraluminal proteolytic degradation of pancreatic enzymes can occur during duodenum-ileal transit (lipolytic activity is the most susceptible to inactivation) [12], (3) the influence of ontogeny of pancreatic and BBM enzymes with age, and the influence of endogenous hormones and exogenous (diet, environmental stressors) factors [13, 14] and (4) secondary or functional exocrine pancreatic insufficiency (EPI) can occur due to impaired entero-endocrine pancreatic signaling from the proximal intestine [15]. These mechanisms underscore the spatial and temporal relevance of the highly organized intestinal delivery and digestion of macronutrients in the intestine.
There is an established duodenal-ileal gradient for absorption of glucose, AA, and peptides; AA absorption is greater in the distal small intestine than proximal, which is reverse in case of di- or tripeptides [13, 16]. Sucrase-isomaltase and β-glycosidase activities are higher in proximal jejunum, while glucoamylase is high in proximal ileum. Peptide and long-chain fatty acid transporters show predominance in the duodenum and jejunum [11]. Lipase and proteases lose ~35% of their activity during the duodenum-jejunal transit, with further loss (>40%) in transit to the ileum, as observed in response to infused macronutrients [12]. Lipolytic activity is the most susceptible to inactivation compared with proteolytic and amylase activity, and loss of elastase is minimal. The survival of the pancreatic enzymes depends on the interaction between delivery of nutrients, and the location/rate of absorption of nutrients and bile acids [12]. The BBM disaccharidase lactase-phlorizin hydrolase (LPH) activity, which is present along the entire length of the small intestine at birth shows a gradual decline with cessation of breastfeeding in many but not all ethnic groups, whereas sucrose-isomaltose (SI) activity and fructose absorption increases with introduction of complementary foods [13]. Pancreatic amylase activity that is undetectable at 1 month of life, increases subsequently with the introduction of complementary foods reaching adult levels by 2 years of age [14]. It is noted that the intestinal response to a diet depends on the age of the child, the duration, and any previous exposure [13]. Overall, studies set to determine digestive and absorptive capacity in early life (<2 years of age), should ensure that there are age-matched controls and consider evaluation of pre-experimental dietary patterns.
However, there is little information on the extent or intensity of damage of the small intestine in EE, as multiple biopsy sampling across the entire length of intestine is not feasible nor ethical in children [3]. In Zambian adults at least, EE does not affect the ileum (P Kelly, unpublished observations). Thus far, studies have only been able to characterize the degree of gross and histopathological lesions at selected sites of the intestine, mainly the duodenum and jejunum, showing partially or fully atrophied villi with loss of secretory cell lineages or a higher density of intraepithelial infiltrates [3, 4, 17, 18]. Children with severe acute malnutrition (SAM), are known to suffer from a severe form of EE, showing marked duodenal villous atrophy [19]. In addition, recent evidence from rhesus monkeys (EE model), where disrupted colonic barrier explained growth faltering, suggests the possibility of the colon being affected in children with EE [20]. This opens up new questions (Box 1) requiring further investigation into the extent of EE, considering the potential contribution of large intestinal dysfunction to the body’s nutrient economy, through energy harvesting and adaptation [21, 22]. With the advent of new technologies (such as wireless capsule endoscopy) these questions can be investigated in children with EE [1].
The present section attempts to enlist mechanisms (Fig. 1, theoretical possibility or evidence) that can cause macronutrient malabsorption, in EE, followed by available experimental evidence in children (if not, in adults), and discusses gaps in research with a note on how to investigate them.
Starting from the gastric cavity, hypochlorhydria reduces pepsin activity (may impair digestion) and leads to dysbiosis in the small intestine. Dysbiosis results in bile acid dysmetabolism and reduced fat absorption. Dysbiosis also plays a role in mucosal damage that reduces surface area for absorption, and transporter density. Enterokinase levels may be affected in mucosal damage leading to reduced pancreatic enzyme activity (may impair digestion). Reduced enteroendocrine cell density because of mucosal damage, reduces CCK release that in turn decreases pancreatic enzyme release, which impairs macronutrient digestion. Elevated levels of cortisol or E-coli toxin LPS could also reduce peptide, glucose and AA transport across the brush border membrane. The downward arrow indicates reduction, upward arrow indicates increase in levels. AA amino acids, CCK cholecystokinin, EE enteroendocrine, LPS lipopolysaccharide. Figure created with BioRender.com.
Mechanisms common for all macronutrients
The human intestinal barrier function is dependent on healthy structure and function of the epithelium, mucus layer, secreted antimicrobial factors, and microbiota [23]. Small intestinal bacterial overgrowth (SIBO), an extreme form of dysbiosis, is a well-known entity in EE [4, 24]. Dysbiosis can trigger dysregulated epithelial apoptosis, which could indirectly disrupt the “pore”, “leak” or “unrestricted” paracellular pathways (global barrier loss) [23]. A vicious cycle of hyperimmune response and intestinal inflammation can ensue, and result in perpetuated mucosal damage [23]. This mucosal damage or villous atrophy, as noted in EE, implies a reduced surface area/transporters for nutrient absorption, impaired enterokinase activity (enzyme converting zymogens to their active form) and entero-endocrine-pancreatic signaling leading to EPI and postcibal asynchrony [15, 25]. Overall, these mechanisms could differentially impair digestion and absorption of the macronutrients. Only if EPI is severe enough (trypsin and lipase <10% of the normal output) will it have a significant impact on absorption of protein and fat, reflecting the high functional reserve capacity of the pancreas [26]. Carbohydrate (CHO) digestion is the least affected, as unlike protein or fats it is well compensated by salivary and gastric amylase, with further breakdown (fermentation) by colonic bacteria [15].
Evidence for EPI
There is evidence of EPI being associated with duodenal enteropathy (of varying etiology), in children [27]. Children showing mild to severe partial villous atrophy or flat mucosa or lymphocytic infiltration of lamina propria on duodenal biopsies had pancreatic insufficiency of varying degree, which was determined using fecal elastase-1 assay [27]. Furthermore, apparently normal Senegalese children (mean age ~1 year) at high risk for EE, were reported to have sub-optimal pancreatic exocrine output, with lower amylase (35% of normal), lipase (6.8% of normal), trypsin and chymotrypsin (~40% of normal) when compared to age-and sex-matched (aged ~1 year) French children, which was then termed “silent pancreatic insufficiency” [28]. The possibility of this relatively low enzyme output causing malabsorption is more likely for fat (with <10% lipase output) than for protein or carbohydrates [15]. A study in adults with severe pancreatic insufficiency (<5% of normal enzyme output) reported a higher (7 times, as energy) ileal cumulative nutrient delivery, that is ~40% of the administered easily digestible low-calorie meal was malabsorbed [29]. In addition to this, there was accelerated gastric and small intestinal transit (2 times) and premature transition from fed to inter-digestive motility pattern compared to normal adults [29].
From these findings, EPI seems to be one of the potential mechanisms, depending on severity, by which EE could cause malabsorption of macronutrients. This suggests the possibility of pancreatic enzyme replacement therapy (PERT) to support digestion, in children with moderate to severe EE, and has been tried before. A reduction in mortality (19% versus 38%) but no difference in weight gain was noted on administration of PERT (containing lipase, amylase and protease), at a dose of 3000U of lipase/kg body weight for 28 days, in children (mean ± SD age of 20 ± 12 months) with SAM, in comparison to those who did not receive therapy [30]. In children (age range 6–30 months) with coeliac disease (similar to EE) with sub-normal pancreatic enzyme output as observed in duodenal aspirates, PERT (first 30 days) has shown a significant percentage increase (9 versus 5%) in weight-for-age, in comparison to a control group on placebo [31].
Mechanisms specific for each nutrient has been described under each macronutrient, below. In EE, these mechanisms may operate individually or synchronously, and an additive effect may substantially reduce nutrient assimilation, which may be buffered by compensatory adaptive mechanisms by the host, mainly in the gut.
Carbohydrates
Mechanism with supporting evidence
Apart from the general mechanisms described above, a direct inhibitory effect of the E-coli toxin lipopolysaccharide (LPS) has been observed on D-glucose transport, in jejunal mucosa of rabbits [32]. LPS seems to alter receptor affinity at the BBM and the basolateral membrane Na+, K+-ATPase activity [32]. The smallest dose (range used 3 µg/mL to 3 × 10−5 µg/mL) of LPS used in this experiment that had an inhibitory effect on the transporters was 3 × 10−5 µg/mL. The plasma LPS concentration in children (2–17 months at recruitment) with non-responsive (4–6 months of nutritional supplementation) stunting and EE has been noted to be in the range of 3–6 × 10−2 µg/mL2. At this concentration, there could be intestinal mucosal or serosal inhibition of nutrient transporters in these children, as observed in the rabbit model. A direct evaluation of this mechanism is difficult in-vivo, especially in children, however, breath tests using carbohydrate substrates (Table 1) can be used to test malabsorption in children with EE and associations can be drawn to circulating LPS concentrations.
Evidence for carbohydrate malabsorption
Table 2 summarizes studies in children with features of EE on small intestinal biopsy samples, and who have undergone measurements for macronutrient malabsorption. Studies in children, with suspected EE (lacking biopsy confirmation), in whom digestion or absorption of macronutrients were tested, are described under each nutrient class herein. These studies do not particularly investigate the possible mechanism by which EE causes malabsorption (as discussed for each nutrient), but some attempt has been made to pin down probable pathways.
The dual sugar assay using lactulose (disaccharide) with mannitol/rhamnose (monosaccharide), to test intestinal permeability and passive absorption respectively, is widely used as a proxy diagnostic for EE [33]. A study examining the breath excretion of 13CO2 following an oral dose (2 g/kg) of stable isotope labeled 13C-sucrose breath test (SBT) in asymptomatic Australian Aboriginal children (n = 18, 95% CI of age 8–16 months, mean length-for-age z-score of −1.9), reported significantly lower (4 versus 6%) cumulative percent dose recovered in breath at 90 min (cPDR90) when compared to healthy non-Aboriginal controls (n = 7, age range 4–60 months, length-for-age z-score not given). A significant inverse correlation was noted between cPDR90 and lactulose rhamnose ratio (LRR) (r = 0.67, 95% CI: 0.42–0.82), in the Aboriginal children (with and without diarrhea), which suggests that impaired intestinal epithelial integrity could lead to sucrose maldigestion [34]. On the contrary, Zambian adults with biopsy confirmed EE who underwent an optimized SBT, showed similar 13CO2 excretion over the experimental duration in comparison to their healthy Scottish counterparts [35]. This could suggest adequate sucrase-isomaltase enzyme (SI) activity even in EE, either compensated by the unaffected intestine or an upregulation of activity as an adaptation to EE or a high sucrose diet. In support, transcriptomic studies suggest that the brush border expression of sucrase-isomaltase enzyme (SI) and sodium-glucose transporter-1 (SGLT-1) were upregulated, in duodenal biopsies of children with EE (from Pakistan) as compared to age-matched healthy North American controls [36]. However, the current protocol of SBT discussed herein lacks sensitivity to detect mild to moderate SI deficiency and may require modification (Table 1) for further use.
An earlier systematic review summarizes studies that have tested carbohydrate malabsorption in children with SAM [37]. The review reports reduced disaccharide and monosaccharide absorption, with a higher prevalence of lactose malabsorption. In the studies reported, a variety of methods to measure carbohydrate malabsorption were used, such as, carbohydrate loading tests, fecal reducing substances or acidic pH, and a few had disaccharidase levels measured in jejunal biopsies. The studies with jejunal biopsy lacked histopathological confirmation of EE, and therefore the link between EE and malabsorption was not established. The disadvantages of these methods are provided in Table 1, and they may not reflect the true deficit in digestion/absorption or compensation by the remaining gut that was not tested in the time duration of the protocol. Nevertheless, the above evidence suggests the possibility that the BBM enzyme lactase is more sensitive/vulnerable in an EE setting and may serve as an early or better marker of malabsorption. Therefore, lactose breath tests can be employed with the suggested modification in Table 1, to evaluate disaccharide malabsorption, in children with EE.
Proteins
Mechanisms with supporting evidence
The high prevalence of hypochlorhydria in malnourished children could theoretically lead to low pepsin activity and downstream consequences of SIBO [38, 39]. There is a great deal of redundant proteolytic activity in the small intestine, with the proportional contribution of pepsin to protein digestion of only ~10–20%. This has been experimentally demonstrated in adults who have undergone gastric bypass surgeries, in whom normal dietary milk protein digestion was reported [40]. On the contrary, reduced protein assimilation has been noted on gastric acid suppression therapy (with proton pump inhibitors for 2 days), but the quantitative contribution may be unimportant [39].
In addition to the consequences of mucosal damage other factors may be implicated in poor absorption. Elevated circulatory cortisol levels as observed in undernutrition with infection or inflammation may impair jejunal peptide transport as was experimentally observed in broilers receiving dexamethasone [7, 41]. In this stress induced (dexamethasone) dose-response study (administered at 0.1, 0.5, and 2.5 mg/kg body weight), the broilers had altered jejunal mucosal morphology akin to EE and showed reduction of glycylsarcosine (artificial dipeptide) transport in an everted jejunal sac experiment, for all three doses, suggesting lower peptide (PePT-1) transporter activity [41]. A direct inhibitory effect of the E-coli toxin LPS has also been observed on Na+-dependent AA transport (leucine), similar to its action on D-glucose transport [32]. On the contrary, rats infected by Cryptosporidium parvum [42], as in EE [43], show a compensatory post-translational upregulation of PePT-1 during acute infection, which maintained the ex-vivo glycylsarcosine transepithelial flux across the ileal mucosa in comparison to non-infected rats [42]. In summary, the extent of local (gut) or systemic infection or inflammatory state could cause protein malabsorption by directly or indirectly inhibiting BBM transporters, with the potential for compensatory upregulation. There is potential for tests using stable isotope AA tracers or glycylsarcosine (Table 1), to investigate these mechanisms in EE.
Evidence for protein malabsorption
There are very few studies in asymptomatic children with EE on the digestive or absorptive capacity of protein or AAs. A recent study conducted in children (aged between 18–24 months) from urban slums in South India, who were classified using a lactulose rhamnose ratio (LRR) cut-off, into EED (LRR ≥ 0.068) and no-EED (LRR < 0.068), showed no statistically significant difference between groups for the systemic availability (after digestion and absorption) of AAs from dietary protein. The dietary protein source tested in this study was an intrinsically labeled mung bean and uniformly labeled spirulina protein [44]. There was also no difference in true phenylalanine digestibility or its absorption index between these EED groups [44]. One possible reason for this observed indifference could be the functional adaptation of the gut epithelium, by either upregulation of PePT-1 transporters or proteases [42].
A study from the past, conducted in adults, point towards AA malabsorption in EE [45]. In healthy asymptomatic Indian men with histopathological features of EE on jejunal biopsy, the mean glycine (AA) and glycylglycine (peptide) absorption from the upper jejunum was lower by 31% when compared to age matched English men [45]. The mechanism for this finding was not investigated, but the researchers suggested theoretical possibilities as pointed out in the mechanisms common for all macronutrients section (above). Contrarily, the finding of lower AA absorption could otherwise imply the role of adaptation to higher protein intakes (mostly animal source) in the English men. Overall, there is some evidence in adults but not children, to indicate AA malabsorption, in EE. Studies using novel approaches using stable isotope AA tracers or glycylsarcosine (Table 1), to determine AA or peptide malabsorption, could be performed after standardization and validation, in children from different geographical settings, with varying degree of EE, preferably confirmed by biopsy.
Fats
Mechanism of fat malabsorption with supporting evidence
Dysbiosis is associated with deconjugation (removal of taurine or glycine) of bile salts (BS) to their constituent bile acids (BA), which could decrease the BS levels below the critical micellar concentration for fat absorption, thus causing fat malabsorption [17]. In the past, the triglyceride load test using margarine was used to assess fat malabsorption in children with biopsy confirmed EE (Table 2). As mentioned in Table 2, children with giardiasis or chronic diarrhea showed only half or one third increase in plasma triglyceride concentration when compared to a control group [17, 46]. The probable mechanistic pathway implicated for reduced fat absorption in these children was the presence of a higher rate of deconjugation of BS in their jejunal aspirates. The reported mean concentration of deconjugated and conjugated BA was 20.1 (SD 15.5) µmol/mL and 18.2 (SD 16.5) µmol/mL, respectively, in children with chronic diarrhea [46]. The level of deconjugated BA in the duodenal aspirates of normal adults (aged 18–45 years) is noted to be <1 µmol/mL [47]. Barring the considerable variation in collection of bile, introduced by time from meals, enterohepatic circulation, synthetic capacity of the liver, and transit time of intestinal contents, the concentration of deconjugated BA seems to be high in these children, suggesting the possibility of causing fat malabsorption.
The Study of Environmental Enteropathy and Malnutrition (SEEM) conducted in Pakistan observed a significant positive correlation (rs = 0.32, 95% CI 0.064, 0.543) between percentage plasma glycocholic acid (a primary conjugated BA) and the total EED histopathological score of Pakistani children (n = 55, aged ~24 months) with EED, who were unresponsive to a 2–3 months nutritional intervention [48]. This finding probably indicates sub-clinical cholestasis, as proposed by the study researchers, which means lower concentration of BS in the intestine. Whereas, in another cross-sectional study conducted in Malawian children aged between 12–59 months, with suspected EE (a lactulose mannitol ratio cut-off of ≥0.15), the total median age adjusted serum BA were significantly lower in children with EE (4.51 versus 5.10 mM/L) compared to those without [49]. Additionally, the proportion of BAs conjugated with taurine instead of glycine was modestly but significantly higher in children with EE, in this study [49]. Both these findings indicate altered bile acid metabolism, but do not directly suggest the possibility of intraluminal deconjugation of BS by SIBO. Overall, this mechanism is not well supported by evidence, although it is likely to occur in children with EE, and therefore needs further investigation, using reliable tests to measure fat malabsorption (Table 1) and linking it to intraluminal (duodenal or jejunal aspirate) BS/BA concentration.
A summary, with gaps in research, and future directions
Multiple interlinking pathophysiological pathways leading to sub-optimal availability of macronutrients are implicated/proposed in EE, which could either act in tandem or in synchrony. There is some experimental evidence of lactose, AA and fat malabsorption in support of these proposed mechanisms. The potential for intestinal plasticity with the available reserve capacity may dampen the impact on growth, and lead to catch-up growth in late childhood or adolescence. On the contrary, the time taken for adaptation may be long enough to cause irreversible deficits in domains (immune system, brain) that have critical time windows for development. Future research should focus on understanding the degree of malabsorption of macronutrient, in different populations of children with EE, by adopting and standardizing available protocols. Where facilities permit, colonic biopsies can be conducted to determine if the large intestine is affected. Additionally, functional colonic contribution to the body’s nutrition economy could be explored. Longitudinal studies to establish causal links between EE related malabsorption and growth faltering are necessary, especially in high-risk settings, for early detection and prevention of deficits. Interventions with PERT, pre-digested fat/peptides could be explored in high prevalence areas to establish whether restoration of key nutrients is beneficial or not. In conclusion, the research gaps identified in this review, paves way for meaningful investigations and interventions in EE.
Change history
01 November 2024
The original online version of this article was revised: affiliation 2 has been corrected from “Manipal Academy of Higher Education, Manipal, India” to “Center for Doctoral Studies, Manipal Academy of Higher Education, Manipal, India”.
07 November 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41430-024-01538-1
References
Thompson AJ, Bourke CD, Robertson RC, Shivakumar N, Edwards CA, Preston T, et al. Understanding the role of the gut in undernutrition: what can technology tell us? Gut. 2021;70:1580–94.
Amadi B, Zyambo K, Chandwe K, Besa E, Mulenga C, Mwakamui S, et al. Adaptation of the small intestine to microbial enteropathogens in Zambian children with stunting. Nat Microbiol. 2021;6:445–54.
Liu TC, VanBuskirk K, Ali SA, Kelly MP, Holtz LR, Yilmaz OH, et al. A novel histological index for evaluation of environmental enteric dysfunction identifies geographic-specific features of enteropathy among children with suboptimal growth. PLoS Negl Trop Dis. 2020;14:e0007975.
Chen RY, Kung VL, Das S, Hossain MS, Hibberd MC, Guruge J, et al. Duodenal microbiota in stunted undernourished children with enteropathy. N Engl J Med. 2020;383:321–33.
Tickell KD, Atlas HE, Walson JL. Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies. BMC Med. 2019;17:1–9.
Inzaghi E, Pampanini V, Deodati A, Cianfarani S. The effects of nutrition on linear growth. Nutrients. 2022;14:1752.
Millward DJ. Nutrition, infection and stunting: the roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr Res Rev. 2017;30:50–72.
Hodges P, Tembo M, Kelly P. Intestinal biopsies for the evaluation of environmental enteropathy and environmental enteric dysfunction. J Infect Dis. 2021;224:S856–63.
Lankisch PG. Secretion and absorption (methods and functions). Best Pr Res Clin Gastroenterol. 2009;23:325–35.
Braden B. Methods and functions: breath tests. Best Pr Res Clin Gastroenterol. 2009;23:337–52.
Goodman BE. Insights into digestion and absorption of major nutrients in humans. Adv Physiol Educ. 2010;34:44–53.
Holtmann GE, Kelly DG, Sternby BE, DiMagno EP. Survival of human pancreatic enzymes during small bowel transit: effect of nutrients, bile acids, and enzymes. Am J Physiol Gastrointest Liver Physiol. 1997;273:G553–8.
Drozdowski LA, Clandinin T, Thomson AB. Ontogeny, growth and development of the small intestine: understanding pediatric gastroenterology. World J Gastroenterol. 2010;16:787.
McClean P, Weaver LT. Ontogeny of human pancreatic exocrine function. Arch Dis Child. 1993;68:62.
Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54:1–28.
Bhutia YD, Ganapathy V. Protein digestion and absorption. Physiol Gastrointest 2018;1:1063–86.
Jové S, Fagundes-Neto U, Wehba J, Machado NL, da Silva Patrício FR. Giardiasis in childhood and its effects on the small intestine. J Pediatr Gastroenterol Nutr. 1983;2:472–7.
Neto UF, Martins MC, Lima FL, Patricio FR, Toledo MR. Asymptomatic environmental enteropathy among slum-dwelling infants. J Am Coll Nutr. 1994;13:51–6.
Amadi B, Besa E, Zyambo K, Kaonga P, Louis-Auguste J, Chandwe K, et al. Impaired barrier function and autoantibody generation in malnutrition enteropathy in Zambia. EBioMedicine. 2017;22:191–9.
Hendrickson SM, Thomas A, Prongay K, Haertel AJ, Garzel LM, Gill L, et al. Reduced infant rhesus macaque growth rates due to environmental enteric dysfunction and association with histopathology in the large intestine. Nat Commun. 2022;13:1–13.
van der Wielen N, Moughan PJ, Mensink M. Amino acid absorption in the large intestine of humans and porcine models. J Nutr. 2017;147:1493–8.
Verbiest A, Jeppesen PB, Joly F, Vanuytsel T. The role of a colon-in-continuity in short bowel syndrome. Nutrients. 2023;15:628.
Ghosh S, Whitley CS, Haribabu B, Jala VR. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol. 2021;11:1463–82.
Vonaesch P, Morien E, Andrianonimiadana L, Sanke H, Mbecko JR, Huus KE, et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc Natl Acad Sci USA 2018;115:E8489–98.
Singh VK, Haupt ME, Geller DE, Hall JA, Diez PM. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017;23:7059.
DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813–5.
Schäppi MG, Smith VV, Cubitt D, Milla PJ, Lindley KJ. Faecal elastase 1 concentration is a marker of duodenal enteropathy. Arch Dis Child. 2002;86:50–3.
Sauniere JF, Sarles H. Exocrine pancreatic function and protein-calorie malnutrition in Dakar and Abidjan (West Africa): silent pancreatic insufficiency. Am J Clin Nutr. 1988;48:1233–8.
Layer PE, von der Ohe MR, Holst JJ, Jansen JB, Grandt DA, Holtmann GE, et al. Altered postprandial motility in chronic pancreatitis: role of malabsorption. Gastroenterology. 1997;112:1624–34.
Bartels RH, Bourdon C, Potani I, Mhango B, van den Brink DA, Mponda JS, et al. Pancreatic enzyme replacement therapy in children with severe acute malnutrition: a randomized controlled trial. J Pediatr. 2017;190:85–92.
Carroccio A, Iacono G, Montalto G, Cavataio F, Lorello D, Greco L, et al. Pancreatic enzyme therapy in childhood celiac disease. Dig Dis Sci. 1995;40:2555–60.
Abad B, Mesonero JE, Salvador MT, Garcia-Herrera J, Rodriguez-Yoldi MJ. Effect of lipopolysaccharide on small intestinal L-leucine transport in rabbit. Dig Dis Sci. 2001;46:1113–9.
Campbell RK, Schulze K, Shaikh S, Mehra S, Ali H, Wu L, et al. Biomarkers of environmental enteric dysfunction among children in rural Bangladesh. J Pediatr Gastroenterol Nutr. 2017;65:40.
Ritchie BK, Brewster DR, Davidson GP, Tran CD, McNeil Y, Hawkes JS, et al. 13C-sucrose breath test: novel use of a noninvasive biomarker of environmental gut health. Pediatr. 2009;124:620–6.
Schillinger RJ, Mwakamui S, Mulenga C, Tembo M, Hodges P, Besa E, et al. 13C-sucrose breath test for the non-invasive assessment of environmental enteropathy in Zambian adults. Front Med. 2022;9:904339.
Abtahi S, Sailer A, Roland JT, Haest X, Chanez-Paredes SD, Ahmad K, et al. Intestinal epithelial digestive, transport, and barrier protein expression is increased in environmental enteric dysfunction. Lab Invest. 2023;103:100036.
Kvissberg MA, Dalvi PS, Kerac M, Voskuijl W, Berkley JA, Priebe MG, et al. Carbohydrate malabsorption in acutely malnourished children and infants: a systematic review. Nutr Rev. 2016;74:48–58.
Sarker SA, Ahmed T, Brüssow H. Hunger and microbiology: is a low gastric acid‐induced bacterial overgrowth in the small intestine a contributor to malnutrition in developing countries? Micro Biotechnol. 2017;10:1025–30.
Evenepoel P. Alteration in digestion and absorption of nutrients during profound acid suppression. Best Pr Res Clin Gastroenterol. 2001;15:539–51.
Bojsen-Møller KN, Jacobsen SH, Dirksen C, Jørgensen NB, Reitelseder S, Jensen JE, et al. Accelerated protein digestion and amino acid absorption after Roux-en-Y gastric bypass. Am J Clin Nutr. 2015;102:600–7.
Chang WH, Li JJ, Zhang S, Zheng AJ, Yuan JL, Cai HY, et al. Effects of glucocorticoid-induced stress on absorption of glycylsarcosine in jejunum of broilers. Poult Sci. 2015;94:700–5.
Marquet P, Barbot L, Plante A, Huneau JF, Gobert JG, Kapel N. Cryptosporidiosis induces a transient upregulation of the oligopeptides transporter (PepT1) activity in neonatal rats. Exp Biol Med. 2007;232:454–60.
Salameh E, Jarbeau M, Morel FB, Zeilani M, Aziz M, Déchelotte P, et al. Modelling undernutrition with enteropathy in mice. Sci Rep. 2020;10:1–5.
Shivakumar N, Kashyap S, Jahoor F, Devi S, Preston T, Thomas T, et al. The systemic availability of indispensable amino acids from orally ingested algal and legume protein in young children at risk of environmental enteric dysfunction. Am J Clin Nutr. 2023;118:96–102.
Hellier MD, Radhakrishnan AN, Ganapathy V, Gammon A, Baker SJ. Intestinal absorption in normal Indian and English people. Br Med J. 1976;1:186–8.
Fagundes-Neto U, Viaro T, Wehba J, Patricio FR, Machado NL. Tropical enteropathy (environmental enteropathy) in early childhood: a syndrome caused by contaminated environment. J Trop Pediatr. 1984;30:204–9.
Mallory A, Kern F Jr, Smith J, Savage D. Patterns of bile acids and microflora in the human small intestine: I. Bile acids. Gastroenterology. 1973;64:26–33.
Zhao X, Setchell KDR, Huang R, Mallawaarachchi I, Ehsan L, Dobrzykowski Iii E, et al. Bile acid profiling reveals distinct signatures in undernourished children with environmental enteric dysfunction. J Nutr. 2021;151:3689–700.
Semba RD, Gonzalez-Freire M, Moaddel R, Trehan I, Maleta KM, Khadeer M, et al. Environmental enteric dysfunction is associated with altered bile acid metabolism. J Pediatr Gastroenterol Nutr. 2017;64:536.
Krasilnikoff PA, Gudmand‐Høyer E, Moltke HH. Diagnostic value of disaccharide tolerance tests in children. Acta Paediatr. 1975;64:693–8.
Patel N, Sellers ZM, Grover A, Liu QY, Maqbool A, Morinville VD, et al. Endoscopic pancreatic function testing (ePFT) in children: a position paper from the NASPGHAN Pancreas Committee. J Pediatr Gastroenterol Nutr. 2021;72:144–50.
James WP. Comparison of three methods used in assessment of carbohydrate absorption in malnourished children. Arch Dis Child. 1972;47:531–6.
Löser C, Möllgaard A, Aygen S, Hennemann O, Fölsch UR. 13C-starch breath test comparative clinical evaluation of an indirect pancreatic function test. Z Gastroenterol. 1997;35:187–94.
Amarri S, Harding M, Coward WA, Evans TJ, Weaver LT. 13C and H2 breath tests to study extent and site of starch digestion in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 1999;29:327–31.
Robayo-Torres CC, Opekun AR, Quezada-Calvillo R, Xavier V, Smith EB, Navarrete M, et al. 13C-breath tests for sucrose digestion in congenital sucrase isomaltase-deficient and sacrosidase-supplemented patients. J Pediatr Gastroenterol Nutr. 2009;48:412–8.
Lee GO, Schillinger R, Shivakumar N, Whyte S, Huq S, Konyole SO, et al. Optimisation, validation and field applicability of a 13C-sucrose breath test to assess intestinal function in environmental enteropathy among children in resource poor settings: study protocol for a prospective study in Bangladesh, India, Kenya, Jamaica, Peru and Zambia. BMJ Open. 2020;10:e035841.
Stellaard F, Koetse HA, Elzinga H, Boverhof R, Tjoonk R, Klimp A, et al. 13C-Carbohydrate breath tests: impact of physical activity on the rate-limiting step in lactose utilization. Scand J Gastroenterol. 2000;35:819–23.
Koetse HA, Stellaard F, Bijleveld CM, Elzinga H, Boverhof R, Van der Meer R, et al. Non-invasive detection of low-intestinal lactase activity in children by use of a combined 13CO2/H2 breath test. Scand J Gastroenterol. 1999;34:35–40.
Shivakumar N, Kashyap S, Kishore S, Thomas T, Varkey A, Devi S, et al. Protein-quality evaluation of complementary foods in Indian children. Am J Clin Nutr. 2019;109:1319–27.
Evenepoel P, Hiele M, Geypens B, Geboes KP, Rutgeerts P, Ghoos Y. 13C-egg white breath test: a non-invasive test of pancreatic trypsin activity in the small intestine. Gut. 2000;46:52–7.
Niederau C, Grendell JH. Diagnosis of chronic pancreatitis. Gastroenterology. 1985;88:1973–95.
Bellentani S, Grisendi A, Rinaldi M, Bertolani P, Costa G, Agostini M, et al. BT-Paba test in the diagnosis of pancreatic exocrine insufficiency in cystic fibrosis: urinary and serum determinations compared. Eur J Pediatr. 1984;143:145–8.
Ishii Y, Kohno T, Ito A, Suzuki S, Kohno T, Takayama T, et al. Measurement of extra-pancreatic secretory function by 13C-dipeptide breath test. Transl Res. 2007;149:298–303.
Lankisch PG, Lembcke B, Wemken G, Creutzfeldt W. Functional reserve capacity of the exocrine pancreas. Digestion. 1986;35:175–81.
Wieczorek-Filipiak M, Drzymala-Czyz S, Szczepanik M, Szydlowski J, Walkowiak D, Nowak JK, et al. Fecal fat concentration and excretion in the first 2 years of life: a cross-sectional study. J Pediatr Gastroenterol Nutr. 2019;68:285–9.
Keller J, Brückel S, Jahr C, Layer P. A modified 13C-mixed triglyceride breath test detects moderate pancreatic exocrine insufficiency. Pancreas. 2011;40:1201–5.
van Dijk-van Aalst K, Van Den Driessche M, van der Schoor S, Schiffelers S, van’t Westeinde T, Ghoos Y, et al. 13 C mixed triglyceride breath test: a noninvasive method to assess lipase activity in children. J Pediatr Gastroenterol Nutr. 2001;32:579–85.
Lembcke B, Braden B, Caspary WF. Exocrine pancreatic insufficiency: accuracy and clinical value of the uniformly labelled 13C-Hiolein breath test. Gut. 1996;39:668–74.
Watkins JB, Klein PD, Schoeller DA, Kirschner BS, Park R, Perman JA. Diagnosis and differentiation of fat malabsorption in children using 13C-labeled lipids: trioctanoin, triolein, and palmitic acid breath tests. Gastroenterology. 1982;82:911–7.
Wali PD, Loveridge-Lenza B, He Z, Horvath K. Comparison of fecal elastase-1 and pancreatic function testing in children. J Pediatr Gastroenterol Nutr. 2012;54:277–80.
Wieczorek-Filipiak M, Drzymała-Czyż S, Szczepanik M, Miśkiewicz-Chotnicka A, Wenska-Chyży E, Moczko JA, et al. Fecal elastase-1 in healthy children up to 2 years of age: a cross-sectional study. J Mother Child Health. 2018;22:123–7.
Uetsuki K, Kawashima H, Ohno E, Ishikawa T, Iida T, Yamamoto K, et al. Measurement of fasting breath hydrogen concentration as a simple diagnostic method for pancreatic exocrine insufficiency. BMC Gastroenterol. 2021;21:1–9.
Bandsma RH, Spoelstra MN, Mari A, Mendel M, van Rheenen PF, Senga E, et al. Impaired glucose absorption in children with severe malnutrition. J Pediatr. 2011;158:282–7.
Kashyap S, Shivakumar N, Sejian V, Deutz NE, Preston T, Sreeman S, et al. Goat milk protein digestibility in relation to intestinal function. Am J Clin Nutr 2021;113:845–53.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: NS wrote the manuscript. All the authors critically read and approved the final manuscript. PK had primary responsibility for final content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shivakumar, N., Morrison, D.J., Hegde, S.G. et al. Is there dietary macronutrient malabsorption in children with environmental enteropathy?. Eur J Clin Nutr 79, 181–194 (2025). https://doi.org/10.1038/s41430-024-01510-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-024-01510-z