Abstract
Assessment of central blood pressure (BP), pulse wave velocity (PWV), and augmentation index (AIx) measurements may improve cardiovascular risk stratification. This study aimed to establish reference office values for central BP, PWV, and AIx by means of a Mobil-O-Graph PWA monitor and to evaluate the impact of cardiovascular risk factors (CVRFs) on these measurements. We cross-sectionally evaluated clinical characteristics, central BP, PWV, AIx, and peripheral BP measurements among 867 apparently healthy individuals (age = 46.0 ± 15.5 years, 39% males) who were free of obesity, hypertension, active smoking, dyslipidemia, and diabetes (CVRF-No) and 5632 individuals (age = 57.0 ± 14.7 years, 44% males) with at least one of these major CVRFs (CVRF-Yes). Reference values for central BP, PWV, and AIx were provided for the CVRF-No and CVRF-Yes groups, stratified by age and sex. PWV and AIx exhibited curvilinear increases with age, and there was an interaction between age and sex for central systolic BP and PWV in both the CVRF-No and CVRF-Yes groups. The results of a multivariable analysis including the whole sample (n = 6499) showed that obesity had a direct association with central BP, while diabetes was directly related to PWV. In addition, alcohol intake was directly associated with central BP, while performance of physical activity was inversely related to AIx. In conclusion, values of office-measured central BP, PWV, and AIx obtained in an apparently healthy population and in a population with CVRFs are now available according to age and sex and may be useful to build thresholds for use in clinical practice.
Similar content being viewed by others
Introduction
Central blood pressure (BP), pulse wave velocity (PWV), and augmentation index (AIx) measurements are established markers of central hemodynamics and arterial stiffness and provide complementary information regarding arterial health [1,2,3,4]. Importantly, these parameters may add predictive value for cardiovascular risk estimation over that of traditional peripheral BP measurements [5,6,7].
The Mobil-O-Graph PWA monitor is part of a generation of devices that allow joint, noninvasive measurement of central BP, AIx, and PWV and has been used in diverse clinical settings [8,9,10]. However, some reports have indicated that the absolute values of central BP, AIx, and PWV measured by the aforementioned monitor are slightly different from those obtained by alternative noninvasive approaches [11,12,13,14], suggesting that the reference values of measurements obtained by distinct devices and techniques assessing central hemodynamics and arterial stiffness might not be interchangeable.
The present study aimed to establish reference office values for central BP, PWV, and AIx by means of the Mobil-O-Graph PWA monitor in apparently healthy individuals without major cardiovascular risk factors (CVRFs) as well as in an alternative sample of individuals with major CVRFs. Furthermore, we aimed to estimate the impact of modifiable and nonmodifiable risk factors on the studied vascular and hemodynamic parameters by multivariable analysis.
Methods
Study population
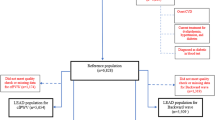
This cross-sectional study retrospectively investigated data from 6499 individuals aged 18 years or older who underwent office measures of central BP, PWV, and AIx at four Brazilian cardiology centers [(1) Clinical Research Center of the Cesmac University Center, Maceió, Alagoas State; (2) Clínica do Coração (Heart Clinic), Aracaju, Sergipe State; (3) Avancor Cardiologia (Avancor Cardiology Clinic), Maringá, Paraná State; and (4) Pedro Ernesto University Hospital, Rio de Janeiro, Rio de Janeiro State] from 2014 to 2017. The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Cesmac University Center, which waived the requirement for informed consent.
Office measures of peripheral and central BP, PWV, and AIx
All participants underwent measurements of office central systolic and diastolic BP, AIx, PWV, and peripheral systolic and diastolic BP using a Mobil‐O‐Graph PWA monitor (IEM Healthcare, Stolberg, Germany), as previously described [10,11,12, 15]. The BP detection unit of this monitor has been validated according to European Society of Hypertension and the British Hypertension Society international protocols [16,17,18]. Furthermore, the device oscillometrically identifies the peripheral pulse wave and builds the central pulse wave using an intrinsic algorithm (ARCSolver), providing central BP measurements similar to those obtained by invasive techniques [19]. PWV measurements are calculated from the time interval between the estimated forward and reflected waves and are comparable with invasive measurements [20]. AIx measurements were calculated as the ratio of the augmentation pressure to the pulse pressure (PP) and were standardized to a pulse rate of 75 beats per min. Each participant underwent three peripheral BP, central BP, PWV, and AIx readings attended by health staff at the office, with 3-min intervals; the measurements were acquired in the sitting position following 5 min of rest. The mean of the three readings was used in the current analysis, and only individuals with three valid readings of all studied parameters were included in the study. Peripheral and central PP were calculated as the difference between peripheral systolic and diastolic BP and between central systolic and diastolic BP, respectively. Peripheral mean arterial pressure (MAP) was calculated as peripheral systolic BP/3 + 2 × peripheral diastolic BP/3.
Clinical characteristics
Information on sex; age; use of antihypertensive, lipid-lowering (statins, fibrates or ezetimibe), or antidiabetic medications; and current smoking status were collected. Individuals using lipid-lowering and antidiabetic medications were labeled as having dyslipidemia and diabetes, respectively. Body mass index was calculated as weight (kg) divided by height (m) squared, and obesity was defined as body mass index ≥30 kg/m2. The participants were also asked regarding physical activity performance and alcohol intake. Individuals who stated that they performed physical activity regularly (e.g., walking, running, cycling, jogging, performing sports, training at the gym) at least 3 days a week were labeled as physically active, while those who stated regularly intaking alcohol at least twice a week were considered as having alcohol intake.
Individuals who had office peripheral systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg or were using antihypertensive medications were considered to have hypertension. Peripheral BP categories were defined as optimal (systolic BP < 120 mmHg and diastolic BP < 80 mmHg), normal (systolic BP = 120–129 mmHg and/or diastolic BP = 80–84 mmHg), high normal (systolic BP = 130–139 mmHg and/or diastolic BP = 85–89 mmHg), stage 1 hypertension (systolic BP = 140–159 mmHg and/or diastolic BP = 90–99 mmHg), stage 2 hypertension (systolic BP = 160–179 mmHg and/or diastolic BP = 100–109 mmHg), and stage 3 hypertension (systolic BP ≥ 180 mmHg and/or diastolic BP ≥ 110 mmHg) [21].
The sample was split according to the presence or absence of a major CVRF. Participants with at least one of the following major risk factors (hypertension, obesity, smoking, diabetes, and dyslipidemia) were labeled as CVRF-Yes, while apparently healthy participants who did not have any of the aforementioned risk factors were labeled as CVRF-No.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation, and categorical variables are presented as proportions. Continuous variables were compared using one-way analysis of variance followed by Bonferroni’s test, while the Bonferroni-corrected chi-square test was used to compare categorical variables. Comparisons of the prevalence of peripheral BP categories were performed solely within the studied groups (CVRF-No and CVRF-Yes). Sex-specific median and 10th, 25th, 75th, and 90th percentile values of central BP, PWV, and AIx within the studied groups are presented stratified by age. The 90th percentile values of central BP, PWV, and AIx of the CVRF-No groups were used as cut-offs for normality to calculate the sensitivity, specificity, positive predictive value, and negative predictive value to identify subjects with CVRFs among CVRF-No and CVRF-Yes participants. Unadjusted restricted cubic splines with three knots assessed the relationship of age with central BP, PWV, and AIx among CVRF-Yes and CVRF-No participants. The likelihood-ratio test was used to test for interactions between age and sex for the studied parameters. Multivariable linear regression analysis for the study parameters including selected variables (age2, sex, age2 × sex interaction term, height, obesity, diabetes mellitus, current smoking, dyslipidemia, alcohol intake, and use of antihypertensive medications) was performed comprising the whole studied sample. For central BP measures, hypertension was also included as an independent variable, while MAP was included as an independent variable for PWV and AIx in multivariable models. P values < 0.05 were considered significant. Statistical analysis was performed using Stata software Version 14.2 (Stata Corp LP, College Station, TX, USA).
Results
Characteristics of the studied participants
The clinical and hemodynamic characteristics of the CVRF-No (n = 867, mean age = 46.0 ± 15.5 years, 39% males) and CVRF-Yes (n = 5632, mean age = 57.0 ± 14.7 years, 44% males) groups split by sex are shown in Table 1. In both groups, men were more likely to be younger, to have a higher body mass index and to consume alcohol. Among CVRF-Yes participants, men were less likely to use antihypertensive medications and to have dyslipidemia but were more likely to have obesity and to smoke than women. Men had greater peripheral and central systolic and diastolic BP and lower AIx than women in both groups, while average PWV values were lower in men among CVRF-Yes participants but similar in both sexes among CVRF-No participants. CVRF-Yes participants were more likely to be older and less likely to perform physical activity and had greater body mass index, peripheral and central BP, PWV and AIx than CVRF-No participants.
Reference values for central BP, PWV, and AIx
Reference values for central BP, PWV, and AIx for the studied groups split by sex and stratified by age are shown in Table 2. The relationship of age with central systolic and diastolic BP as well as with peripheral systolic and diastolic BP is further explored in Fig. 1. In both the CVRF-No and CVRF-Yes groups, men had a greater average central systolic BP than women at younger ages, while women tended to have a greater average central systolic BP than men at older ages due to progressive increases in these BP measures with age in female participants. Average central diastolic BP values decreased with age among participants older than ≈50–60 years and were greater in men than in women throughout the age spectrum in the CVRF-No and CVRF-Yes groups. Compared with central BP, peripheral BP had a similar relationship with age in both sexes and studied groups, even though the average peripheral systolic BP was greater than the central systolic BP and the average central diastolic BP was modestly greater than the peripheral diastolic BP across the age spectrum (Fig. 1 and Table 2). The relationship between PWV and AIx and age is also shown in Fig. 2. The average PWV increased with age in a curvilinear fashion in both sexes, although the values tended to be slightly higher in men at younger ages and modestly higher in women at older ages in the CVRF-No and CVRF-Yes groups. Average AIx values also increased curvilinearly with age among male and female participants in both the CVRF-No and CVRF-Yes groups, even though women had greater AIx values than men across the entire age spectrum (Fig. 2 and Table 2).
Relationship between blood pressure values and age in men and women with and without major CVRFs. CVRFs cardiovascular risk factors, BP blood pressure. P for interaction between age and sex <0.001 for central and peripheral systolic BP in both studied groups and for central and peripheral diastolic BP in the CVRF-Yes group and >0.05 for central and peripheral diastolic BP in the CVRF-No group
Relationship between pulse wave velocity and augmentation index and age in men and women with and without major CVRFs. CVRFs cardiovascular risk factors, PWV pulse wave velocity, AIx augmentation index. P for the interaction between age and sex was at least <0.007 for PWV in both groups and >0.05 for AIx in both groups
To investigate the additional usefulness of the reference values shown in Table 2, we used the 90th percentile values of central BP, PWV, and AIx of the CVRF-No group as cut-offs for normality to calculate the sensitivity, specificity, positive predictive value, and negative predictive value in identifying subjects with CVRFs among CVRF-No and CVRF-Yes participants (Supplementary Table 1). In general, these cut-offs had high specificity and positive predictive value but low sensitivity and negative predictive value to identify participants with CVRFs.
As a secondary analysis, we provided reference values for central BP, PWV, and AIx for the CVRF-Yes group, excluding participants with a diagnosis of hypertension (Supplementary Table 2). These reference values were usually similar to those of the complete CVRF-Yes group (Table 2), albeit absolute median values tended to be slightly lower in the analysis excluding hypertensive participants (Supplementary Table 2).
Determinants of BP, PWV, and AIx
Multivariable linear regression analysis including all studied participants (n = 6499) was performed to identify the variables that were independently related to central BP, PWV, and AIx (Table 3). Given the nonlinear relationship between the studied parameters and age and the significant interaction between sex and age, we included the age2 and age2 × sex interaction term as independent variables in the multivariable models. Central systolic BP was directly related to age2, male sex, obesity, alcohol intake, and hypertension and was inversely associated with dyslipidemia, antihypertensive medication use and age2 × sex interaction, while central diastolic BP was directly related to male sex, obesity, alcohol intake, and hypertension and was inversely associated with age, dyslipidemia, diabetes, and antihypertensive medication use. Peripheral and central systolic BP shared similar determinants, except for alcohol intake, which was only associated with central systolic BP, and height and physical activity, which were solely and inversely associated with peripheral systolic BP (Supplementary Table 3). Conversely, peripheral and central diastolic BP shared exactly the same determinants (Supplementary Table 3). PWV showed a direct association with age2, male sex, diabetes mellitus, peripheral MAP and was inversely related to age2 × sex interaction and antihypertensive medication use, while AIx was directly related to age2, female sex and peripheral MAP and had an inverse association with height and physical activity (Table 3).
Discussion
This multicenter study evaluating a large sample of individuals who underwent assessment of central hemodynamics and arterial stiffness has three major findings. First, office reference values of central BP, PWV, and AIx were reported for individuals with and without CVRFs using the Mobil-O-Graph PWA device, which may be useful to build thresholds for clinical practice use. Second, there was an interaction between age and sex for central systolic BP and PWV. Third, modifiable and nonmodifiable CVRFs exerted distinct impacts on the parameters of central hemodynamics and arterial stiffness, strengthening the notion that central BP, PWV, and AIx assess different aspects of vascular health.
In the current report, we found a curvilinear association between age and PWV, with a steeper relationship among older participants, confirming widespread evidence [22, 23]. In addition, we observed a significant interaction between age and sex, with younger men exhibiting greater PWV than younger women and older women showing greater PWV than older men. Similar trends have also been reported in alternative samples [24], suggesting that differences in PWV between men and women might be influenced by age. Consistent with former evidence, we also found that PWV had a direct association with MAP [23, 25] and diabetes [26] and an inverse relationship with antihypertensive medications [27], further strengthening the validity of our findings. Conversely, median values of PWV in our sample were usually lower than those reported in other populations of similar age who underwent a PWV assessment using alternative methods [22, 23]. This finding appears to agree with the notion that PWV measurements obtained with the Mobil-O-Graph PWA device provide slightly lower absolute values than those obtained with Sphygmocor, Complior, and Arteriograph [11, 12], suggesting that the reference values of measurements obtained by distinct devices and techniques assessing central hemodynamics and arterial stiffness might not be interchangeable. Another potential explanation for the discrepancies in age-specific PWV values between our study and the aforementioned reports could be that our measures were performed in the sitting rather than in the supine position. However, this hypothesis seems less probable since PWV values are reported to not significantly vary when measured in supine or sitting positions [28]. Furthermore, it cannot be discounted that differences in clinical and ethnic characteristics among the studied populations might have played a role in this regard.
In agreement with previous reports [29], average values of peripheral systolic BP were higher than those of central systolic BP, while the average values of central diastolic BP were slightly higher than those of peripheral diastolic BP in our sample. Conversely, peripheral and central BP measures shared several acknowledged determinants, including age, sex, and obesity [21]. Central and peripheral systolic BP values were greater in men than in women at younger ages, but this sex-related difference decreased with age, corroborating available evidence [30]. Interestingly, central and peripheral BP values were slightly, albeit significantly, lower among our participants with the diagnosis of dyslipidemia. Although this result might seem unexpected at first glance, it can be explained by the definition of dyslipidemia used in the current analysis, which was based on the use of lipid-lowering medications. Given that statins were the most commonly used lipid-lowering medications in our sample (>90%; data not shown) and statin use is known to slightly reduce BP values [29, 31], it can be argued that the lower BP values among our participants with dyslipidemia were related to the use of statins. Our analysis also confirmed that some BP components had a direct relationship with alcohol intake and an inverse association with physical activity [21]. However, central systolic BP had a direct association with alcohol intake but a neutral relationship with physical activity, while peripheral systolic BP showed an inverse association with physical activity but a neutral relationship with alcohol intake. Although the reasons for such differences are not apparent in our analysis, these findings suggest that central and peripheral systolic BP might be differentially affected by distinct factors.
The results of our unadjusted and adjusted analyses reproduced the acknowledged notion that women have greater AIx than men regardless of the presence of CVRF [32,33,34]. Although the greater height in men could potentially explain the lower AIx in this sex due to later arterial wave reflections [33], we found that sex-related differences in AIx were independent of height, indicating that alternative, albeit unestablished, mechanisms leading to faster wave reflection in women might play a role in this regard [32,33,34]. In addition, we found a curvilinear increase in AIx with age, as previously reported [32, 33, 35]. One potential explanation for this finding can be related to an earlier return of the reflected wave due to age-related increases in PWV [33]. In agreement with this assumption, both AIx and PWV showed parallel increases with age in our sample, particularly among individuals older than ≈50–60 years. Last, AIx values were significantly lower in participants reporting the performance of physical activities. Exercising might result in greater release of nitric oxide due to increased shear stress on the vascular endothelium, leading to reductions in systemic vascular resistance and, eventually, in AIx [36, 37]. In addition, physical activity could reduce AIx by decreasing arterial stiffness and hence arterial wave reflections [36]. The lack of a significant relationship between PWV and physical activity in our sample would make this hypothesis less probable. However, it is important to acknowledge that the effects of exercise, particularly aerobic exercise, on PWV tend to be less evident in devices that evaluate central PWV indices, such as the Mobil-O-Graph PWA monitor, than in alternative devices that evaluate peripheral PWV indices [36], which might have contributed to underestimating the relationship between PWV and physical activity in our analysis.
Some limitations of this report must be acknowledged. First, although PWV measures provided by Mobil-O-Graph PWV values have been validated by invasive techniques [20], this device estimates PWV only at a single site and therefore has been considered to have limited ability to estimate large artery stiffening [38, 39]. Second, the diagnosis of diabetes and dyslipidemia was based on the use of antidiabetic and lipid-lowering medications, respectively, which could have led to underestimation of these risk factors. Third, information on relevant covariates, including lipid profile and glycemia, was not available. Fourth, the precise amounts of alcohol intake and physical activity performance were not quantified. Therefore, these lifestyle habits were treated as categorical variables. Fifth, data on adverse outcomes at follow-up were not available, thus precluding our ability to evaluate the prognostic importance of the studied parameters. Sixth, the presented reference values were derived from a convenient sample from four Brazilian cardiology centers, which could be different from those derived from a prospectively designed population-based sample.
In conclusion, this study provided office reference values for central BP, PWV, and AIx in populations with and without CVRF, according to age and sex, by means of the Mobil-O-Graph PWA monitor, which may be useful to build thresholds for clinical practice use. The present results also demonstrated an interaction between age and sex in central systolic BP and PWV. In addition, modifiable and nonmodifiable CVRFs exerted distinct impacts on the parameters of central hemodynamics and arterial stiffness, strengthening the notion that central BP, PWV, and AIx assess different aspects of vascular health.
References
Palatini P, Casiglia E, Gąsowski J, Głuszek J, Jankowski P, Narkiewicz K, et al. Arterial stiffness, central hemodynamics, and cardiovascular risk in hypertension. Vasc Health Risk Manag. 2011;7:725–39.
Protogerou AD, Safar ME, Papaioannou TG, Zhang Y, Agnoletti D, Papadogiannis D, et al. The combined effect of aortic stiffness and pressure wave reflections on mortality in the very old with cardiovascular disease: the PROTEGER Study. Hypertens Res. 2011;34:803–8.
Sougawa Y, Miyai N, Utsumi M, Miyashita K, Takeda S, Arita M. Brachial-ankle pulse wave velocity in healthy Japanese adolescents: reference values for the assessment of arterial stiffness and cardiovascular risk profiles. Hypertens Res. 2020;43:331–41.
Sun P, Yang Y, Cheng G, Fan F, Qi L, Gao L, et al. Noninvasive central systolic blood pressure, not peripheral systolic blood pressure, independently predicts the progression of carotid intima-media thickness in a Chinese community-based population. Hypertens Res. 2019;42:392–9.
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. Eur Heart J. 2010;31:1865–1871.
McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719–1725.
Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646.
Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, Recio-Rodriguez JI, Frontera G, Ramos R, et al. The association between the cardio-ankle vascular index and other parameters of vascular structure and function in caucasian adults: MARK study. J Atheroscler Thromb. 2015;22:901–11.
Resende LAPR, Silva MAV, Resende JAM, Resende EAMR, Silva VJD, Correia D. Comparison of pulse wave analysis parameters by oscillometry in hypertensive diabetic and nondiabetic patients in a Brazilian outpatient care. Medicine. 2019;98:e18100.
Paiva AMG, Gomes MICM, Campana ÉMG, Feitosa ADM, Sposito AC, Mota-Gomes MA, et al. Impact of hypertension phenotypes on the office and 24-h pulse wave velocity and augmentation index in individuals with or without antihypertensive medication use. Hypertens Res. 2019;42:1989–95.
Sarafidis PA, Georgianos PI, Karpetas A, Bikos A, Korelidou L, Tersi M, et al. Evaluation of a novel brachial cuff-based oscillometric method for estimating central systolic pressure in hemodialysis patients. Am J Nephrol. 2014;40:242–50.
Benas D, Kornelakis M, Triantafyllidi H, Kostelli G, Pavlidis G, Varoudi M, et al. Pulse wave analysis using the Mobil-O-Graph, Arteriograph and Complior device: a comparative study. Blood Press. 2019;28:107–13.
Papaioannou TG, Thymis J, Benas D, Triantafyllidi H, Kostelli G, Pavlidis G, et al. Measurement of central augmentation index by three different methods and techniques: agreement among Arteriograph, Complior, and Mobil-O-Graph devices. J Clin Hypertens. 2019;21:1386–92.
Gotzmann M, Hogeweg M, Seibert FS, Rohn BJ, Bergbauer M, Babel N, et al. Accuracy of fully automated oscillometric central aortic blood pressure measurement techniques. J Hypertens. 2020;38:235–42.
Paiva AMG, Brandão AA, Feitosa ADM, Novais GCA, Cantarelli EM, Gomes MICM, et al. Correlation between office and 24-hour ambulatory measures of pulse wave velocity, central augmentation index and central blood pressure. J Clin Hypertens. 2019;21:335–7.
Jones CR, Taylor K, Chowienczyk P, Poston L, Shennan AH. A validation of the Mobil O Graph (version 12) ambulatory blood pressure monitor. Blood Press Monit. 2000;5:233–8.
Franssen PM, Imholz BP. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press Monit. 2010;15:229–31.
Weiss W, Tolle M, Zidek W, van der Giet M. Validation of the mobil-O-Graph: 24 h-blood pressure measurement device. Blood Press Monit. 2010;15:225–8.
Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, Mayer CC, et al. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension. 2011;58:825–32.
Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood Press Monit. 2013;18:173–6.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018;36:2284–309.
Khoshdel AR, Thakkinstian A, Carney SL, Attia J. Estimation of an age-specific reference interval for pulse wave velocity: a meta-analysis. J Hypertens. 2006;24:1231–7.
Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–50.
Liang X, Su S, Hao G, Snieder H, Treiber F, Kapuku G, et al. Determinants of pulse wave velocity trajectories from youth to young adulthood: the Georgia Stress and Heart Study. J Hypertens. 2019;37:563–71.
Baldo MP, Cunha RS, Ribeiro ALP, Lotufo PA, Chor D, Barreto SM, et al. Racial differences in arterial stiffness are mainly determined by blood pressure levels: results from the ELSA-Brasil study. J Am Heart Assoc. 2017;6:e005477.
Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–1090.
Ye L, Yang X, Hu J, Chen Q, Wang J, Li X. Impact of antihypertensive agents on arterial stiffness in hypertensive patients. Int J Cardiol. 2018;273:207–12.
Nürnberger J, Michalski R, Türk TR, Opazo Saez A, Witzke O, Kribben A. Can arterial stiffness parameters be measured in the sitting position? Hypertens Res. 2011;34:202–8.
Lamarche F, Agharazii M, Nadeau-Fredette AC, Madore F, Goupil R. Central and brachial blood pressures, statins, and low-density lipoprotein cholesterol: a mediation analysis. Hypertension. 2018;71:415–21.
Kang MG, Kim KH, Koh JS, Park JR, Hwang SJ, Hwang JY, et al. Association between pulse pressure and body mass index in hypertensive and normotensive populations in the Korea National Health and Nutrition Examination Survey V, 2010–2. J Clin Hypertens. 2017;19:395–401.
Spósito AC, Mansur AP, Coelho OR, Nicolau JC, Ramires JA. Additional reduction in blood pressure after cholesterol-lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin-converting enzyme inhibitors (enalapril or lisinopril). Am J Cardiol. 1999;83:1497–9.
Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45.
Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E. Aortic augmentation index: reference values in a large unselected population by means of the SphygmoCor device. Am J Hypertens. 2010;23:180–5.
Costa-Hong VA, Muela HCS, Macedo TA, Sales ARK, Bortolotto LA. Gender differences of aortic wave reflection and influence of menopause on central blood pressure in patients with arterial hypertension. BMC Cardiovasc Disord. 2018;18:123.
Eeftinck Schattenkerk DW, van Gorp J, Snijder MB, Zwinderman AH, Agyemang CO, Peters RJ, et al. Ethnic differences in arterial wave reflection are mostly explained by differences in body height-cross-sectional analysis of the HELIUS study. PLoS One. 2016;11:e0160243.
Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9:e110034.
Padilla J, Harris RA, Rink LD, Wallace JP. Characterization of the brachial artery shear stress following walking exercise. Vasc Med. 2008;13:105–11.
Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722.
Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1237–63.
Funding
The study was supported by the Brazilian National Council for Scientific and Technological Development (CNPq; grant 306154/2017–0, awarded to WN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Paiva, A.M.G., Mota-Gomes, M.A., Brandão, A.A. et al. Reference values of office central blood pressure, pulse wave velocity, and augmentation index recorded by means of the Mobil‐O‐Graph PWA monitor. Hypertens Res 43, 1239–1248 (2020). https://doi.org/10.1038/s41440-020-0490-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-020-0490-5
Keywords
This article is cited by
-
Age- and sex-related reference values for central blood pressure parameters in middle-aged and older Japanese adults: the Wakayama study
Hypertension Research (2025)
-
Mean arterial pressure differences between cuff oscillometric and invasive blood pressure
Hypertension Research (2025)
-
Physical activity level and influencing factors in pediatric Wolff-Parkinson-White syndrome patients: A case–control study
European Journal of Pediatrics (2025)
-
Intracranial Pressure and Vascular Aging: A Narrative Review on its Role in Monitoring Cognitive Decline
Artery Research (2025)
-
Evaluation of arterial stiffness and quality of life in the treatment of moderate to severe obstructive sleep apnea with Continuous Positive Airway Pressure or Mandibular Advancement Appliance: a cross-sectional study
BMC Cardiovascular Disorders (2024)