Abstract
Premature infants suffer from conditions such as necrotising enterocolitis and sepsis, whose risk is reduced by breastmilk. Rates of breastfeeding are lower in premature infants compared to term infants. Insufficient breastmilk is the most commonly cited reason for breastfeeding termination. Herbal medicines are commonly used for promoting breastmilk production, but their safety and efficacy are unclear. We wanted to assess whether specific herbal galactagogues can safely and effectively increase lactation in mothers who delivered prematurely. Six databases were searched (Medline, Embase, CINAHL, AMED, COCHRANE library, ProQuest Dissertations and Theses Global) with no language or date restrictions. We included randomised controlled trials (RCTs) of herbal galactagogue use in preterm infant mothers. Ten RCTs were included, each investigating a different galactagogue or mixture. Two scored ‘high’ for risk of bias, the remainder scored ‘some concerns’. There was low certainty evidence of an increase in milk volumes by day 7 of the intervention period with barley malt and lemon balm (mean difference 149 ml, 95% CI: 38–260); silymarin in combination with phosphatidylserine and Galega (mean difference 105 ml, 95% CI: 27–183); Pimpinella anisum seed tea (mean difference 98 ml, 95% CI: 63–133); and Latuca sativa (lettuce) syrup (mean difference 82 ml, 95% CI: 60–105). There is a lack of high-quality RCTs on herbal galactagogues within the preterm population. There is low certainty evidence that Barley malt with lemon balm, silymarin phytosomes with Galega, Pimpinella anisum seed tea, Moringa oleifera leaf capsules and Latuca sativa (lettuce) syrup increase breastmilk production. Higher-quality trials are needed to confirm this effect.
Similar content being viewed by others
Introduction
Annually, approximately 11% of births (14 million babies) are born premature (at less than 37 completed weeks of gestation) [1]. 1 million of these newborns die each year, and it is the second leading cause of death in the under 5 s [2]. The World Health Organisation recommends exclusive breastfeeding for the first 6 months of life [3, 4]. If breastfeeding rates were increased to near universal levels, 12% of deaths in the under 5 s could be averted [5].
Necrotising enterocolitis (NEC) and sepsis are important causes of mortality and morbidity in premature infants; 70% of cases of NEC occur in premature infants [6]. In the US, the incidence of early-onset sepsis in premature infants is 13.5 per 1000 premature births [7], compared to 1 per 1000 of total live births [8]. A 2019 Cochrane review found that donor human milk reduces the risk of NEC compared to infant formula [9]. It is possible that raw maternal milk has an even greater beneficial effect than processed donor milk [10]. Despite this beneficial effect, infants born prematurely are less likely to be breastfed than term infants; in premature infants who are breastfed, the duration of breastfeeding is shorter than in infants born at term [11].
Insufficient breastmilk production is an important cause of breastfeeding cessation. According to the 2017 Scottish Maternal and Infant Nutrition survey [12], 86% of women who stopped breastfeeding reported concerns with milk production. Successfully breastfeeding premature infants presents additional challenges due to immature or absent coordination of swallowing and breathing compared to term babies [13, 14]. This means that many mothers of premature infants in newborn care units need to express their milk [15], often for extended periods of time, which can be difficult [15].
Premature babies are often separated from their mothers in intensive care units, making it more difficult for mothers to maintain their milk supply [16]. Premature babies are more likely to have been born by caesarean section, which also reduces the chance of breastfeeding [17]: in the US the overall caesarean delivery rate was 32.1% in 2022. This fell to 26.3% when only term babies were accounted for. Only 10.38% of babies were born preterm in 2022, but accounted for almost 6% of caesareans [18].
Galactagogues are substances that increase breastmilk production [19, 20]. The two most commonly used pharmacological agents, metoclopramide and domperidone, are both used off-label to increase lactation [21, 22]. The evidence regarding their efficacy has been mixed [23, 24]. A meta-analysis on metoclopramide found that despite significantly increasing serum prolactin, there was no increase in milk production compared to placebo [23]. All included studies gave the intervention group metoclopramide 10 mg TDS except one which gave 10 mg BD. In contrast, the most recent systematic review on domperidone showed the drug increased daily milk volumes after 14 days by 88.3 ml/day (95% CI: 56.8–119.8) compared to placebo [24]. The most significant barrier to the use of these agents in clinical practice is concern about side effects. For example, domperidone is not available in the USA due to case reports of patients receiving domperidone suffering from cardiac arrhythmias, cardiac arrests and sudden death [25].
Herbal galactagogues have been used for thousands of years and remain a popular choice. As early as the first century AD, Dioscorides wrote that both fennel and anise ‘draw down the milk’ [26]. In modern times, inquiries regarding herbal galactagogues at an Australian medicine information centre rose from 0 in 2001 to 23% of calls concerning galactagogue use in 2014 [27]. In a US survey of over 1200 breastfeeding women, 27.7% reported using herbal galactagogues. A survey of 82 US healthcare workers found that 70.4% recommended galactagogues; with fenugreek recommended most commonly [28].
However, the safety of using herbal alternatives while breastfeeding remains largely unknown [29]. The Academy of Breastfeeding Medicine has stated that while herbs have historically been used, which is reassuring, there is little scientific evidence regarding their safety and efficacy. Caution should be exercised around their use due to lack of standardised dosing outside of trial settings and the potential for contaminants in preparations [30].
Preclinical studies have shown that herbs may act through several mechanisms. A hydroalcoholic extract of fennel fruits was able to increase prolactin levels in mice [31]. Isoflavones from chickpea sprouts have an oestrogenic effect in ovariectomized rats by increasing levels of FSH and LH and increasing uterine weight [32]. A scoping review looking at 80 studies found that multiple polyphenols may affect milk production in humans, but currently there is a profound lack of information regarding the mechanisms of action of herbal galactagogues in the existing literature [33].
A number of systematic reviews have previously been carried out looking at herbal galactagogues, with variable findings. We identified one review looking solely at mothers of preterm babies and this investigated non-herbal galactagogues [24] (a previous review by Donovan and Buchanan [34] included two studies, which were included in the aforementioned review; as such, we have not included this study). We identified a further study looking at non-herbal galactagogues in preterm infants, but term infants were also included in this study population [35]. There have been five previous systematic reviews including only herbal galactagogues [36,37,38,39,40], two included only term infants [36, 40], one did not specify the study population [37], and the remaining two included both term and preterm infants [38, 39]. We also identified two reviews that included herbal and non-herbal galactagogues [20, 41], but only one included preterm infants [41].
All these reviews illustrated that herbal galactagogues were able to have some positive effect on breastmilk volumes (Supplementary Table 1).
This review aims to answer whether there are specific herbal galactagogues that can safely and effectively increase lactation in mothers of preterm babies.
Methods
The review protocol was registered on PROSPERO (CRD42023476811). Electronic searches were conducted in Medline, Embase, CINAHL, AMED, COCHRANE library and ProQuest dissertations and theses global from inception to August 2024. There were no language or date restrictions. We also searched references in relevant studies to identify additional studies. We used search terms for ‘preterm babies’, ‘breastfeeding’, ‘herbal galactogogues’ and ‘randomised controlled trials’ (Supplementary Table 2).
Inclusion criteria
We included studies in lactating mothers of preterm infants (born at less than 37 weeks of gestational age) who received any herbal medicine to increase milk volume. The primary outcome was increase in breastmilk quantity. Secondary outcomes were safety, adverse effects of treatment and increase in preterm infant weight. Only randomised controlled trials (RCTs) were included. Herbal medicines were compared to placebo, no intervention or a different intervention.
Study selection
Title, abstracts and full texts were screened in Rayyan [42] by two independent reviewers (AC, SD). Inconsistencies were resolved by discussion involving a third reviewer (MW).
Data extraction
Data extraction forms were created in Microsoft Excel. One reviewer extracted the data (AC), which was then checked by a second reviewer (MW) to confirm accuracy before analyses. Data extracted included: number and type of participants, details of intervention and control, outcomes measured, and any conflicts of interest/funding source reported by the study authors. We extracted all available data on each outcome: milk volumes for days 1, 4 and 7 of the intervention, total milk volume for intervention duration and infant weights for day 7 of the intervention. When data were not provided in a usable format, we contacted the authors for more information. Where we were unsuccessful in contacting authors, WebPlotDigitizer [43] was used to extract data from published figures (IL).
Study quality appraisal and risk of bias
Quality of the included RCTs was assessed by two independent reviewers (AC, SD) using the Cochrane Risk of Bias 2 Tool [44].
Data synthesis
Heterogeneity between study interventions prevented meta-analysis in most cases, therefore we performed a narrative synthesis.
The mean difference between intervention and control groups was used to measure effect size for each outcome, for studies which provided sufficient data. Publication bias was assessed using funnel plots where possible. Statistical analysis used Stata SE v18.0 [45].
When results were reported in grams or ounces rather than millilitres/cubic centimetres, we converted this to millilitres using the density of human milk as 1.03 g/ml [46].
Certainty of evidence
We assessed the certainty of evidence in the included trials using the GRADE system (IL, MW, AC) [47]. We evaluated methodological limitations (risk of bias), inconsistency, indirectness, imprecision, and publication bias.
Results
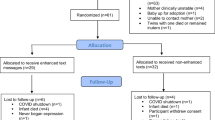
Database searching identified 1474 papers (Fig. 1) [48]. 170 duplicates were removed, leaving 1304 papers for title and abstract screening, of which 12 were selected for full text screening. Citation searching of full texts identified one additional study, and another additional study was provided by an expert. After screening, ten studies, including 856 participants, were eligible for inclusion [49,50,51,52,53,54,55,56,57,58].
Characteristics of included studies
Each study evaluated a different agent, or a mixture of agents (Table 1). Four trials investigated single herbs, as a preparation of the raw herb. Estrella et al. studied Moringa oleifera leaf capsules [58]. Reeder et al. investigated fenugreek seed capsules in a small trial of only 26 women [53]. Ranade and Mudgalkar studied Lepidium sativum seeds soaked in warm water for 30 min [54], Izaaddoost et al. trialled Lactuca sativa (lettuce) syrup [57]. Khalili et al. assessed Anise (Pimpinella anisum) seed tea [56].
Two trials investigated silymarin, an extract of Silybum marianum: Peila et al. [52]. used micronised silymarin (BIO-C®), while Zecca et al. [50]. investigated phytosomes of silymarin with phosphatidylserine and Galega (Piùlatte®).
Three commercial combination products were included in the review. Wesolowska et al. trialled ‘Femaltiker®’ (5 g barley malt with 80 mg lemon balm) [55]. Dermirci et al. investigated ‘Motherlove®: More Milk Plus Alcohol Free’, which contained a mixture of fenugreek, nettle, fennel and blessed thistle—as it is a proprietary blend, the proportions of each herb in the mixture were not specified [49]. This study was a very small feasibility trial (9 participants). Ozalkaya et al. investigated a commercially available tea, ‘Hipp Natal®’, containing a proprietary blend of 1.0% stinging nettle and six other herbs (Lemon balm (Melisa officinalis L.), caraway (Carum carvi L.), anise (Pimpinella anisum), fennel (Foeniculum vulgare), goat’s rue (Galega officinalis), and lemon grass (Cymbopogon citratus)) [51].
Most studies used placebo as a comparator [50,51,52,53, 55, 56, 58]. Ranade and Mudgalkar compared to no intervention [54], and Demirci et al. compared to meditation, which they considered an active intervention [49]. Three studies had two control groups; in all cases, one was given a placebo. Ozalkaya et al. gave the other advice on supportive measures [51]. Khalili et al. and Izaddoost et al. gave no treatment [56, 57].
Measurement of milk quantity varied slightly between studies. Six of the included studies [50, 51, 53,54,55, 58] measured milk quantity pumped within a 24-h period using an electric breast pump (the number of times per day women were advised to pump varied between studies from 6 times per day to 8–10 times per day). One study used test weighing of the infant before and after feeds in combination with measuring expressed milk volumes [52]. Two studies [56, 57] used infant weight as an outcome in addition to breastmilk volumes; in one of these studies all infants were weighed at day 0, day 3 and day 7 of the intervention to assess weight gain [56]; in the second study all infants were weighed at day 1, day 3, day 5 and day 7 of the intervention. One study used test weighing or measurement of expressed milk quantity twice a day only, at the time that the mother felt her milk supply was most abundant [49].
Risk of bias
Eight of the RCTs had ‘some concerns’ for risk of bias overall [50,51,52, 54,55,56,57], with two assessed as ‘high risk’ [49, 53] (Fig. 2) [59].
Four studies were assessed as ‘some concerns’ for domain one (bias arising from the randomisation process) due to insufficient information about allocation concealment for two studies [51, 54] and imbalance in baseline characteristics for two [49, 57].
Only four studies were assessed as ‘low risk’ for domain two[49, 50, 57, 58] (bias arising due to deviations from the intended intervention); six were assessed as ‘some concerns’ due to a failure to carry out intention-to-treat analysis, resulting in a small number of participants being excluded from data analysis. One was assessed as ‘high risk’ due to a failure to carry out intention-to-treat analysis, resulting in a large number of participant exclusions [53].
Three studies were assessed as ‘some concerns’ for bias in domain 3 (bias due to missing outcome data). One provided no reasons for the loss to follow up of participants [51]. While two provided some reasons for loss to follow-up, there was insufficient detail to be certain that participant drop-out was unrelated to outcome measurement [53, 55]. Reeder et al. [53]. lost over half their participants to follow-up, which was similar between the two study arms.
All studies except one were assessed as ‘low risk’ in domain four (bias in measurement of the outcome), as appropriate methods to measure the outcome were used. The remaining study was assessed as ‘high risk’ for this domain as an inappropriate method, which is prone to bias, was used to measure the desired outcome [49] (mothers pumped milk twice per day at times when milk was perceived to be most abundant).
No study provided a pre-specified statistical analysis plan, so all were assessed as ‘some concerns’ for domain five (bias in selection of the reported result).
There were other limitations of the included studies. One was a feasibility trial with a small sample size [49]. Peila et al. [52]. presented some of their results only as graphs, which could not be extracted with full accuracy. We attempted to contact both study authors but received no reply.
Synthesis of results
Milk volume at day 1 (Supplementary Table 3 and Supplementary Fig. 1)
Four studies provided milk volumes after day 1 of the intervention [54, 56,57,58]. Mean differences and 95% confidence intervals were calculable for all (Supplementary Table 3 and supplementary Fig. 1). Moringa oleifera leaf capsules produced a small increase in milk volume at day 1 compared to placebo, which was not statistically significant (26.9 ml, 95% CI: 22.1–31.6, P = 0.052). However, on days 2 and 3, the capsules produced much larger increases in milk volume, which were highly significant (day 2, 66.2 ml, p = 0.007; day 3, 199.5 ml, p = 0.000) [58]. Two others (Lepidium sativum and Pimpinella anisum) produced a small increase in milk volume compared to no treatment.
Milk volume at day 4 (Supplementary Table 4 and Supplementary Fig. 2)
Five studies provided milk volumes for day 4 of the intervention [50, 53, 55,56,57] ‘Piulatte®’ (silymarin with phosphatidylserine and galega) produced the largest increase (81.8 ml, 95% CI: 17.3–146.4 [50]); followed by ‘Femaltiker®’ (barley malt and lemon balm) (54.1 ml, 95% CI: −16.7 to 124.9, P = 0.13 [55]), Anise (44.9 ml, 95% CI: 16.7–73.2, P < 0.001 [56]) and Latuca sativa (lettuce) syrup (39.2 ml, 95% CI: 22.4–56.0, P < 0.001 [57]). In the case of ‘Femaltiker®’ (barley malt and lemon balm), this result was not statistically significant. The study on fenugreek seed capsules [53] (−46 ml, 95% CI: −234 to 142, P = 0.645) did not show an increase in milk volume at day 4.
Milk volume at days 7–10 (Fig. 3a, Supplementary Table 5)
Milk volumes at day 7 or 10 of the intervention period were obtained from nine studies [49,50,51,52,53,54,55,56,57] (Fig. 3a, Supplementary Table 5). The average participant was between day 9 and day 23 after birth at this time point across the different studies.
There is low certainty evidence that Femaltiker® (barley malt and lemon balm) increases milk volume at day 7 (MD: 149.1 ml, 95% CI: 38.1–260.1; one study, 80 participants) [55]. The certainty of evidence was downgraded by one level for risk of bias and one level for imprecision.
There is low certainty evidence that Piùlatte® (Silybum marianum phytosomes with Galega) increases milk volume at day 7(MD: 105 ml, 95% CI: 27.1–182.9) [50, 52]. The certainty of evidence was downgraded by one level for risk of bias and one level for imprecision. However, a different preparation of micronised Silybum marianum (Bio-C) did not have a significant effect on milk volume at day 7 [52].
There is low certainty evidence that Pimpinella anisum tea increases milk volume at day 7 (MD: 98.0 ml, 95% CI: 63.2–132.8; one study, 129 participants) [56]. The certainty of evidence was downgraded by one level for risk of bias and one level for imprecision.
There is low certainty evidence that ‘Hipp Natal®’ granules (containing 1.0% stinging nettle, Pimpinella anisum and five other herbs) increased milk volume at day 7 (MD: 99.8 ml, 95% CI: not calculable, p = 0.22; one study, 85 participants) [51]. The certainty of evidence was downgraded by one level for risk of bias and one level for imprecision.
There is low certainty evidence that Latuca sativa (lettuce) syrup increases milk volume at day 7 (MD: 82.0 ml, 95% CI: 59.5–104.5; one study, 140 participants, p < 0.001). The certainty of evidence was downgraded by one level for risk of bias and one level for imprecision.
There is low certainty evidence that Lepidium sativum seeds do not change milk volume at day 7 (MD: −0.2 ml, 95% CI: −11.0 to 10.6; one study, 46 participants) [54]. The certainty of evidence was downgraded by one level for risk of bias and one level due to imprecision.
There is very low certainty evidence that ‘Motherlove®: More Milk Plus Alcohol Free’ does not increase milk volume at day 7 (MD: 23.3 ml, 95% CI: −15.8 to 62.5; one study, 9 participants) [49]. The certainty of evidence was downgraded two levels due to risk of bias and one level for imprecision.
There is very low certainty evidence that fenugreek seed capsules do not increase milk volume at day 10 (MD: −63.0 ml, 95% CI: −354.6 to 228.6; one study, 26 participants) [53]. The certainty of evidence was downgraded two levels due to risk of bias and one level for imprecision.
A funnel plot of these studies (Fig. 4) suggests publication bias towards studies with a positive effect (five studies had a positive effect, and only two studies with a negative effect were published).
Milk volume at day 28 (Fig. 3b, Supplementary Table 6)
There is low certainty evidence that Lepidium sativum seeds increase milk volume at day 28 (MD: 67.2 ml, 95% CI: −2.9 to 137.3; one study, 46 participants) [54]. The certainty of evidence was downgraded by one level for risk of bias and one level for imprecision.
There is very low certainty evidence that micronised silymarin does not increase milk volume at day 28 (MD: 31.2 ml, 95% CI: −165.1 to 227.5; one study, 48 participants) [52]. The certainty of evidence was downgraded by one level for risk of bias and two levels for imprecision.
Milk quantity over the whole intervention period (Fig. 5, Supplementary Table 7)
There is very low certainty evidence that Piulatte® (silymarin phytosomes with phosphatidylserine and galega) increases milk volume throughout the intervention period (25 days) (MD: 2387.0 ml, 95% CI: 531.3–4242.7; one study, 100 participants) [50]. The certainty of evidence was downgraded by one level for risk of bias and two levels for imprecision.
There is very low certainty evidence that Femaltiker® (barley malt and lemon balm) increases milk volume throughout the intervention period (14 days) (MD: 1827 ml, 95% CI: 650.7–3003.4 ml; one study, 80 participants) [55]. The certainty of evidence was downgraded by one level for risk of bias and two levels for imprecision. With Piùlatte®, the increase in milk volume was statistically significant from day 4 onwards [50]. With Femaltiker® (barley malt and lemon balm), milk volumes were measured from days 4 to 11, and the increase in milk volume became statistically significant from day 6 [55].
Infant weight at day 7 of the intervention (Fig. 6, supplementary Table 8)
Four studies [49, 51, 56, 57] reported infant weight as an outcome on day 7 of the intervention (Fig. 6, Supplementary Table 8). In Khalili et al. and Ozalkaya et al.’s studies, infants were also fed formula milk [51, 56], which could confound results.
There is low certainty evidence that Pimpinella anisum tea does not increase infant weight (MD: 9.2 g, 95% CI: −55.8 to 74.2; one study, 90 participants) [56]. The certainty of evidence was downgraded by one level for risk of bias and one level for imprecision.
There is low certainty evidence that ‘Hipp Natal®’ granules (containing 1.0% stinging nettle, Pimpinella anisum and five other herbs) do not increase infant weight (MD: −3 g, 95% CI: not calculable, p = 0.99; one study, 64 participants) [51]. The certainty of evidence was downgraded by one level for risk of bias and one level for imprecision.
There is low certainty evidence that Latuca sativa (lettuce) syrup does not increase infant weight (MD: −27.2 g, 95% CI: −74.6 to 20.2, p = 0.822; one study, 140 participants). The certainty of evidence was downgraded by one level for risk of bias and one level for imprecision.
There is very low certainty of evidence that ‘Motherlove®: More Milk Plus Alcohol Free’ does not increase infant weight (MD: −223.9 g, 95% CI: −879.0 to 431.2, one study, 9 participants) [49]. The certainty of evidence was downgraded two levels for risk of bias and one level for imprecision.
Safety of the interventions
Nine studies reported that no participants experienced any side effects; however, only three studies clearly stated how the presence of adverse effects was assessed [51, 54, 55]. Only one study (‘Motherlove®: More Milk Plus Alcohol Free’ [49]) reported a number of minor maternal side effects: headache (n = 1), nausea (n = 1), bodily smell of maple-syrup (n = 2), increased perspiration (n = 1). No infant side effects were reported by any included study.
Discussion
Summary of findings and comparison with existing literature
Our search identified only 10 eligible RCTs of herbal galactagogues in mothers of preterm babies, all of different products. There were no safety concerns. From the limited available evidence, barley malt with lemon balm powder, silymarin phytosomes with phosphatidylserine and Galega, Moringa oleifera leaf capsules, Anise seed tea and Latuca sativa (lettuce) syrup appear most promising for further research.
Barley malt
Barley malt is the major constituent of Femaltiker®, which had a significant effect on breastmilk production from day 6 of intervention [55]. Beer was traditionally consumed to increase lactation; it has been shown that barley malt is the active component [60]. Experiments on ewes found that intravenous administration of barley malt increased blood prolactin levels, suggesting this is the mechanism behind its effect on breastmilk [60]. Barley malt is a safe, inexpensive and widely available plant product, which could be recommended to breastfeeding mothers.
Silymarin
Silymarin is an extract of the milk thistle plant (Silybum marianum), and is believed to be responsible for its therapeutic effects [61]. Silymarin includes four flavonolignans (silybin, silychristin, silydianin and isosilybin), which are phytoestrogens [50]. These may increase breastmilk volumes by acting at D2 receptors to increase prolactin levels [62].
Of the two studies of silymarin, only one showed a significant increase in milk volume after 7 days of using a combination of 400 mg Silybum marianum with 130 mg phosphatidylserine, and 150 mg Galega officinalis (‘Piulatte®’). The silymarin was combined with the phospholipid in order to create ‘phytosomes’, which are claimed to improve intestinal absorption of the insoluble silymarin.
Although the clinical trial of micronised silymarin (‘BIO-C®’) showed no significant effect [52], a different trial of the same preparation in lactating mothers of term babies [63] (excluded from this review) reported significant increases in milk volume over 63 days compared to placebo (85.9% v 32.1%). The reasons for this different result are not clear, because the same preparation and dose were used; however, the eligibility criteria were not clear in the study of term babies.
Anise
There was low certainty evidence that Anise (Pimpinella anisum) seed tea [56] increased milk volumes in the short term, as the study by Khalili et al. only lasted for 7 days and there was no long-term follow-up of participants.
Hipp Natal® tea was used by Ozalkaya et al. [51]. (which also contained anise) resulted in a statistically significant increase in breastmilk volumes when adjusting for baseline milk volumes (but not at day seven alone as reported above). However, the proportions of the different herbs used in this product were not specified, so the specific role of Anise cannot be deduced from this.
Anise contains anethole, whose structure is similar to dopamine [64]. Dopamine inhibits the release of prolactin. Anethole may compete with dopamine, thus preventing inhibition of prolactin release, allowing prolactin levels to rise [64].
However, both anise and lemon balm contain the aromatic compound estragole [65], which has a dose-dependent genotoxic effect [66]. Animal studies suggested that this effect would likely be minimal in the dose range of 1–10 mg/kg, which is 100–1000 times greater than the expected human exposure to estragole [67]. However, the Herbal Medicinal Products Committee of the European Medicines Agency recently updated its guidance [67], recommending that breastfeeding women should not exceed a daily dose of 0.05 mg of estragole, making regulatory agencies potentially cautious about its use. However, it is unlikely that the dose in a herbal galactagogue preparation would exert a harmful effect. Anise has a long history of use in lactating women, with no known problems, at doses which do not greatly exceed amounts used in foods [68].
Latuca sativa
Latuca sativa, commonly known as lettuce, contains lignan, a component of the phytoestrogen family [69] and flavonoids [70], which may be responsible for its galactagogue effect. Lettuce is widely cultivated [70], making it cheap and readily available. As it is a food, it is very safe for human consumption.
Moringa oleifera
M. oleifera, commonly referred to as the ‘drumstick tree’ because of its large seed pods, is native to the western sub-Himalayan regions of India, Pakistan, Bangladesh and Afghanistan, but has been cultivated for food and medicine in tropical Asia, sub-Saharan Africa, Latin America and the Caribbean [71, 72]. In the Philippines, Moringa is commonly known as ‘Malunggay’, where almost all of the plant is used in multiple industries such as food, cosmetics and herbal medicine [73]. Its leaves, pods and flowers are commonly eaten. The leaves are especially rich in protein, essential amino acids, iron, copper, calcium, Vitamin C and carotenoids [71, 74] and have been promoted as a nutritional supplement for malnourished children and lactating women [75].
Therefore, various non-governmental organisations and governments have supported large-scale planting of Moringa [76]. The safety of the leaves has been confirmed by laboratory experiments in rats—even at doses of 2000 mg/kg, no mortality ensued [77].
Moringa has a long history of use in traditional medicine, with both the leaves and seed pods being used [78]. It is believed to increase lactation by increasing prolactin levels; however, its mechanism of action is still unclear [37].
Fenugreek
There was very low certainty evidence about any effect of fenugreek for mothers of preterm infants in the two included studies, which were both extremely small, so no conclusions can be drawn in this population.
Although fenugreek is commonly used as a galactagogue, its mechanism of action is poorly understood [36]. A 2020 study in rats, which used a dry water extract of fenugreek seeds at 1 g/kg body weight/day, indicated that fenugreek may increase milk production by stimulating insulin secretion and a modulation of the insulin/GH/IGF-1 axis [79].
There has been a previous systematic review of five RCTs of fenugreek as a galactagogue in the mothers of term infants and preterm infants. Aside from the study by Reeder et al, this review included three studies of fenugreek herbal tea and one of fenugreek seed capsules. The review suggested that fenugreek increased breastmilk production more than a placebo but was less effective than two other herbal products, namely Coleus amboinicus and palm dates [38].
A number of side effects from fenugreek use have been reported [80]. Fenugreek has the potential to cause anticoagulant effects, and therefore should not be used by women who currently take anticoagulants or women who have a history of clotting disorders [80]. Fenugreek has been associated with nausea, vomiting and diarrhoea [80]. Fenugreek may cause hypotension, so it should be used with caution in those with existing low blood pressure or patients who take anti-hypertensives; however, a previous systematic review concluded it did not cause a significant reduction in systolic or diastolic blood pressure unless doses of 15 g/day or more over a minimum of 12 weeks were used [81]. Therefore, it is unlikely that the dose used to promote lactation would have a harmful effect.
Additionally, fenugreek has been linked to hypoglycaemia [82], and so is not recommended for use in women with insulin-dependent diabetes.
Study strengths and limitations
The strengths of this review include the following. This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [83]. We conducted a comprehensive search of six databases, including grey literature. The citations of articles meeting our inclusion criteria were also searched. The search strategy used was checked by a librarian with specialist knowledge. We attempted to contact the authors of studies where possible when clarification was required. There were no language or date restrictions. All included studies were double-screened. Data extraction and risk of bias assessments conducted by one reviewer were checked by a second independent reviewer.
Limitations of this review include the following. More articles might have been identified by searching other databases, such as Chinese language databases for herbal medicines. We were only able to identify a small number of studies which met our inclusion criteria, and these studies had a small number of participants. Although we attempted to contact several authors for clarification, not all responded. Meta-analysis was only possible between two studies, which had similar interventions; no other meta-analysis was possible due to heterogeneity between interventions. The funnel plot showed that it is likely that there is publication bias as a whole in the field of herbal galactagogues, and that, therefore, there are probably unpublished studies with negative results.
Our methodology could have been improved by calculating the mean difference in change from baseline between study arms to adjust for differences in milk production at baseline. As not all included studies provided baseline values, this was not possible.
We changed one inclusion criterion from the protocol registered on PROSPERO, which specified we would only include mothers with lactational insufficiency. We broadened this to include all mothers of preterm babies, as there is no clear definition of ‘lactational insufficiency’.
Implications for policy and practice
All included outcomes were rated as either having low or very low certainty of evidence, so any recommendations must be cautious. Nevertheless, breastfeeding mothers of preterm babies may request advice on the most useful herbal galactagogues and it is important to give evidence-based recommendations. This review suggests that the safest, most effective and affordable ‘natural’ galactagogues for mothers of preterm babies are barley malt, lettuce syrup, and Moringa oleifera leaf capsules. Lepidium sativum [54] took 28 days to have a significant effect. Despite fenugreek’s common recommendation by healthcare professionals [28]; based on one study in our review, there is insufficient evidence on its efficacy in mothers of preterm babies. There was evidence in favour of silymarin phytosomes (combined with phosphatidylserine) with Galega (Piùlatte® [50]), but as a branded supplement, this is expensive. A box of sachets, which would last for 14 days, currently retails for ~£25 [84], so would not be accessible for all lactating mothers, and would likely not be available in many countries.
Priorities for future research
Our results suggest that the top priorities are high-quality RCTs of barley malt, Latuca sativa (lettuce) syrup, Moringa oleifera leaf capsules, and Silymarin phytosomes with Galega in breastfeeding mothers of preterm infants. Given the widespread use of fenugreek in practice, a large RCT would also be useful for this. Trials would also be useful to evaluate the safety and effectiveness of other herbal products which have a significant effect on mothers of term babies, but have not yet been studied in mothers of preterm babies. There is little evidence on the optimal doses required to exert a therapeutic effect for any of these agents, so dose-finding studies [85] are also needed to define the optimal therapeutic doses.
It is important to note that published RCTs used only one form of the aforementioned galactagogues, so we can only draw conclusions based on the effectiveness of those preparations rather than the effectiveness of the herb in general. Different preparations, for example, a capsule containing the dried herb or an aqueous preparation such as a tea of the same herb, may have a different effect. Different parts of the same plant may also produce different results. How each galactagogue would affect milk production in a different form (for example, a tea or tincture), may be a consideration for future research. It is also important to consider that several of our studies used capsules, which is very different to the traditional form in which herbs were traditionally consumed. In future, researchers should consider using herbs in traditional preparations when considering their efficacy. However, this would make blinding difficult, which is why capsules may be more useful when conducting a double-blind trial.
Future RCTs should be correctly powered to allow clinically relevant conclusions to be drawn; double-blinded where possible (although this could be challenging for some products with a distinctive taste or smell); and should provide a pre-specified analysis plan. Confounding factors such as maternal age, milk removal frequency, and delivery method should be recorded and controlled for in the analysis, especially if groups are not well balanced [86]. When using herbal medicines, it is important to ensure product quality and consistency due to factors such as geographical variations in plants and differences in cultivation methods between regions [87].
Our included studies measured outcomes such as breastmilk volumes, infant weight and serum prolactin levels. It could be argued that an increase in breastmilk volume is not an end in itself. For example, the percentage of mothers who continue to breastfeed until their child is 6 months of age is an important outcome, which was not assessed in any included trials. However, the maternal anxiety that surrounds milk volumes following a preterm birth means it is still an important direct outcome to measure.
Four of our included studies measured infant weight as an outcome [49, 51, 56, 57]. Two of these studies [49, 51] stated that infant formula was used as a supplement to maternal milk. In the other two studies [56, 57], it is unclear if infants were given infant formula. The time of day when infants were weighed was only reported by two studies [56, 57]. Standardising when infants are weighed may not be possible in a busy hospital setting. If mothers are responsible for weighing their own infant, this relies on all participants adhering to the study protocol. If milk volumes are insufficient and supplementary infant formula is used, the amount of infant formula used should be measured and included in the analysis. If infant weight is used as an outcome, care should be taken to measure it in such a way that the results are able to provide meaningful information. Seven days is a very short period over which to conduct a study, so future studies should have longer follow-up periods.
More efforts should be made to reduce losses to follow-up by providing incentives for participants to continue, such as offering vouchers, and keeping them informed about the relevance and progress of the study [88].
Conclusions
Low-certainty evidence suggests that Femaltiker® (barley malt with lemon balm), Anise (Pimpinella anisum) seed tea, silymarin phytosomes (with Galega), Moringa oleifera leaf capsules and Latuca sativa (lettuce) syrup, may be of benefit to lactating mothers of preterm infants. Further high-quality clinical trials are needed for all these, and other herbal galactogogues which are effective in the mothers of term babies. Based on the only currently available study of fenugreek use in this population, there is at present insufficient evidence to recommend fenugreek or other herbal galactogogues for mothers of preterm infants.
References
Ohuma EO, Moller A-B, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. 2023;402:1261–71.
Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. 2022;6:106–15.
Binns C, Lee M, Low WY. The Long-Term Public Health Benefits of Breastfeeding. Asia Pac J Public Health. 2016;28:7–14.
World Health Organisation. Breastfeeding. 2023 [cited 2023 Sep 21]. Available from: https://www.who.int/health-topics/breastfeeding#tab=tab_2.
Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–90.
Ginglen JG, Butki N. Necrotizing enterocolitis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
Flannery DD, Edwards EM, Puopolo KM, Horbar JD. Early-onset sepsis among very preterm infants. Pediatrics. 2021;148:e2021052456.
Stoll BJ, Puopolo KM, Hansen NI, Sánchez PJ, Bell EF, Carlo WA, et al. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 2020;174:e200593.
Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;7:CD002971.
Hård AL, Nilsson AK, Lund AM, Hansen-Pupp I, Smith LE, Hellström A. Review shows that donor milk does not promote the growth and development of preterm infants as well as maternal milk. Acta Paediatr. 2019;108:998–1007.
Donath SM, Amir LH. Effect of gestation on initiation and duration of breastfeeding. Arch Dis Child Fetal Neonatal Ed. 2008;93:F448–50.
Scottish Government. Scottish Maternal and Infant Nutrition Survey 2017. Edinburgh: Scottish Government; 2018.
Briere CE, Lucas R, McGrath JM, Lussier M, Brownell E. Establishing breastfeeding with the late preterm infant in the NICU. J Obstet Gynecol Neonatal Nurs. 2015;44:102–13.
Carpay NC, Kakaroukas A, D Embleton N, van Elburg RM. Barriers and facilitators to breastfeeding in moderate and late preterm infants: a systematic review. Breastfeed Med. 2021;16:370–84.
Asztalos EV. Supporting mothers of very preterm infants and breast milk production: a review of the role of galactogogues. Nutrients. 2018;10:600.
Hallowell SG, Spatz DL. The relationship of brain development and breastfeeding in the late-preterm infant. J Pediatr Nurs. 2012;27:154–62.
Chaplin J, Kelly J, Kildea S. Maternal perceptions of breastfeeding difficulty after caesarean section with regional anaesthesia: a qualitative study. Women Birth. 2016;29:144–52.
Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: Final Data for 2022. Natl Vital Stat Rep. 2024;73:1–56. .
Gabay MP. Galactogogues: medications that induce lactation. J Hum Lact. 2002;18:274–9.
Foong SC, Tan ML, Foong WC, Marasco LA, Ho JJ, Ong JH. Oral galactagogues (natural therapies or drugs) for increasing breast milk production in mothers of non-hospitalised term infants. Cochrane Database Syst Rev. 2020;5:CD011505.
McGuire TM. Drugs affecting milk supply during lactation. Aust Prescr. 2018;41:7–9.
Grzeskowiak LE, Wlodek ME, Geddes DT. What evidence do we have for pharmaceutical galactagogues in the treatment of lactation insufficiency?—A narrative review. Nutrients. 2019;11:974.
Hussain NHN, Noor NM, Ismail SB, Zainuddin NA, Sulaiman Z. Metoclopramide for milk production in lactating women: a systematic review and meta-analysis. Korean J Fam Med. 2021;42:453–63.
Grzeskowiak LE, Smithers LG, Amir LH, Grivell RM. Domperidone for increasing breast milk volume in mothers expressing breast milk for their preterm infants: a systematic review and meta-analysis. BJOG. 2018;125:1371–8.
Food and Drug Administration. FDA Talk Paper: FDA warns against women using unapproved drug, domperidone, to increase milk production. 2004. Available from: https://www.fda.gov/drugs/information-drug-class/fda-talk-paper-fda-warns-against-women-using-unapproved-drug-domperidone-increase-milk-production.
Gunther RT. The Greek herbal of dioscorides. London and New York: Hafner Publishing Co.; 1968. pp. 299 and 314.
Grzeskowiak LE, Hill M, Kennedy DS. Phone calls to an Australian pregnancy and lactation counselling service regarding use of galactagogues during lactation–the MotherSafe experience. Aust N Z J Obstet Gynaecol. 2018;58:251–4.
Bazzano AN, Littrell L, Brandt A, Thibeau S, Thriemer K, Theall KP. Health provider experiences with galactagogues to support breastfeeding: a cross-sectional survey. J Multidiscip Healthc. 2016;9:623–30.
Budzynska K, Gardner ZE, Dugoua JJ, Low Dog T, Gardiner P. Systematic review of breastfeeding and herbs. Breastfeed Med. 2012;7:489–503.
Brodribb W. Medicine AoB. ABM Clinical Protocol# 9: Use of galactogogues in initiating or augmenting maternal milk production, second revision 2018. Breastfeed Med. 2018;13:307–14.
Sadeghpour N, Khaki A, Najafpour A, Dolatkhah H, Montaseri A. Study of Foeniculum vulgare (fennel) seed extract effects on serum level of estrogen, progesterone and prolactin in mouse. Crescent J Med Biol Sci. 2015;2:23–7.
Ma HR, Wang J, Qi HX, Gao YH, Pang LJ, Yang Y, et al. Assessment of the estrogenic activities of chickpea (Cicer arietinum L) sprout isoflavone extract in ovariectomized rats. Acta Pharmacol Sin. 2013;34:380–6.
Kelleher SL, Burkinshaw S, Kuyooro SE. Polyphenols and lactation: molecular evidence to support the use of botanical galactagogues. Mol Nutr Food Res. 2024;68:2300703.
Donovan TJ, Buchanan K. Medications for increasing milk supply in mothers expressing breastmilk for their preterm hospitalised infants. Cochrane Database Syst Rev. 2012;2012:CD005544.
Shen Q, Khan KS, Du M-C, Du W-W, Ouyang Y-Q. Efficacy and safety of domperidone and metoclopramide in breastfeeding: a systematic review and meta-analysis. Breastfeed Med. 2021;16:516–29.
Mortel M, Mehta SD. Systematic review of the efficacy of herbal galactogogues. J Hum Lact. 2013;29:154–62.
King J, Raguindin PF, Dans L. Moringa oleifera (Malunggay) as a galactagogue for breastfeeding mothers: a systematic review and meta-analysis of randomized controlled trials. Philipp J Pediatr. 2013;61:34–42.
Khan TM, Wu DB, Dolzhenko AV. Effectiveness of fenugreek as a galactagogue: a network meta-analysis. Phytother Res. 2018;32:402–12.
Kwan SH, Abdul-Rahman PS. Clinical study on plant galactagogue worldwide in promoting women’s lactation: a scoping review. Plant Foods Hum Nutr. 2021;76:257–69.
Dilokthornsakul W, Rinta A, Dhippayom T, Dilokthornsakul P. Efficacy and safety of ginger regarding human milk volume and related clinical outcomes: a systematic review of randomized controlled trials. Complement Med Res. 2022;29:67–73.
Bazzano AN, Hofer R, Thibeau S, Gillispie V, Jacobs M, Theall KP. A review of herbal and pharmaceutical galactagogues for breast-feeding. Ochsner J. 2016;16:511–24.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Rev. 2016;5:1–10.
Rohatgi A. WebPlotDigitizer [software]. Version 4.5. Pacifica (CA): Ankit Rohatgi; 2021. Available from: https://automeris.io/WebPlotDigitizer
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
StataCorp. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC; 2023.
Hughes SW. Measuring liquid density using Archimedes’ principle. Phys Educ. 2006;41:445.
Ryan R, Hill S. How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group Version 3.0. 2016.
Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. 2022;18:e1230.
Demirci JR, Bare S, Cohen SM, Bogen DL. Feasibility and acceptability of two complementary and alternative therapies for perceived insufficient milk in mothers of late preterm and early term infants. Altern Complement Ther. 2016;22:196–203.
Zecca E, Zuppa AA, D’Antuono A, Tiberi E, Giordano L, Pianini T, et al. Efficacy of a galactogogue containing silymarin-phosphatidylserine and galega in mothers of preterm infants: a randomized controlled trial. Eur J Clin Nutr. 2016;70:1151–4.
Ozalkaya E, Aslandoğdu Z, Özkoral A, Topcuoğlu S, Karatekin G. Effect of a galactagogue herbal tea on breast milk production and prolactin secretion by mothers of preterm babies. Niger J Clin Pract. 2018;21:38–42.
Peila C, Coscia A, Tonetto P, Spada E, Milani S, Moro G, et al. Evaluation of the galactogogue effect of silymarin on mothers of preterm newborns (<32 weeks). Pediatr Med Chir. 2015;37:1–5.
Reeder C, Legrand A, O’Connor-Von SK. The effect of fenugreek on milk production and prolactin levels in mothers of preterm infants. Clin Lact. 2013;4:159–65.
Ranade M, Mudgalkar N. The efficacy of diet supplemented with Lepidium sativum (Chandrashoor) on expressed breast milk volume in hypogalactic mothers—an open-label noncross-over randomized trial. Ayu. 2021;42:35–8.
Wesolowska A, Pietrzak B, Kociszewska-Najman B, Wielgos M, Czajkowski K, Wietrak E, et al. Barley malt-based composition as a galactagogue—a randomized, controlled trial in preterm mothers. Ginekol Pol. 2021;92:118–25.
Khalili S, Amiri-Farahani L, Haghani S, Bordbar A, Shojaii A, Pezaro S. The effect of Pimpinella Anisum herbal tea on human milk volume and weight gain in the preterm infant: a randomized controlled clinical trial. BMC Complement Med Ther. 2023;23:19.
Izaddoost N, Amiri-Farahani L, Haghani S, Bordbar A, Shojaii A, Pezaro S. The effect of orally consumed Lactuca sativa syrup on human milk volume and weight gain in the preterm infant: a randomized controlled clinical trial. Sci Rep. 2023;13:18896.
Estrella MCP, Mantaring J, David G. A double-blind, randomized controlled trial on the use of malunggay (Moringa oleifera) for augmentation of the volume ofbreastmilk among non-nursing mothers of preterm infants. Phillipp J Pediatr. 2000;49:3–6.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61.
Sawagado L, Houdebine LM. Identification of the lactogenic compound present in beer. Ann Biol Clin. 1988;46:129–34.
Karimi G, Vahabzadeh M, Lari P, Rashedinia M, Moshiri M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran J Basic Med Sci. 2011;14:308.
Capasso R, Aviello G, Capasso F, Savino F, Izzo AA, Lembo F, et al. Silymarin BIO-C®, an extract from Silybum marianum fruits, induces hyperprolactinemia in intact female rats. Phytomedicine. 2009;16:839–44.
Di Pierro F, Callegari A, Carotenuto D, Tapia MM. Clinical efficacy, safety and tolerability of BIO-C (micronized Silymarin) as a galactagogue. Acta Biomed. 2008;79:205–10.
Albert-Puleo M. Fennel and anise as estrogenic agents. J Ethnopharmacol. 1980;2:337–44.
De Vincenzi M, Silano M, Maialetti F, Scazzocchio B. Constituents of aromatic plants: II. Estragole. Fitoterapia. 2000;71:725–9.
Smith R, Adams T, Doull J, Feron V, Goodman J, Marnett L, et al. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances—methyl eugenol and estragole. Food Chem Toxicol. 2002;40:851–70.
European Medicines Agency. Public statement on the use of herbal medicinal products containing estragole (EMA/HMPC/137212/2005 Rev 1 Corr 1 Committee on Herbal Medicinal Products (HMPC)). London: EMA; 2023 [cited 2023 Dec 21]. Available from: https://www.ema.europa.eu/en/documents/other/public-statement-use-herbal-medicinal-products-containing-estragole-revision-1_en.pdf.
Barnes J, Anderson LA, Phillipson JD. Herbal medicines: a guide for healthcare professionals. 2nd ed. London: Pharmaceutical Press; 2002.
Di Gioia F, Petropoulos SA. Phytoestrogens, phytosteroids and saponins in vegetables: biosynthesis, functions, health effects and practical applications. Adv Food Nutr Res. 2019;90:351–421.
Shi M, Gu J, Wu H, Rauf A, Emran TB, Khan Z, et al. Phytochemicals, nutrition, metabolism, bioavailability, and health benefits in lettuce—a comprehensive review. Antioxidants. 2022;11:1158.
Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005;1:1–15.
Morton JF. The horseradish tree, Moringa pterygosperma (Moringaceae)—a boon to arid lands?. Economic Bot. 1991;45:318–33.
Palada MC (ed.) The moringa industry in the Philippines: status, challenges and opportunities. In: I International Symposium on Moringa; 2015 Nov 15-18; Manila, Philippines. Leuven, Belgium: International Society for Horticultural Science; 2017. Acta Horticulturae 1158.
Fuglie LJ. L’arbre de la vie: les multiples attributs du moringa. Dakar, Sénégal, New York, NY: CTA; Church World Service; 2002.
Pandey A, Pradheep K, Gupta R, Nayar ER, Bhandari D. ‘Drumstick tree’(Moringa oleifera Lam.): a multipurpose potential species in India. Genet Resour Crop Evol. 2011;58:453–60.
Thurber MD, Fahey JW. Adoption of Moringa oleifera to combat under-nutrition viewed through the lens of the “Diffusion of Innovations” theory. Ecol Food Nutr. 2009;48:212–25.
Adedapo A, Mogbojuri O, Emikpe B. Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. J Med Plants Res. 2009;3:586–91.
Othman N, Lamin RAC, Othman CN. Exploring Behavior on the Herbal Galactagogue Usage among Malay Lactating Mothers in Malaysia. Procedia Soc Behav Sci. 2014;153:199–208.
Sevrin T, Boquien C-Y, Gandon A, Grit I, de Coppet P, Darmaun D, et al. Fenugreek stimulates the expression of genes involved in milk synthesis and milk flow through modulation of insulin/GH/IGF-1 axis and oxytocin secretion. Genes. 2020;11:1208.
Shawahna R, Qiblawi S, Ghanayem H. Which benefits and harms of using fenugreek as a galactogogue need to be discussed during clinical consultations? A Delphi study among breastfeeding women, gynecologists, pediatricians, family physicians, lactation consultants, and pharmacists. Evid Based Complement Altern Med. 2018;2018:2418673.
Amini MR, Payandeh N, Sheikhhossein F, Pourreza S, Ghalandari H, Askarpour M, et al. The effects of fenugreek seed consumption on blood pressure: a systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc Prev. 2023;30:123–33.
Vijayakumar MV, Singh S, Chhipa RR, Bhat MK. The hypoglycaemic activity of fenugreek seed extract is mediated through the stimulation of an insulin signalling pathway. Br J Pharmacol. 2005;146:41–8.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
PharmacyClub. Humana Piulatte 14 Sachets. 2023 [cited 2023 Dec 27]. Available from: https://www.pharmacyclub.net/uk/supplements/pregnancy/humana-piulatte-14-sachets.
Schmidt R. Dose-finding studies in clinical drug development. Eur J Clin Pharmacol. 1988;34:15–9.
Dong D, Ru X, Huang X, Sang T, Li S, Wang Y, et al. A prospective cohort study on lactation status and breastfeeding challenges in mothers giving birth to preterm infants. Int Breastfeed J. 2022;17:6.
Wang H, Chen Y, Wang L, Liu Q, Yang S, Wang C. Advancing herbal medicine: enhancing product quality and safety through robust quality control practices. Front Pharm. 2023;14:1265178.
Nunan D, Aronson J, Bankhead C. Catalogue of bias: attrition bias. BMJ Evid Based Med. 2018;23:21–2.
Acknowledgements
The authors would like to thank the UHS librarian, Ms Paula Sands, for her help in refining the search strategy to ensure the authors would find all results relevant to the inclusion criteria. The authors would also like to thank the UHS library department in general for supplying us with loans of inter-library articles when required. The authors are very grateful to an anonymous reviewer for producing a very thorough and knowledgeable review, which helped us to greatly improve this paper.
Funding
MLW’s salary was funded by the NIHR (grant NIHR302412). The authors did not receive a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AC wrote the initial draft of the manuscript, proposed the initial search strategy, screened full texts and abstracts, contributed to risk of bias assessment, and contributed to data extraction. MW proposed the initial research question, provided feedback on and helped write the manuscript, reviewed the risk of bias assessment, and reviewed data extraction. IL provided feedback on the search strategy used, provided feedback on and helped write the manuscript, and contributed to data extraction and analysis. SD screened full texts and abstracts and contributed to the risk of bias assessment. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cragg, A., Levene, I., Darabi, S. et al. Herbal galactagogues to improve breastmilk production and lactation in mothers of preterm babies: a systematic review of clinical trials. Eur J Clin Nutr (2025). https://doi.org/10.1038/s41430-025-01679-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41430-025-01679-x