Abstract
Fauna is highly abundant and diverse in soils worldwide, but surprisingly little is known about how it affects soil organic matter stabilization. Here, we review how the ecological strategies of a multitude of soil faunal taxa can affect the formation and persistence of labile (particulate organic matter, POM) and stabilized soil organic matter (mineral-associated organic matter, MAOM). We propose three major mechanisms - transformation, translocation, and grazing on microorganisms - by which soil fauna alters factors deemed essential in the formation of POM and MAOM, including the quantity and decomposability of organic matter, soil mineralogy, and the abundance, location, and composition of the microbial community. Determining the relevance of these mechanisms to POM and MAOM formation in cross-disciplinary studies that cover individual taxa and more complex faunal communities, and employ physical fractionation, isotopic, and microbiological approaches is essential to advance concepts, models, and policies focused on soil organic matter and effectively manage soils as carbon sinks, nutrient stores, and providers of food.
Similar content being viewed by others
Introduction
Soil fauna is highly abundant, diverse, and active in soils worldwide, even in the most extreme environments, such as Antarctica or deserts1,2,3,4,5. Soil fauna affects various soil biophysicochemical properties, including microbial diversity, soil structure/texture, and soil nutrients, via ingesting and transforming organic matter into more or less decomposable forms, mixing this organic matter with mineral soil, and grazing on microorganisms6,7,8,9,10. These processes are essential in driving biogeochemical cycles and may substantially affect soil organic matter (SOM) dynamics11,12,13,14,15,16. However, despite this recognized relevance of soil fauna to soil processes, knowledge of its involvement in the formation and stabilization of SOM is very scarce, except for that of earthworms17,18,19,20,21,22. This key knowledge gap hampers our understanding and modeling of global biogeochemical cycles and thus effective management of soils.
Current paradigms consider the formation of stabilized SOM to be regulated by the efficiency with which microorganisms transform plant litter (leaf and root) and root exudates into microbial biomass23,24,25, which is strongly affected by litter/exudate quality26. When microbial biomass eventually turns into necromass, it readily interacts with mineral surfaces and thus accumulates as mineral-associated organic matter (MAOM; “microbial pathway” of SOM formation), which can persist for centuries to millennia27 (see also Kleber et al.28). This stabilized SOM pool can also form via direct sorption of dissolved organic matter on reactive mineral surfaces, which requires little or no microbial pre-processing of organic matter (“direct sorption pathway” of SOM formation)29,30. Notably, soil fauna might substantially affect both of these SOM formation pathways by (1) altering the quantity, quality, and location of organic matter in the soil via bioturbation and transformation processes, (2) altering microbial biomass and community composition via active and/or indirect grazing on bacteria and fungi, (3) generating dissolved organic matter via processing of litter and other organic matter, and (4) influencing soil texture (and mineralogy)6,31,32,33. All of these processes also have the potential to affect the share of comparatively labile pools in soils, such as particulate organic matter (POM). This pool is composed of partly decomposed plant fragments that have, if not occluded within aggregate structures, comparatively short residence time in soils (<15 years)34,35,36.
Particulate OM is directly linked to the formation of MAOM, in which effective decomposition of bioavailable POM can boost MAOM formation via the microbial pathway, while “recalcitrant” POM can hamper the formation of MAOM but foster relative accumulation of POM37. The proportion of labile (POM) and stabilized pools of SOM (MAOM) determines how this SOM, and carbon (C) within, responds to altered environmental conditions, such as induced by land use, climate change38, or management focused on increasing or maintaining C storage37, nutrient stores, or crop yields39.
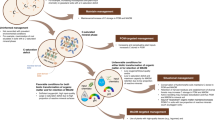
Despite strong indications that soil fauna may substantially affect the pathways of MAOM and POM formation, knowledge on these effects, except for earthworms, is virtually absent (Fig. 1). We argue that such knowledge is crucially needed to manage soils as C sinks and accurately predict SOM dynamics in the face of climate change. In this review, we summarize the state of the art of how macro-, meso-, and microfauna affect soil properties, such as SOM quantity and chemistry, microbial biomass and community composition, or soil texture. We categorize these effects into three main processes - transformation, translocation, and grazing - by which soil fauna can alter the share between and the formation of POM and MAOM (Fig. 2). We discuss how these processes can be altered by interactions among soil faunal taxa and environmental change and propose future research directions (Fig. 3).
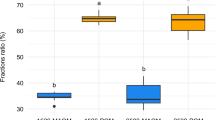
a Studies by process (translocation, transformation, grazing; n = 180) and b investigated fraction (POM, MAOM, aggregates; n = 87) based on our literature search; % values in a indicate the proportion of studies (research articles) dedicated to translocation, transformation, and/or grazing independent of taxon, respectively. Percentage values in b to the right indicate the proportion of studies that investigated POM, MAOM, and aggregates independent of taxon, respectively, while % values within the alluvial graph indicate the proportion of studies on earthworms relative to the whole number of studies. Aggregates are grayed out because they were not in the focus of our review (consisting of both POM and MAOM).
Soil fauna transforms litter and other organic matter into more or less decomposable forms, such as feces and litter fragments. This organic matter is then translocated via bioturbation into the mineral soil, where it can be further transformed or translocated vertically or horizontally and eventually accumulate as POM (as “free” POM or occluded within aggregates). This POM can then be microbially processed and either be stabilized as MAOM or mineralized to CO2. Transformation of organic matter by soil fauna can also result in the release of dissolved organic matter from litter layers or, specifically, feces (both in the litter layer or mineral soil), which may have altered chemistry compared to the initial litter (indicated by different drop colors). This dissolved organic matter can directly sorb on mineral surfaces and thus form MAOM and/or desorb previously sorbed organic matter, which can then be mineralized to CO2. Grazing on and translocation of microbial communities by fauna may affect microbial physiological traits and community composition and abundance and thus have an influence on the microbial pathway of MAOM formation (and decomposition of POM).
*Investigation of POM and MAOM in feces of nematodes and protists is likely unfeasible due to the small amounts of feces produced by these taxa relative to the amount necessary for physical fractionation. Some icons have been created using BioIcons.com (DBCLS; springtails and termites; CC-BY 4.0) and BioRender.com (earthworms, snails, beetles, ants, woodlice, protists). Icons created with BioRender.com are released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
We ultimately call for laboratory and field studies that focus on the direction and magnitude of transformation, translocation, and grazing effects of individual soil faunal taxa and whole communities on the formation and/or decomposition of POM and MAOM, and how environmental factors such as land use, climate, or soil type alter these processes. The results of such studies are envisioned to help refine current concepts on SOM formation and soil organic C-related models to be eventually implemented at the management and policy level.
Literature review
We focused our systematic literature search on soil macro-, meso-, and microfaunal taxa with high abundance in soil and potential influence on POM and MAOM40, including ants (Formicidae), beetles (Carabidae), earthworms (Lumbricina/Crassiclitellata), fly larvae (Diptera), millipedes (Diplopoda), mites (mainly Mesostigmata/Prostigmata/Oribatida), nematodes (Nematoda), potworms (Enchytraeidae), protists (Protozoa), snails (Gastropoda), springtails (Collembola), termites (Isoptera), and woodlice (Isopoda). We used strings [“TITLE-ABS-KEY (//taxon AND soil AND fraction* OR MAOM OR POM OR soil organic matter OR SOM OR carbon OR nitrogen OR phosphorus OR lipid* OR lignin OR protein OR necromass OR aggregate OR “Organo-mineral association*“ OR “mineral-associated organic matter” OR NMR OR FTIR OR GCMS OR biomarker* OR “microbial biomass” OR “microbial carbon” OR fung* OR bacteria*)” and “((AB = (//taxa) AND AB = (soil) AND AB = (fraction*) AND AB = (MAOM OR POM OR soil organic matter OR SOM OR carbon OR nitrogen OR phosphorus OR lipids OR lignin OR protein OR necromass OR aggregate OR “organo-mineral association*“ OR “mineral-associated organic matter” OR NMR OR FTIR OR GCMS OR biomarker* OR “microbial biomass” OR “microbial carbon” OR fung* OR bacteria*)”] to scan the Scopus and Web of Science databases for suitable articles and complement our expertize on the topic. Although soil fauna may also influence the formation and share of POM and MAOM via effects on plant growth and resource allocation41,42,43, we did not include a detailed review of this topic because it is beyond the scope of this study. The initial number of articles returned was 1,794 and 1811 for the Scopus and Web of Science databases, respectively. After screening of titles and abstracts and excluding articles out of scope, we finally based our review on 180 core articles about the effects of one or more faunal taxa on one or more soil properties related to SOM formation (Table S1). We then read the whole articles and extracted information on the types of soil fauna and their major effects on the soil properties investigated.
Based on this review of literature and our expertise, we identified three major processes by which soil fauna may influence formation of POM and MAOM: (i) transformation, in which soil fauna alters soil mineralogy or transforms organic matter into more or less decomposable forms via ingestion, digestion, and egestion, (ii) translocation, in which soil fauna moves soil particles, organic matter, or microorganisms from one location to another, and (iii) grazing on microorganisms, in which soil fauna modifies soil microbial community composition, biomass, or activity via targeted or incidental grazing. Many soil faunal taxa perform more than one of these processes and are thus repeatedly referred to in the following sections (cf. Fig. 1).
Transformation
Feces/casts
An important process by which soil fauna can influence the chemistry and stability of SOM is the ingestion of mineral particles and/or litter/organic matter, partial digestion in the gut, and egestion of the altered material as feces (Fig. 2). Depending on the ingested material, taxon and environmental context, this process can result in more labile, recalcitrant, or stabilized organic matter in feces compared to the ingested material.
Earthworm casts
The most prominent and researched feces (commonly termed casts) are those of earthworms. Earthworms burrow through the soil and ingest SOM or litter and/or mineral particles and mix this material with mucus in their guts. The egested casts often turn into stabilized (micro-)aggregate structures upon ageing44,45,46,47,48,49, in which POM is occluded (i.e., protected from decomposition), and in which MAOM is more stable as compared to that outside of aggregates50. Casts can have a distinct molecular composition compared to uningested bulk soil9,19,47,51,52,53,54,55, with enhanced microbial diversity, richness, and activity, and altered community composition56,57,58, and are often enriched in microbial biomass59,60,61,62. This earthworm-induced stimulation of the soil microbial community has recently been conceptualized to boost the microbial pathway of MAOM formation63, as earthworms co-locate microorganisms, microbial energy sources, and mineral particles, and potentially alleviate nutrient limitations by the addition of mucus. This is supported by studies showing increased microbial C use efficiencies64,65, microbial necromass (which preferentially associates with minerals)19,22, and MAOM in casts20,66 as compared to non-ingested soil. The stabilizing effect of earthworms on both POM and MAOM is highly relevant to SOM dynamics, given the high earthworm abundance and biomass in many soils worldwide2,63. However, earthworm-induced stimulation of soil microbial activity and biomass can also lead to increased mineralization and emission of CO2 and/or N2O from soils67,68,69,70. The net effect of earthworms on the C balance of soils is not solved and may be strongly modulated by the respective environmental conditions (e.g., climate, soil properties, plant, and faunal communities), experimental design (length, water regime, SOM content), and earthworm ecological groups, species, and/or traits71,72. More, preferably long-term, field studies involving earthworms (and other soil fauna) and quantification of C fluxes and pools are clearly needed (Fig. 3). Moreover, research on earthworm casts typically compares cast properties to those of uningested bulk soil, thereby omitting the contribution of ingested litter (e.g., leaf or root litter) to cast properties. Future studies should thus compare cast properties with those expected from all ingested substrates (decomposing litter and soil) weighed by their ingestion rate, or at least with a control mixture of mineral soil and litter19.
Millipedes, woodlice, snails, fly larvae, springtails, mites, termites, and potworms
Apart from earthworms, many other faunal taxa may also substantially affect SOM dynamics via the production of feces. Because most other soil faunal taxa are more abundant in the litter layer and feed on decomposing dead leaves, studies typically compared feces properties to those of uningested litter. Generally, feces of taxa other than earthworms feature physicochemical characteristics that are favorable for microbial proliferation and leaching as compared to non-ingested litter. These include higher nitrogen or phosphorus contents, lower contents of recalcitrant compounds such as lignin and secondary metabolites, higher contents of dissolved organic C and nitrogen, and higher surface area and water-holding capacity. Such features have often been reported for feces of millipedes73,74,75,76,77,78,79, woodlice6,80,81, snails6,80, and partly, mites82. In contrast, some studies on woodlice and fly larvae reported feces properties rather unfavorable for microbial proliferation, such as higher contents of lignin or lower contents of nitrogen as compared to non-ingested litter81,83,84,85,86,87,88. These contrasted results, however, do not appear to be taxa-specific but depend on the age of feces83,84 and the quality of ingested litter. Indeed, Joly et al.6 reported that across diverse taxa (millipedes, woodlice, and snails), the positive conversion effect on feces quality was stronger for low- than for high-quality litter. The magnitude of the change in quality, however, was species-specific, indicating that generalizations across species and taxa remain difficult.
The changes in physicochemical characteristics following litter conversion into feces may have important consequences for the gradual transformation of litter into SOM. Several studies found that these changes were associated with generally higher mass loss rates during decomposition for feces compared to uningested litter6,75,77, with only few cases of slower decomposition for feces compared to uningested litter84. These decomposition rates may be related to the addition of gut microbiota involved in the degradation of organic matter73,89 and facilitated leaching of dissolved organic compounds6. This suggests that conversion of litter into feces, by facilitating microbial proliferation and/or leaching, may boost the production of microbial necromass and leachates and thus the stabilization of litter as MAOM via the microbial and/or direct sorption pathways. Alternatively, conversion into feces, being composed of a myriad of minute particles, may increase the accumulation of POM, specifically for those feces that decompose more slowly than the uningested litter. Feces may also form nuclei for aggregate formation, such as reported for springtails, millipedes, potworms90,91,92,93, or constitute microaggregates themselves, as reported for termites94, thus increasing the persistence of POM within. The conversion effect of litter into feces on MAOM formation and accumulation of POM may also depend on the location at which feces are produced. If feces are located in or transferred to the mineral soil, actively or passively (e.g., via ingestion and transport by earthworms), they may affect both POM and MAOM. Feces remaining on the soil surface, however, may affect MAOM mainly via dissolved organic matter but have likely little effect on POM in mineral soil layers.
Feces-related research on taxa other than earthworms has mainly been focused on the conversion of litter into feces in the absence of mineral soil6. While this provides valuable information on physical and chemical changes of litter upon ingestion, digestion, and egestion, this does not allow to track the ultimate fate of these feces, i.e., their mineralization to CO2 or transfer to and stabilization in mineral soil. On the other hand, earthworm-related research has focused more on SOM dynamics in mineral soil, with less emphasis on litter decomposition. We thus advocate for a systematic consideration of litter and mineral soil in studies on the feces of earthworms and other taxa to identify general effects across the diversity of faunal taxa on the formation of POM and MAOM (Fig. 3).
Mineral composition
Mineral composition affects the reactive surface area available for the sorption of organic matter95. Few faunal taxa can directly change this composition and affect the capacity of soils to store organic matter in MAOM. Under controlled conditions, Jouquet et al.7,96 showed that termites were able to extract otherwise un-exchangeable potassium from illites likely via saliva and stimulation of microorganisms, thus forming expandable smectite layers, which have higher capacity to sorb organic matter97. This process may explain the enrichment of expandable clays and the higher cation exchange capacity observed in termite nests98,99 and potentially increase MAOM formation. Earthworm activity may also alter mineral weathering100,101, with yet unknown consequences for the sorption of organic matter. Studies on how certain faunal taxa such as termites and earthworms affect MAOM formation may thus want to involve mineralogical analyzes (e.g., via X-ray diffraction102) and/or specific surface area measurements (e.g., via the BET method103).
Translocation
Bioturbation
Incorporation of organic matter from litter/organic horizons into the mineral soil, and its further relocation therein, is substantially affected by the bioturbation activity of soil fauna (broadly defined as “the enhanced dispersal of particles resulting from sediment [soil] reworking by burrowing animals”10,104). Soil fauna has been estimated to remove up to 73 and 100% of the annual litterfall from the soil surface in tropical and temperate ecosystems, respectively, which highlights the high relevance of bioturbation across biomes32. This transfer of organic matter to the mineral soil as plant fragments or feces (see “Transformation” section above) fuels soil food webs, biochemical processes, and likely plays an important role in the formation and dynamics of POM and MAOM (Fig. 2).
Earthworm burrows/drilosphere
Earthworms have a disproportionately large influence on bioturbation105, as they not only dominate this process in the majority of ecosystems10,106, but also directly influence the share between POM and MAOM via bioturbation. When fragmenting and removing litter from the soil surface and incorporating it into the mineral soil, which can increase the amount of POM in soil107, anecic (deep burrowers) and endo-epigeic (soil-/litter-dwelling) earthworm species create vertical and horizontal burrows, respectively108,109. Burrows of endogeic and endo-epigeic species are mostly confined to the upper mineral soil and frequently refilled with soil, while those of anecic earthworms are relatively stable and can occur at large soil depths (up to 3 m)110. Although these burrows only occupy a minor volume of the whole soil111, they can be hotspots for biochemical and physical processes112,113,114. For example, burrow walls are compacted though exertion of axial and radial pressures and mucus deposition by the earthworm115, resulting in smaller pores and pore neck diameters116, in which small POM may be less accessible to microbial decomposers117. Burrow walls have also been reported to feature high microbial biomass, enzyme activities, and C mineralization rates118,119,120,121,122, being hotspots for C turnover and, perhaps, the concurrent formation of MAOM via the microbial pathway. This is supported by higher amounts of sugars detected in burrow walls123. Moreover, direct sorption of earthworm mucus to reactive mineral surfaces in burrow walls can increase MAOM124. However, Don et al.125 did not observe enhanced adsorption of C to minerals in burrow walls, and the higher C stocks of burrow walls in that study mineralized relatively quickly (in 3-5 years). The net effect of POM stabilization, MAOM formation, and microbial decomposition on C in burrow walls remains to be explored (Fig. 3).
Ants, termites, and dung beetles
While earthworms have clearly been the focus of a plethora of studies, other taxa may have considerable effects on organic matter dynamics via their bioturbation activity as well10,126,127,128, particularly under conditions less favorable for earthworms, such as in acid forests or dry ecosystems. However, we have not found any study on how bioturbation of soil fauna other than earthworms influences the formation of and share between POM and MAOM, although especially the characteristic mound- and gallery-building activity of ants and termites or bioturbation of dung beetles could have distinct effects on these fractions.
For example, the bioturbation and nest-building activity of ants and termites may induce particle sorting, with relatively higher and lower amounts of clay in nests and the underlying soil, respectively129,130,131,132,133 (but see also Whitford and Eldritch134). This process has been reported to soil depths well exceeding 1 m135,136 and may even reverse lessivation (the downward movement of clay particles)133. This may substantially decrease the capacity of soils below ant and termite nests to store organic matter as MAOM. In contrast, the transport of clay particles from deeper soil layers, which are likely far from C-saturation137, to the surface may increase this capacity in nest soil. Particle sorting and incorporation of organic matter into the soil has also been reported for dung beetles, specifically for tunnelers. This may increase the contents of clay, likely originating from deeper soil layers138,139, C, and nutrients in soil140, potentially boosting MAOM formation through co-location of organic matter and reactive mineral surfaces and alleviation of microbial nutrient limitations (also in combination with the effects of beetles on dissolved organic matter; see below).
Transport of forage to ant and termite nests and preferential feeding of dung beetles may also influence POM. For example, litter- and wood-feeding ants and termites, respectively, transport “recalcitrant” compounds to their nests133,141,142, potentially resulting in higher amounts of POM as compared to those in the surrounding bulk soil or in nests of ants and termites feeding on less recalcitrant substrates, such as honeydew or grasses141. Termites have also been shown to substantially alter wood decay rates, with potential consequences for POM (and MAOM) in the underlying mineral soil143. Likewise, dung beetles preferentially ingest small particles (8–50 µm in size) and leave large plant fragments widely unaffected144, perhaps increasing the transfer of POM to the mineral soil upon bioturbation.
While the effects of dung beetle bioturbation on the share between POM and MAOM are likely confined to the soil below dung pads (to ~10 cm depth), those of ant and termite bioturbation could be traced beyond the perimeter of nests (up to 0.5 ha)133,145 and were still perceivable 20 years after nest abandonment142. However, it is difficult to make general statements about the influence of ant/termite bioturbation on POM and MAOM, as this influence will likely strongly be modulated by the respective environmental conditions (e.g., climate or soil properties). Such conditions have been shown to influence factors important to the decomposition of POM and formation of MAOM in nests, such as microbial activity, pH, and temperature132,146,147,148,149,150,151.
Other soil fauna
Other groups of soil fauna, apart from earthworms, ants/termites, and beetles, perform bioturbation as well10. However, much less is known about the relevance of bioturbation by those groups. For example, woodlice, millipedes, and springtails can significantly increase litter decomposition across litter species152,153,154,155,156,157,158,159,160,161,162. Recent evidence also suggests that invertebrates (and vertebrates) contribute globally to deadwood decay163. Since animals are able to use only a fraction of the ingested litter for their own nutrition, most of this litter is converted into feces164. Additionally, woodlice and millipedes have been reported to feed preferentially on leaf lamina, leaving the leaf vein mostly untouched164, which may increase the accumulation of recalcitrant compounds and POM at the soil surface. Animal species that actively move vertically may mix some of this litter and POM with the mineral soil (either as litter fragments or feces). Such vertical movement through the soil to forage, lay eggs, hibernate, or estivate has been reported for insect larvae and adults, millipedes, and centipedes164,165,166,167,168,169,170. The rate of this mixing, however, is yet to be quantified in controlled experiments, and in situ using various biochemical tools (Fig. 3), and linked to the formation MAOM and accumulation (or decomposition) of POM.
Dissolved compounds
Some faunal taxa can have substantial effects on fluxes of dissolved organic matter and thus affect the direct sorption pathway of MAOM formation. For example, potworms dwelling in organic horizons, such as the forest floor or peat deposits, have often been related to increased production and leaching of dissolved organic matter31,171,172,173,174,175, likely via stimulation of microbial activity and thus decomposition of organic matter176. Increased leaching of dissolved organic matter from litter and forest floors has also been reported in the presence of springtails177,178, likely induced by feces production, and for dung pats reworked by beetles179. Given that this dissolved organic matter encounters reactive mineral surfaces, such as when percolating through forest or rangeland soils, soil fauna may indirectly influence sorption (or desorption180) of dissolved compounds to mineral surfaces and thus MAOM formation.
Dissolved organic matter rich in nutrients, such as that released from organic horizons or dung pats in the presence of potworms or beetles, respectively171,175,181, has also been reported to increase total nitrogen and phosphorus and ammonium-nitrogen in mineral soil181,182,183,184, potentially alleviating microbial nutrient limitations and boosting the microbial pathway of MAOM formation. This notion is supported by studies reporting microbial biomass stimulation in the presence of potworms and dung beetles185,186,187 (but see also Liiri et al.175 and Menendez et al.179). However, microbial traits considered important to the microbial pathway of MAOM formation, such as microbial C use efficiency25, have not been investigated in tandem with potworms or beetles and hardly with springtails188 (Fig. 3).
While the potential effects of beetles on the formation of MAOM via increased leaching of dissolved organic matter is relevant mainly in direct vicinity to dung pats (to ~10 cm depth)181, those of potworms and springtails may be important on larger scales. Potworms are typically abundant in wet, acid forest soils of the boreal and tundra zones, where they contribute up to 20 and 50% to the total animal biomass, respectively, while springtails are present in high abundance in virtually all ecosystems worldwide, being the most abundant in tundra soils3. Notably, the transfer of dissolved organic matter to deeper soil horizons can be enhanced by animals creating long and continuous vertical burrows, such as anecic earthworms189. Such preferential flow paths enable the rapid migration of dissolved organic matter, by which fauna could extend the relevance of dissolved organic matter to MAOM formation via direct sorption to deeper soil layers.
Transport of bacteria and fungi
Microorganisms are key players in the decomposition of fresh organic matter and POM as well as the formation of MAOM. The mobility of microorganisms is generally limited, and any vector promoting microbial dispersal may help the exploitation of resources previously out of reach190. This may be specifically relevant for bacteria, as their mobility is generally more strongly limited than that of fungi191,192,193. Vertical and horizontal transport of bacteria, for example, by nematodes194,195,196, may have importance in SOM dynamics if influencing emergent traits relevant to MAOM formation and/or accumulation (or decomposition) of POM, such as microbial diversity or C use efficiency25,197. Moreover, transport of fungal spores in the gut or on the body surface has been reported for a magnitude of invertebrate soil faunal species (i.e., snails, termites, ants, woodlice, mites, potworms, springtails, nematodes, millipedes, beetles, and earthworms)198,199,200,201. Such transport might have a recognizable effect on SOM dynamics in ant and termite nests202,203 or in disturbed sites, such as post-mining areas, where it might affect plant establishment in early successional stages204. However, generalizable statements on whether certain faunal taxa preferentially (or exclusively) transport certain microorganisms and whether such transport is indeed relevant to the formation of POM and MAOM remain unsupported by a lack of studies and data198 (Fig. 3).
Grazing on microorganisms
Microorganisms are the main food source for various faunal taxa, with preference for either fungi or bacteria40,114. Microorganisms can also be accidentally consumed by faunal taxa that prefer litter or SOM as food sources. Such targeted and incidental grazing can substantially alter microbial biomass, community composition, and activity, and thus potentially the amount and proportion of POM and MAOM in soils (Fig. 2).
Grazing on bacteria
Bacteria are the preferred food source for protists (but see Geisen205) and some nematode species. Protists appear to generally decrease bacterial abundance in bulk and rhizosphere soil206,207,208. In turn, bacterial abundance was found to either increase or decrease upon nematode grazing209,210, which may relate to nematode density in soil as well as altered microbial community structure and growth dynamics in response to grazing211,212. However, a common response to grazing by both protists and nematodes is an increase in bacterial activity196,208,213,214, specifically of gram-negative bacteria for nematodes (both bacterivorous and fungivorous species)8,196,210,214,215,216 and gram-positive bacteria for protists217,218,219. Shifts in the dominance of these bacterial groups upon grazing41 may alter utilization of rather labile (gram-negative) and more complex (gram-positive) organic matter220,221. Dominance of either bacterial group may also affect the turnover time of bacterial biomass in soil, which can be ~40% longer for gram-positive as compared to gram-negative bacteria222. Because adsorption of necromass from gram-negative bacteria to microbial necromass already present on reactive mineral surfaces can be higher than that of necromass from gram-positive bacteria223, grazing may ultimately alter the formation of MAOM via the microbial pathway. Changes in microbial community composition in response to protist and/or nematode grazing, specifically in rhizosphere soil, can also alter root growth/architecture224 and exudation rates225 (see also “microbial loop”41,194), perhaps affecting accumulation of POM (via increased structural root inputs) or the direct sorption pathway of MAOM formation (via exudates). Protists may also actively contribute to microbial necromass formation by using the cytoplasmic contents of bacteria for growth and releasing undigested cell walls and other recalcitrant materials as waste226, which may then interact with minerals and form MAOM227. Increased excretion of extracellular polymeric substances by bacteria in response to grazing by protists has also been reported to foster aggregate formation228, which could render POM within more persistent.
Grazing on fungi
Woodlice, mites, millipedes, springtails, and fungivorous nematodes generally prefer fungi over bacteria as their main food source40,229,230,231,232,233. This can have consequences for mycelial growth, the outcomes of interspecific competition232,234,235,236, fungal biomass153,237, and microbial diversity and community composition8,216. Earthworms can also preferentially feed on certain fungal species238, but the influence of this on soil microbial communities remains to be elucidated.
These multiple effects by fungal grazers on the soil microbiome may have various consequences for the stability of SOM. For example, although woodlice hampered mycelial growth, fungal decomposition of organic matter increased due to prevention of exclusion of other fungi and increased fungal diversity239,240. This may promote the microbial pathway of MAOM formation (and decomposition of POM), as microbial diversity has been linked to microbial necromass in MAOM197 and microbial C-use efficiency241, which in turn is related to the formation of MAOM24,242. Likewise, fungivorous nematodes can decrease microbial alpha diversity and C-use efficiency but increase the biomass of gram-negative bacteria8,216, with potentially opposite effects on MAOM formation. Finally, density-dependent promotion or impairment of arbuscular mycorrhizal (AM) or ectomycorrhizal (EM) fungal growth243, specifically by springtails244, may influence the share between POM and MAOM based on the respective nutrient acquisition strategy of these fungi. Arbuscular mycorrhizal fungi are unable to directly extract nitrogen and phosphorus from organic tissues, so that they release C to stimulate the growth and activity of saprotrophic microorganisms to “mine” these nutrients for them245. This can boost the microbial pathway of MAOM formation246. In contrast, EM fungi have retained some of their capacity to directly degrade organic matter so that competition for nutrients may suppress free-living saprotrophs247 and the decomposition of POM and formation of MAOM248. Thus, soils dominated by EM fungi will likely harbor more POM and less MAOM, while soils dominated by AM fungi will likely harbor more MAOM245,246. However, soil fauna generally appears to prefer saprotrophic over mycorrhizal fungi230,243,249, which might suppress decomposition of organic matter and, perhaps, foster the accumulation of POM. Recent evidence also suggests that impairment or promotion of individual fungal species by springtails can affect aggregate formation250 and thus the stability of POM and MAOM within50.
Interactions across soil faunal taxa and influence of climate and land use
Faunal taxa do not live isolated in soil but coexist with other taxa embedded in complex soil food webs251. Interactions among fauna on higher trophic levels can trickle down through the food web and affect multiple taxa on various lower trophic levels252 and vice versa. However, since experimental studies on soil food webs are difficult, many studies have investigated how individual taxa or groups of taxa interact to suppress or boost each other’s biomass/abundance and/or activity. Such amensalism or commensalism has been reported for interactions between microarthropods, such as mites or springtails, and nematodes and potworms, and between earthworms or ants and microarthropods113,253,254,255,256,257,258. For example, the presence of microarthropods can reduce the densities of nematodes and potworms through predation, while endogeic earthworms can suppress microarthropod densities through amensalism113. In contrast, anecic earthworms and ants appear to create habitat conditions, such as stable burrows, middens, or nest soil rich in nutrients and microorganisms, that enable microarthropods to thrive113,257. The activity of certain faunal taxa can also alter the effect other taxa have on ecosystems. In the presence of isopods, and their feces in particular, endogeic earthworms preferentially fed on these feces259, which are energetically less demanding to process, and formed lower amounts of aggregates160. Similarly, termites reduced their foraging rates in response to ant predation, with potential consequences for the amount of POM in termite nests260. Such interactions, however, are likely strongly influenced by environmental conditions such as soil fertility, organic matter inputs, and specifically, land use and climate change261,262.
While wetter and warmer sites will likely experience an increase263,264,265,266,267,268,269,270 and those affected by drought a decrease in soil faunal biomass266,268,271,272,273,274,275,276, climate change effects are not necessarily uniform across faunal taxa277. For example, increased warming or precipitation resulted in higher diversity of fungivorous mites278 or increased the relative abundance of fungivorous nematodes279, indicating changes in food-web structure beyond mere increases or decreases in biomass. These effects may further be amplified (or dampened) by land-use change. Specifically, land-use intensification appears to consistently reduce the biomass, abundance, richness, and diversity of soil fauna, apparently largely consistently across taxa277,280,281,282,283,284,285,286,287,288.
We here clarify that the potential effects of individual soil faunal taxa on POM and MAOM do not necessarily reflect their effects when being part of the complex food webs encountered in the multitude of soil types present on Earth or when being affected by climate and/or land-use change. Studies that aim to mechanistically explore the role of soil fauna in SOM dynamics and stability ideally take these dependencies into account and, apart from investigating individual taxa, examine interactions among taxa under variable environmental conditions, such as in different soil types, under different land uses and land-use intensities, or in simulated future climates.
Research gaps and recommendations
We emphasize that soil fauna may have a strong influence on the formation of and share between labile (POM) and stabilized (MAOM) SOM via three major processes – transformation, translocation, and grazing – with potentially far-reaching, global implications for management focused on increasing or maintaining soil C storage, nutrient stores, or crop yields. However, studies on the relevance of soil fauna to SOM stability beyond earthworms are widely lacking (Fig. 1). This might be due to the long history of ecological experiments with earthworms, their large body size and simple handling in the field and in the lab, their high biomass and ecosystem effects, as well as their significant contribution to decomposition processes289. We thus highlight the necessity to quantitatively assess the extent to which the wide diversity of soil fauna contributes to the formation (or decomposition) of POM and MAOM via each of the three major processes identified in this review (Figs. 2 and 3), how interactions among faunal taxa or within whole faunal communities alter this contribution, and whether it remains stable under varying environmental conditions, such as related to land-use or climate change. To this end, we recommend the use of controlled, micro- or meso-scale laboratory experiments290,291,292 along with standardized fractionation schemes293,294, which allow for separation of soil into compartments with varying stability, such as POM and MAOM. Potential constraints related to representative results from experiments with smaller organisms (meso-/microfauna) may be overcome by adapting the experimental size, duration, and number of replicates, and designing collaborative experiments distributed among research institutes. Such laboratory approaches will allow mechanistic insights into individual and combined effects of soil fauna on SOM dynamics, which can then be upscaled to field settings to investigate the relevance of land use, climate change, soil depth, or other environmental factors in this nexus295. Bioturbation rates and flows of C among different SOM pools (e.g., from litter to POM and aggregate-occluded POM to MAOM, from litter to dissolved organic matter to MAOM, and/or from litter to feces to MAOM) can be traced and quantified via stable isotope labeling, such as with 13C, coupled with compound-specific isotope measurements296,297 and physical fractionation19,20,298. This is specifically relevant for feces, as these structures, boosting microbial growth and leaching of dissolved organic matter, could strongly affect the formation of MAOM both via the microbial and direct sorption pathways. Likewise, the contribution of soil faunal necromass to SOM dynamics remains largely unknown but could be quantified by tracing the fate of isotopically labeled carcasses299. Combined with DNA analyzes250 of gut microbiomes, feces, and the surrounding soil, such isotopic and physical fractionation techniques could further provide insights into the relevance of microbial community changes to SOM dynamics and stability induced by soil fauna via grazing on microorganisms, transfer of gut microbiota to soil/feces, and physical and chemical transformation of organic matter in feces. Use of X-ray diffraction102 and/or BET analysis103, specifically in studies on termites (but also earthworms), would enable detailed insights into how mineralogical changes caused by soil fauna affect MAOM formation. Faunal effects on the whole C budget of soil should be quantified via monitoring of fluxes of C to and from the soil with different animal communities300, i.e., by quantifying heterotrophic respiration, leaching of dissolved organic matter, and C in bulk soil and/or fractions. The inclusion of living plants in controlled experiments could further help disentangle the role of soil fauna-plant interactions in SOM dynamics290, such as related to the microbial loop41. Finally, assessment and manipulation of soil faunal diversity in studies related to POM and MAOM could provide valuable insights into the relevance of biodiversity conservation efforts for establishing soils, as the most biodiverse systems on Earth301, as C sinks.
We are convinced that such experiments and the related knowledge gain are indispensable in accounting for the relevance of soil fauna in SOM dynamics. Environmental alterations are changing the composition and functioning of soil communities and processes302, which are key to soil C dynamics and feedbacks to climate change. Soil fauna are subject to many of these environmental changes277 and play decisive roles in SOM dynamics by regulating POM and MAOM formation through organic matter transformation, translocation, and grazing on microbial communities. Addressing this major research frontier requires novel interdisciplinary approaches to inform Earth system models and manage multifunctional soils in sustainable ways. We encourage cross-disciplinary cooperation among soil zoologists and chemists, microbial ecologists, and other related disciplines, as we envision that the vast remaining research gaps are most efficiently tackled with joint forces.
Data availability
No data have been generated in the preparation of this review.
References
Johnson, K. A. & Whitford, W. G. Foraging ecology and relative importance of subterranean termites in chihuahuan desert ecosystems. Environ. Entomol. 4, 66–70 (1975).
Phillips, H. R. P. et al. Global distribution of earthworm diversity. Science 366, 480–485 (2019).
Potapov, A. M. et al. Globally invariant metabolism but density-diversity mismatch in springtails. Nat. Commun. 14, 674 (2023).
van den Hoogen, J. et al. Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198 (2019).
Rosenberg, Y. et al. The global biomass and number of terrestrial arthropods. Sci. Adv. 9, 1–13 (2023).
Joly, F.-X. et al. Detritivore conversion of litter into faeces accelerates organic matter turnover. Commun. Biol. 3, 660 (2020).
Jouquet, P., Mamou, L., Lepage, M. & Velde, B. Effect of termites on clay minerals in tropical soils: fungus-growing termites as weathering agents. Eur. J. Soil Sci. 53, 521–528 (2002).
Kane, J. L., Kotcon, J. B., Freedman, Z. B. & Morrissey, E. M. Fungivorous nematodes drive microbial diversity and carbon cycling in soil. Ecology 104, e3844 (2023).
Van Groenigen, J. W. et al. How fertile are earthworm casts? A meta-analysis. Geoderma 338, 525–535 (2018).
Wilkinson, M. T., Richards, P. J. & Humphreys, G. S. Breaking ground: pedological, geological, and ecological implications of soil bioturbation. Earth-Sci. Rev. 97, 257–272 (2009).
Briones, M. J. I. Soil fauna and soil functions: a jigsaw puzzle. Front. Environ. Sci. 2, 7 (2014).
Grandy, A. S., Wieder, W. R., Wickings, K. & Kyker-Snowman, E. Beyond microbes: Are fauna the next frontier in soil biogeochemical models? Soil Biol. Biochem. 102, 40–44 (2016).
Osler, G. H. R. & Sommerkorn, M. Toward a complete soil C and N cycle: incorporating the soil fauna. Ecology 88, 1611–1621 (2007).
Filser, J. et al. Soil fauna: Key to new carbon models. Soil 2, 565–582 (2016).
Vidal, A. et al. Chapter One - The role of earthworms in agronomy: Consensus, novel insights and remaining challenges. in (ed. Sparks, D. L.) vol. 181 1–78 (Academic Press, 2023).
Frouz, J. Plant-soil feedback across spatiotemporal scales from immediate effects to legacy. Soil Biol. Biochem. 189, 109289 (2024).
Pulleman, M. M., Six, J., Uyl, A., Marinissen, J. C. Y. & Jongmans, A. G. Earthworms and management affect organic matter incorporation and microaggregate formation in agricultural soils. Appl. Soil Ecol. 29, 1–15 (2005).
Bossuyt, H., Six, J. & Hendrix, P. F. Protection of soil carbon by microaggregates within earthworm casts. Soil Biol. Biochem. 37, 251–258 (2005).
Angst, G. et al. Earthworms act as biochemical reactors to convert labile plant compounds into stabilized soil microbial necromass. Commun. Biol. 2, 441 (2019).
Vidal, A. et al. Earthworm cast formation and development: a shift from plant litter to mineral associated organic matter. Front. Environ. Sci. 7, 1–15 (2019).
Le Mer, G. et al. Inferring the impact of earthworms on the stability of organo-mineral associations, by Rock-Eval thermal analysis and 13C NMR spectroscopy. Org. Geochem. 144, 104016 (2020).
Kellerová, A., Angst, G. & Jílková, V. Earthworms facilitate stabilization of both more-available maize biomass and more-recalcitrant maize biochar on mineral particles in an agricultural soil. Soil Biol. Biochem. 189, 109278 (2024).
Castellano, M. J., Mueller, K. E., Olk, D. C., Sawyer, J. E. & Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 21, 3200–3209 (2015).
Cotrufo, M. F., Wallenstein, M. D., Boot, C. M., Denef, K. & Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 19, 988–995 (2013).
Sokol, N. W. et al. Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 20, 415–430 (2022).
Kallenbach, C. M., Grandy, A. S., Frey, S. D. & Diefendorf, A. F. Microbial physiology and necromass regulate agricultural soil carbon accumulation. Soil Biol. Biochem. 91, 279–290 (2015).
Lehmann, J. et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 13, 529–534 (2020).
Kleber, M. et al. Dynamic interactions at the mineral – organic matter interface. Nat. Rev. Earth Environ. 2, 402–421 (2021).
Cotrufo, M. F. & Lavallee, J. M. Chapter One - Soil organic matter formation, persistence, and functioning: A synthesis of current understanding to inform its conservation and regeneration. in (ed. Sparks, D. L.) vol. 172 1–66 (Academic Press, 2022).
Sokol, N. W., Sanderman, J. & Bradford, M. A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 25, 12–24 (2019).
Cole, L., Bardgett, R. D., Ineson, P. & Hobbs, P. J. Enchytraeid worm (Oligochaeta) influences on microbial community structure, nutrient dynamics and plant growth in blanket peat subjected to warming. Soil Biol. Biochem. 34, 83–92 (2002).
Heděnec, P. et al. Global distribution of soil fauna functional groups and their estimated litter consumption across biomes. Sci. Rep. 12, 17362 (2022).
Jiang, X., Denef, K., Stewart, C. E. & Cotrufo, M. F. Controls and dynamics of biochar decomposition and soil microbial abundance, composition, and carbon use efficiency during long-term biochar-amended soil incubations. Biol. Fertil. Soils 52, 1–14 (2016).
Bol, R., Poirier, N., Balesdent, J. & Gleixner, G. Molecular turnover time of soil organic matter in particle-size fractions of an arable soil. Rapid Commun. Mass Spectrom. 23, 2551–2558 (2009).
Mueller, C. W. & Koegel-Knabner, I. Soil organic carbon stocks, distribution, and composition affected by historic land use changes on adjacent sites. Biol. Fertil. Soils 45, 347–359 (2009).
von Lützow, M. et al. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions - a review. Eur. J. Soil Sci. 57, 426–445 (2006).
Angst, G. et al. Unlocking complex soil systems as carbon sinks: multi-pool management as the key. Nat. Commun. 14, 2967 (2023).
Lugato, E., Lavallee, J. M., Haddix, M. L., Panagos, P. & Francesca Cotrufo, M. Different climate sensitivity of particulate and mineral-associated soil organic matter. Nat. Geo 14, 295–300 (2021).
Crowther, T. W. et al. The global soil community and its influence on biogeochemistry. Science 365, eaav0550 (2019).
Potapov, A. M. et al. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biol. Rev. 97, 1057–1117 (2022).
Bonkowski, M. Protozoa and plant growth: the microbial loop in soil revisited. N. Phytol. 162, 617–631 (2004).
Scheu, S. Effects of earthworms on plant growth: patterns and perspectives: The 7th international symposium on earthworm ecology · Cardiff · Wales · 2002. Pedobiologia (Jena.) 47, 846–856 (2003).
Brown, G. G., Edwards, C. A. & Brussaard, L. How Earthworms Affect Plant Growth: Burrowing into the Mechanisms. in Earthworm Ecology (ed. Edwards, C. A.) 13–49 (CRC Press, 2004). https://doi.org/10.1201/9781420039719.
Angst, Š. et al. Stabilization of soil organic matter by earthworms is connected with physical protection rather than with chemical changes of organic matter. Geoderma 289, 29–35 (2017).
Bossuyt, H., Six, J. & Hendrix, P. F. Rapid incorporation of carbon from fresh residues into newly formed stable microaggregates within earthworm casts. Eur. J. Soil Sci. 55, 393–399 (2004).
Fahey, T. J. et al. Earthworm effects on the incorporation of litter C and N into soil organic matter in a sugar maple forest. Ecol. Appl. 23, 1185–1201 (2013).
Guggenberger, G., Thomas, R. J. & Zech, W. Soil organic matter within earthworm casts of an anecic-endogeic tropical pasture community. Colomb. Appl. Soil Ecol. 3, 263–274 (1996).
Sánchez-de León, Y. et al. Endogeic earthworm densities increase in response to higher fine-root production in a forest exposed to elevated CO2. Soil Biol. Biochem. 122, 31–38 (2018).
Wachendorf, C., Potthoff, M., Ludwig, B. & Joergensen, R. G. Effects of addition of maize litter and earthworms on C mineralization and aggregate formation in single and mixed soils differing in soil organic carbon and clay content. Pedobiologia (Jena.). 57, 161–169 (2014).
Angst, G., Mueller, K. E., Kögel-Knabner, I., Freeman, K. H. & Mueller, C. W. Aggregation controls the stability of lignin and lipids in clay-sized particulate and mineral associated organic matter. Biogeochemistry 132, 307–324 (2017).
Hong, H. N. et al. How do earthworms influence organic matter quantity and quality in tropical soils? Soil Biol. Biochem. 43, 223–230 (2011).
Ma, Y. et al. The combined controls of land use legacy and earthworm activity on soil organic matter chemistry and particle association during afforestation. Org. Geochem. 58, 56–68 (2013).
Vidal, A., Quenea, K., Alexis, M. & Derenne, S. Molecular fate of root and shoot litter on incorporation and decomposition in earthworm casts. Org. Geochem. 101, 1–10 (2016).
Zhang, X., Wang, J., Xie, H., Wang, J. & Zech, W. Comparison of organic compounds in the particle-size fractions of earthworm casts and surrounding soil in humid Laos. Appl. Soil Ecol. 23, 147–153 (2003).
Angst, G., Angst, Š., Frouz, J., Peterse, F. & Nierop, K. G. J. Preferential degradation of leaf- vs. root-derived organic carbon in earthworm-affected soil. Geoderma 372, 114391 (2020).
Hoeffner, K., Monard, C., Santonja, M. & Cluzeau, D. Feeding behaviour of epi-anecic earthworm species and their impacts on soil microbial communities. Soil Biol. Biochem. 125, 1–9 (2018).
McLean, M. A., Migge-Kleian, S. & Parkinson, D. Earthworm invasions of ecosystems devoid of earthworms: Effects on soil microbes. Biol. Invasions Belowgr. Earthworms as Invasive Species 57–73 https://doi.org/10.1007/978-1-4020-5429-7_7 (2006).
Singh, J., Eisenhauer, N., Schädler, M. & Cesarz, S. Earthworm gut passage reinforces land-use effects on soil microbial communities across climate treatments. Appl. Soil Ecol. 164, 103919 (2021).
Bernard, L. et al. Endogeic earthworms shape bacterial functional communities and affect organic matter mineralization in a tropical soil. ISME J. 6, 213–222 (2012).
Chang, C. H., Szlavecz, K. & Buyer, J. S. Species-specific effects of earthworms on microbial communities and the fate of litter-derived carbon. Soil Biol. Biochem. 100, 129–139 (2016).
Jouquet, P., Maron, P. A., Nowak, V. & Tran Duc, T. Utilization of microbial abundance and diversity as indicators of the origin of soil aggregates produced by earthworms. Soil Biol. Biochem. 57, 950–952 (2013).
Scullion, J. & Malik, A. Earthworm activity affecting organic matter, aggregation and microbial activity in soils restored after opencast mining for coal. Soil Biol. Biochem. 32, 119–126 (2000).
Angst, G. et al. Earthworms as catalysts in the formation and stabilization of soil microbial necromass. Glob. Chang. Biol. 28, 4775–4782 (2022).
Barthod, J., Dignac, M. F. & Rumpel, C. Effect of decomposition products produced in the presence or absence of epigeic earthworms and minerals on soil carbon stabilization. Soil Biol. Biochem. 160, 108308 (2021).
Bohlen, P. J., Edwards, C. A., Zhang, Q., Parmelee, R. W. & Allen, M. Indirect effects of earthworms on microbial assimilation of labile carbon. Appl. Soil Ecol. 20, 255–261 (2002).
Barthod, J. et al. How do earthworms affect organic matter decomposition in the presence of clay-sized minerals? Soil Biol. Biochem. 143, 107730 (2020).
Lubbers, I. M. et al. Greenhouse-gas emissions from soils increased by earthworms. Nat. Clim. Chang. 3, 187–194 (2013).
Marhan, S., Auber, J. & Poll, C. Additive effects of earthworms, nitrogen-rich litter and elevated soil temperature on N2O emission and nitrate leaching from an arable soil. Appl. Soil Ecol. 86, 55–61 (2015).
Marhan, S., Langel, R., Kandeler, E. & Scheu, S. Use of stable isotopes (13C) for studying the mobilisation of old soil organic carbon by endogeic earthworms (Lumbricidae). Eur. J. Soil Biol. 43, S201–S208 (2007).
Lubbers, I. M., Pulleman, M. M. & Van Groenigen, J. W. Can earthworms simultaneously enhance decomposition and stabilization of plant residue carbon? Soil Biol. Biochem. 105, 12–24 (2017).
Capowiez, Y., Marchán, D., Decaëns, T., Hedde, M. & Bottinelli, N. Let earthworms be functional - Definition of new functional groups based on their bioturbation behavior. Soil Biol. Biochem. 188, 109209 (2024).
Bottinelli, N., Hedde, M., Jouquet, P. & Capowiez, Y. An explicit definition of earthworm ecological categories – Marcel Bouché’s triangle revisited. Geoderma 372, 114361 (2020).
Ambarish, C. N. & Sridhar, K. R. Chemical and microbial characterization of feed and faeces of two giant pill-millipedes from forests in the Western Ghats of India. Pedosphere 26, 861–871 (2016).
Ambarish, C. N. & Sridhar, K. R. Production and quality of pill-millipede manure: a microcosm study. Agric. Res. 2, 258–264 (2013).
Coulis, M., Hättenschwiler, S., Coq, S. & David, J.-F. Leaf litter consumption by macroarthropods and burial of their faeces enhance decomposition in a mediterranean ecosystem. Ecosystems 19, 1104–1115 (2016).
da Silva, V. M. et al. Influence of the tropical millipede, Glyphiulus granulatus (Gervais, 1847), on aggregation, enzymatic activity, and phosphorus fractions in the soil. Geoderma 289, 135–141 (2017).
Joly, F.-X., Coq, S., Coulis, M., Nahmani, J. & Hättenschwiler, S. Litter conversion into detritivore faeces reshuffles the quality control over C and N dynamics during decomposition. Funct. Ecol. 32, 2605–2614 (2018).
Ganault, P. et al. Leaf litter morphological traits, invertebrate body mass and phylogenetic affiliation explain the feeding and feces properties of saprophagous macroarthropods. Eur. J. Soil Biol. 109, 103383 (2022).
Coulis, M., Hättenschwiler, S., Rapior, S. & Coq, S. The fate of condensed tannins during litter consumption by soil animals. Soil Biol. Biochem. 41, 2573–2578 (2009).
Joly, F.-X., Coq, S. & Subke, J.-A. Soil fauna precipitate the convergence of organic matter quality during decomposition. Oikos 2023, e09497 (2023).
Špaldoňová, A. & Frouz, J. Decomposition of forest litter and feces of armadillidium vulgare (isopoda: oniscidea) produced from the same litter affected by temperature and litter quality. Forests 10, 939 (2019).
Wickings, K. & Grandy, A. S. The oribatid mite Scheloribates moestus (Acari: Oribatida) alters litter chemistry and nutrient cycling during decomposition. Soil Biol. Biochem. 43, 351–358 (2011).
Frouz, J. et al. Utilization of dietary protein in the litter-dwelling larva of bibio marci (Diptera: Bibionidae). Eurasia. Soil Sci. 52, 1583–1587 (2019).
Frouz, J. & Šimek, M. Short term and long term effects of bibionid (Diptera: Bibionidae) larvae feeding on microbial respiration and alder litter decomposition. Eur. J. Soil Biol. 45, 192–197 (2009).
Jia, Y. et al. Insight into the indirect function of isopods in litter decomposition in mixed subtropical forests in China. Appl. Soil Ecol. 86, 174–181 (2015).
Kaneda, S., Frouz, J., Baldrian, P., Cajthaml, T. & Krištůfek, V. Does the addition of leaf litter affect soil respiration in the same way as addition of macrofauna excrements (of Bibio marci Diptera larvae) produced from the same litter? Appl. Soil Ecol. 72, 7–13 (2013).
Špaldoňová, A. & Frouz, J. The role of Armadillidium vulgare (Isopoda: Oniscidea) in litter decomposition and soil organic matter stabilization. Appl. Soil Ecol. 83, 186–192 (2014).
Frouz, J., Špaldoňová, A., Lhotáková, Z. & Cajthaml, T. Major mechanisms contributing to the macrofauna-mediated slow down of litter decomposition. Soil Biol. Biochem. 91, 23–31 (2015).
Buse, T., Ruess, L. & Filser, J. Collembola gut passage shapes microbial communities in faecal pellets but not viability of dietary algal cells. Chemoecology 24, 79–84 (2014).
Chan, K. Y. & Heenan, D. P. Occurrence of Enchytraeid worms and some properties of their casts in an Australian soil under cropping. Soil Res. 33, 651–657 (1995).
Maaß, S., Caruso, T. & Rillig, M. C. Functional role of microarthropods in soil aggregation. Pedobiologia (Jena.) 58, 59–63 (2015).
Makoto, K., Arai, M. & Kaneko, N. Change the menu? Species-dependent feeding responses of millipedes to climate warming and the consequences for plant–soil nitrogen dynamics. Soil Biol. Biochem. 72, 19–25 (2014).
Fujimaki, R., Sato, Y., Okai, N. & Kaneko, N. The train millipede (Parafontaria laminata) mediates soil aggregation and N dynamics in a Japanese larch forest. Geoderma 159, 216–220 (2010).
Jungerius, P. D., van den Ancker, J. A. M. & Mücher, H. J. The contribution of termites to the microgranular structure of soils on the Uasin Gishu Plateau, Kenya. CATENA 34, 349–363 (1999).
Kaiser, K. & Guggenberger, G. Mineral surfaces and soil organic matter. Eur. J. Soil Sci. 54, 219–236 (2003).
Jouquet, P., Bottinelli, N., Lata, J.-C., Mora, P. & Caquineau, S. Role of the fungus-growing termite Pseudacanthotermes spiniger (Isoptera, Macrotermitinae) in the dynamic of clay and soil organic matter content. An experimental analysis. Geoderma 139, 127–133 (2007).
Blume, H.-P. & Schad, P. Bodenkunde in Stichworten p. 238 (Springer Berlin Heidelberg, 2015).
Lejoly, J. et al. Effects of termite sheetings on soil properties under two contrasting soil management practices. Pedobiologia (Jena.). 76, 150573 (2019).
Mujinya, B. B. et al. Clay composition and properties in termite mounds of the Lubumbashi area, D.R. Congo. Geoderma 192, 304–315 (2013).
Carpenter, D., Hodson, M. E., Eggleton, P. & Kirk, C. Earthworm induced mineral weathering: Preliminary results. Eur. J. Soil Biol. 43, S176–S183 (2007).
Hušák, M., Frouz, J. & Had, J. Can earthworm gut passage modify clay minerals structure: preliminary results. in Contributions to Soil Zoology in Central Europe 1 (eds. Tajovský, K., Schlaghamerský, J. & Pižl, V.) 37–39 (2005).
Eusterhues, K. et al. Characterization of ferrihydrite-soil organic matter coprecipitates by x-ray diffraction and mössbauer spectroscopy. Environ. Sci. {} Technol. 42, 7891–7897 (2008).
Brunauer, S., Emmett, P. H. & Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 60, 309–319 (1938).
Meysman, F. J. R., Middelburg, J. J. & Heip, C. H. R. Bioturbation: a fresh look at Darwin’s last idea. Trends Ecol. Evol. 21, 688–695 (2006).
Darwin, C. The Formation of Vegetable Mould, Through the Action of Worms: With Observations on Their Habits. p. 326 (Murray, J., 1892).
Paton, T. R., Humphreys, G. S. & Mitchell, P. B. Soils: A New Global View. p. 234 (Yale University Press, 1995).
Ma, Y., Filley, T. R., Szlavecz, K. & McCormick, M. K. Controls on wood and leaf litter incorporation into soil fractions in forests at different successional stages. Soil Biol. Biochem. 69, 212–222 (2014).
Capowiez, Y., Bottinelli, N., Sammartino, S., Michel, E. & Jouquet, P. Morphological and functional characterisation of the burrow systems of six earthworm species (Lumbricidae). Biol. Fertil. Soils 51, 869–877 (2015).
Pham, Q. V. et al. Using morpho-anatomical traits to predict the effect of earthworms on soil water infiltration. Geoderma 429, 116245 (2023).
Lee, K. E. Earthworms: Their Ecology and Relationships with Soils and Land Use. p. 411 (Academic Press, 1985).
Bastardie, F., Capowiez, Y. & Cluzeau, D. 3D characterisation of earthworm burrow systems in natural soil cores collected from a 12-year-old pasture. Appl. Soil Ecol. 30, 34–46 (2005).
Kuzyakov, Y. & Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 83, 184–199 (2015).
Eisenhauer, N. The action of an animal ecosystem engineer: Identification of the main mechanisms of earthworm impacts on soil microarthropods. Pedobiologia (Jena.). 53, 343–352 (2010).
Brown, G. G. How do earthworms affect microfloral and faunal community diversity? BT - The Significance and Regulation of Soil Biodiversity: Proceedings of the International Symposium on Soil Biodiversity, held at Michigan State University, East Lansing, May 3–6, 199. in (eds. Collins, H. P., Robertson, G. P. & Klug, M. J.) 247–269 (Springer Netherlands, 1995). https://doi.org/10.1007/978-94-011-0479-1_22.
Brown, G. G., Barois, I. & Lavelle, P. Regulation of soil organic matter dynamics and microbial activityin the drilosphere and the role of interactionswith other edaphic functional domains. Eur. J. Soil Biol. 36, 177–198 (2000).
Görres, J. H., Savin, M. C. & Amador, J. A. Soil micropore structure and carbon mineralization in burrows and casts of an anecic earthworm (Lumbricus terrestris). Soil Biol. Biochem. 33, 1881–1887 (2001).
Schlüter, S. et al. Microscale carbon distribution around pores and particulate organic matter varies with soil moisture regime. Nat. Commun. 13, 2098 (2022).
Görres, J. H., Savin, M. C. & Amador, J. A. Dynamics of carbon and nitrogen mineralization, microbial biomass, and nematode abundance within and outside the burrow walls of anecic earthworms (Lumbricus terrestris). Soil Sci. 162, 666–671 (1997).
Hoang, D. T. T., Razavi, B. S., Kuzyakov, Y. & Blagodatskaya, E. Earthworm burrows: Kinetics and spatial distribution of enzymes of C-, N- and P- cycles. Soil Biol. Biochem. 99, 94–103 (2016).
Hoang, D. T. T., Bauke, S. L., Kuzyakov, Y. & Pausch, J. Rolling in the deep: Priming effects in earthworm biopores in topsoil and subsoil. Soil Biol. Biochem. 114, 59–71 (2017).
Lipiec, J., Frąc, M., Brzezińska, M., Turski, M. & Oszust, K. Linking microbial enzymatic activities and functional diversity of soil around earthworm burrows and casts. Front. Microbiol. 7, 1361 (2016).
Parkin, T. B. & Berry, E. C. Microbial nitrogen transformations in earthworm burrows. Soil Biol. Biochem. 31, 1765–1771 (1999).
Andriuzzi, W. S. et al. Organic matter composition and the protist and nematode communities around anecic earthworm burrows. Biol. Fertil. Soils 52, 91–100 (2016).
Guhra, T., Stolze, K., Schweizer, S. & Totsche, K. U. Earthworm mucus contributes to the formation of organo-mineral associations in soil. Soil Biol. Biochem. 145, 107785 (2020).
Don, A. et al. Organic carbon sequestration in earthworm burrows. Soil Biol. Biochem. 40, 1803–1812 (2008).
Taylor, A. R., Lenoir, L., Vegerfors, B. & Persson, T. Ant and earthworm bioturbation in cold-temperate ecosystems. Ecosystems 22, 981–994 (2019).
Viles, H. A., Goudie, A. S. & Goudie, A. M. Ants as geomorphological agents: A global assessment. Earth-Sci. Rev. 213, 103469 (2021).
Noriega, J. A. et al. Dung removal increases under higher dung beetle functional diversity regardless of grazing intensification. Nat. Commun. 14, 8070 (2023).
Cammeraat, E. L. H. & Risch, A. C. The impact of ants on mineral soil properties and processes at different spatial scales. J. Appl. Entomol. 132, 285–294 (2008).
Ehrle, A. et al. Yellow-meadow ant (Lasius flavus) mound development determines soil properties and growth responses of different plant functional types. Eur. J. Soil Biol. 81, 83–93 (2017).
Fall, S., Brauman, A. & Chotte, J.-L. Comparative distribution of organic matter in particle and aggregate size fractions in the mounds of termites with different feeding habits in Senegal: Cubitermes niokoloensis and Macrotermes bellicosus. Appl. Soil Ecol. 17, 131–140 (2001).
Frouz, J. & Jilková, V. The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmecological N. 11, 191–199 (2008).
Rückamp, D. et al. Soil genesis and heterogeneity of phosphorus forms and carbon below mounds inhabited by primary and secondary termites. Geoderma 170, 239–250 (2012).
Whitford, W. G. & Eldridge, D. J. Effects of ants and termites on soil and geomorphological processes. Treatise Geomorphol. 12, 281–292 (2013).
Jouquet, P. et al. Where do South-Indian termite mound soils come from? Appl. Soil Ecol. 117–118, 190–195 (2017).
Nkem, J. N., Lobry De Bruyn, L. A., Grant, C. D. & Hulugalle, N. R. The impact of ant bioturbation and foraging activities on surrounding soil properties. Pedobiologia (Jena.). 44, 609–621 (2000).
Chen, S. et al. Fine resolution map of top- and subsoil carbon sequestration potential in France. Sci. Total Environ. 630, 389–400 (2018).
Cheik, S., Harit, A., Bottinelli, N. & Jouquet, P. Bioturbation by dung beetles and termites. Do they similarly impact soil and hydraulic properties? Pedobiologia (Jena.) 95, 150845 (2022).
Kalisz, P. J. & Stone, E. L. Soil mixing by scarab beetles and pocket gophers in North-Central Florida. Soil Sci. Soc. Am. J. 48, 169–172 (1984).
Yamada, D., Imura, O., Shi, K. & Shibuya, T. Effect of tunneler dung beetles on cattle dung decomposition, soil nutrients and herbage growth. Grassl. Sci. 53, 121–129 (2007).
Cao, Q. et al. Differentiated impacts of the feeding-habits of three ant species on carbon mineralization in tropical forest soils. Eur. J. Soil Biol. 110, 103403 (2022).
Kristiansen, S. M. & Amelung, W. Abandoned anthills of Formica polyctena and soil heterogeneity in a temperate deciduous forest: morphology and organic matter composition. Eur. J. Soil Sci. 52, 355–363 (2001).
Zanne, A. E. et al. Termite sensitivity to temperature affects global wood decay rates. Science 377, 1440–1444 (2022).
Holter, P., Scholtz, C. H. & Wardhaugh, K. G. Dung feeding in adult scarabaeines (tunnellers and endocoprids): even large dung beetles eat small particles. Ecol. Entomol. 27, 169–176 (2002).
Meyer, S. T. et al. Leaf-cutting ants as ecosystem engineers: topsoil and litter perturbations around Atta cephalotes nests reduce nutrient availability. Ecol. Entomol. 38, 497–504 (2013).
Cammeraat, L. H., Willott, S. J., Compton, S. G. & Incoll, L. D. The effects of ants’ nests on the physical, chemical and hydrological properties of a rangeland soil in semi-arid Spain. Geoderma 105, 1–20 (2002).
Frouz, J., Holec, M. & Kalčík, J. The effect of Lasius niger (Hymenoptera, Formicidae) ant nest on selected soil chemical properties. Pedobiologia (Jena.). 47, 205–212 (2003).
Jílková, V., Matějíček, L. & Frouz, J. Changes in the pH and other soil chemical parameters in soil surrounding wood ant (Formica polyctena) nests. Eur. J. Soil Biol. 47, 72–76 (2011).
Jouquet, P., Harit, A., Bottinelli, N. & Eldridge, D. J. Termite bioturbation: Fungal versus non-fungal building strategies lead to different soil sheeting stability. Soil Biol. Biochem. 176, 108868 (2023).
Lenoir, A., D’Ettorre, P., Errard, C. & Hefetz, A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599 (2001).
Véle, A., Frouz, J., Holuša, J. & Kalčík, J. Chemical properties of forest soils as affected by nests of Myrmica ruginodis (Formicidae). Biol. (Bratisl.). 65, 122–127 (2010).
Coûteaux, M.-M., Aloui, A. & Kurz-Besson, C. Pinus halepensis litter decomposition in laboratory microcosms as influenced by temperature and a millipede, Glomeris marginata. Appl. Soil Ecol. 20, 85–96 (2002).
Des Marteaux, L. E., Kullik, S. A., Habash, M. & Schmidt, J. M. Terrestrial isopods porcellio scaber and oniscus asellus (crustacea: isopoda) increase bacterial abundance and modify microbial community structure in leaf litter microcosms: a short-term decomposition study. Microb. Ecol. 80, 690–702 (2020).
Yang, X., Shao, M. & Li, T. Effects of terrestrial isopods on soil nutrients during litter decomposition. Geoderma 376, 114546 (2020).
Förster, B., Muroya, K. & Garcia, M. Plant growth and microbial activity in a tropical soil amended with faecal pellets from millipedes and woodlice. Pedobiologia (Jena.). 50, 281–290 (2006).
Hanisch, J., Engell, I., Linsler, D., Scheu, S. & Potthoff, M. The role of Collembola for litter decomposition under minimum and conventional tillage. J. Plant Nutr. Soil Sci. 185, 529–538 (2022).
HÄttenschwiler, S. & Bretscher, D. Isopod effects on decomposition of litter produced under elevated CO2, N deposition and different soil types. Glob. Chang. Biol. 7, 565–579 (2001).
Hedde, M., Bureau, F., Akpa-Vinceslas, M., Aubert, M. & Decaëns, T. Beech leaf degradation in laboratory experiments: Effects of eight detritivorous invertebrate species. Appl. Soil Ecol. 35, 291–301 (2007).
Irmler, U. Changes in the fauna and its contribution to mass loss and N release during leaf litter decomposition in two deciduous forests. Pedobiologia (Jena.). 44, 105–118 (2000).
Loranger-Merciris, G., Laossi, K.-R. & Bernhard-Reversat, F. Soil aggregation in a laboratory experiment: Interactions between earthworms, woodlice and litter palatability. Pedobiologia (Jena.). 51, 439–443 (2008).
Patoine, G. et al. Plant litter functional diversity effects on litter mass loss depend on the macro-detritivore community. Pedobiologia (Jena.). 65, 29–42 (2017).
Veldhuis, M. P., Laso, F. J., Olff, H. & Berg, M. P. Termites promote resistance of decomposition to spatiotemporal variability in rainfall. Ecology 98, 467–477 (2017).
Seibold, S. et al. The contribution of insects to global forest deadwood decomposition. Nature 597, 77–81 (2021).
David, J. F. The role of litter-feeding macroarthropods in decomposition processes: A reappraisal of common views. Soil Biol. Biochem. 76, 109–118 (2014).
Booth, S., Kurtz, B., de Heer, M. I., Mooney, S. J. & Sturrock, C. J. Tracking wireworm burrowing behaviour in soil over time using 3D X-ray computed tomography. Pest Manag. Sci. 76, 2653–2662 (2020).
Adachi, H., Ozawa, M., Yagi, S., Seita, M. & Kondo, S. Pivot burrowing of scarab beetle (Trypoxylus dichotomus) larva. Sci. Rep. 11, 14594 (2021).
Bryson, H. R. The identification of soil insects by their burrow characteristics. Trans. Kans. Acad. Sci. 42, 245–253 (1939).
Hembree, D. I. Neoichnology of burrowing millipedes: linking modern burrow morphology, organism behavior, and sediment properties to interpret continental ichnofossils. Palaios 24, 425–439 (2009).
Bowen, J. J. & Hembree, D. I. Neoichnology of two spirobolid millipedes: Improving the understanding of the burrows of soil detritivores. Palaeontol. Electron. 17, 17.1.18A (2014).
Mele, G., Buscemi, G., Gargiulo, L. & Terribile, F. Soil burrow characterization by 3D image analysis: Prediction of macroinvertebrate groups from biopore size distribution parameters. Geoderma 404, 115292 (2021).
Briones, M. J. I., Carreira, J. & Ineson, P. Cognettia sphagnetorum (Enchytraeidae) and nutrient cycling in organic soils: a microcosm experiment. Appl. Soil Ecol. 9, 289–294 (1998).
Carrera, N., Barreal, M. E., Rodeiro, J. & Briones, M. J. I. Interactive effects of temperature, soil moisture and enchytraeid activities on C losses from a peatland soil. Pedobiologia (Jena.). 54, 291–299 (2011).
Lappalainen, M. et al. Release of carbon in different molecule size fractions from decomposing boreal mor and peat as affected by enchytraeid worms. Water, Air, Soil Pollut. 229, 240 (2018).
Laurén, A. et al. Nitrogen and carbon dynamics and the role of enchytraeid worms in decomposition of l, f and h layers of boreal mor. Water, Air, Soil Pollut. 223, 3701–3719 (2012).
Liiri, M., Ilmarinen, K. & Setälä, H. The significance of Cognettia sphagnetorum(Enchytraeidae) on nitrogen availability and plant growth in wood ash-treated humus soil. Plant Soil 246, 31–39 (2002).
Cole, L., Bardgett, R. D. & Ineson, P. Enchytraeid worms (Oligochaeta) enhance mineralization of carbon in organic upland soils. Eur. J. Soil Sci. 51, 185–192 (2000).
Cragg, R. G. & Bardgett, R. D. How changes in soil faunal diversity and composition within a trophic group influence decomposition processes. Soil Biol. Biochem. 33, 2073–2081 (2001).
Filser, J. The role of Collembola in carbon and nitrogen cycling in soil: Proceedings of the Xth international Colloquium on Apterygota, České Budějovice 2000: Apterygota at the Beginning of the Third Millennium. Pedobiologia (Jena.). 46, 234–245 (2002).
Menéndez, R., Webb, P. & Orwin, K. H. Complementarity of dung beetle species with different functional behaviours influence dung–soil carbon cycling. Soil Biol. Biochem. 92, 142–148 (2016).
Kaiser, K. & Kalbitz, K. Cycling downwards - dissolved organic matter in soils. Soil Biol. Biochem. 52, 29–32 (2012).
Evans, K. S. et al. Soil fauna accelerate dung pat decomposition and nutrient cycling into grassland soil. Rangel. Ecol. Manag. 72, 667–677 (2019).
Barragán, F. et al. The rolling dung master: an ecosystem engineer beetle mobilizing soil nutrients to enhance plant growth across a grassland management intensity gradient in drylands. J. Arid Environ. 197, 104673 (2022).
Haimi, J. & Siira-Pietikäinen, A. Activity and role of the enchytraeid worm Cognettia sphagnetorum (Vejd.) (Oligochaeta: Enchytraeidae) in organic and mineral forest soil. Pedobiologia (Jena.). 47, 303–310 (2003).
Kaleri, A. R. et al. Effects of dung beetle-amended soil on growth, physiology, and metabolite contents of bok choy and improvement in soil conditions. J. Soil Sci. Plant Nutr. 20, 2671–2683 (2020).
Engell, I. et al. Crop residue displacement by soil inversion: Annelid responses and their impact on carbon and nitrogen dynamics in a lab-based mesocosm study. Appl. Soil Ecol. 167, 104151 (2021).
Gan, H., Liang, C. & Wickings, K. Root herbivores accelerate carbon inputs to soil and drive changes in biogeochemical processes. Rhizosphere 6, 112–115 (2018).
Kaleri, A. R. et al. Dung beetle improves soil bacterial diversity and enzyme activity and enhances growth and antioxidant content of chinese cabbage (Brassica rapa ssp. pekinensis). J. Soil Sci. Plant Nutr. 21, 3387–3401 (2021).
Coulibaly, S. F. M. et al. Functional assemblages of collembola determine soil microbial communities and associated functions. Front. Environ. Sci. 7, 52 (2019).
Franklin, S. M. et al. The unexplored role of preferential flow in soil carbon dynamics. Soil Biol. Biochem. 161, 108398 (2021).
Erktan, A., Or, D. & Scheu, S. The physical structure of soil: Determinant and consequence of trophic interactions. Soil Biol. Biochem. 148, 107876 (2020).
Brownlee, C. & Jennings, D. H. Long distance translocation in Serpula lacrimans: Velocity estimates and the continuous monitoring of induced perturbations. Trans. Br. Mycol. Soc. 79, 143–148 (1982).
Connolly, J. H. & Jellison, J. Two-way translocation of cations by the brown rot fungus Gloeophyllum trabeum. Int. Biodeterior. Biodegrad. 39, 181–188 (1997).
Yang, P. & van Elsas, J. D. Mechanisms and ecological implications of the movement of bacteria in soil. Appl. Soil Ecol. 129, 112–120 (2018).
Bonkowski, M., Villenave, C. & Griffiths, B. Rhizosphere fauna: the functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 321, 213–233 (2009).
Dighton, J., Jones, H. E., Robinson, C. H. & Beckett, J. The role of abiotic factors, cultivation practices and soil fauna in the dispersal of genetically modified microorganisms in soils. Appl. Soil Ecol. 5, 109–131 (1997).
Standing, D., Knox, O. G. G., Mullins, C. E., Killham, K. K. & Wilson, M. J. Influence of Nematodes on Resource Utilization by Bacteria—an in vitro Study. Microb. Ecol. 52, 444–450 (2006).
Angst, G. et al. Stabilized microbial necromass in soil is more strongly coupled with microbial diversity than the bioavailability of plant inputs. Soil Biol. Biochem. 190, 109323 (2024).
Vašutová, M. et al. Taxi drivers: the role of animals in transporting mycorrhizal fungi. Mycorrhiza 29, 413–434 (2019).
Lunde, L. F. et al. Beetles provide directed dispersal of viable spores of a keystone wood decay fungus. Fungal Ecol. 63, 101232 (2023).
Aanen, D. K. & Boomsma, J. J. Social-insect fungus farming. Curr. Biol. 16, R1014–R1016 (2006).
Kitabayashi, K., Kitamura, S. & Tuno, N. Fungal spore transport by omnivorous mycophagous slug in temperate forest. Ecol. Evol. 12, e8565 (2022).
Swanson, A. C. et al. Welcome to the Atta world: A framework for understanding the effects of leaf-cutter ants on ecosystem functions. Funct. Ecol. 33, 1386–1399 (2019).
Ahmad, F. et al. Multipartite symbioses in fungus-growing termites (Blattodea: Termitidae, Macrotermitinae) for the degradation of lignocellulose. Insect Sci. 28, 1512–1529 (2021).
Gange, A. C. Translocation of mycorrhizal fungi by earthworms during early succession. Soil Biol. Biochem. 25, 1021–1026 (1993).
Geisen, S. The bacterial-fungal energy channel concept challenged by enormous functional versatility of soil protists. Soil Biol. Biochem. 102, 22–25 (2016).
Clarholm, M. Protozoan grazing of bacteria in soil—impact and importance. Microb. Ecol. 7, 343–350 (1981).
Kuikman, P. J., Jansen, A. G., van Veen, J. A. & Zehnder, A. J. B. Protozoan predation and the turnover of soil organic carbon and nitrogen in the presence of plants. Biol. Fertil. Soils 10, 22–28 (1990).
Rosenberg, K. et al. Soil amoebae rapidly change bacterial community composition in the rhizosphere of Arabidopsis thaliana. ISME J. 3, 675–684 (2009).
Ingham, R. E., Trofymow, J. A., Ingham, E. R. & Coleman, D. C. Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecol. Monogr. 55, 119–140 (1985).
Jiang, Y. et al. Nematodes and microbial community affect the sizes and turnover rates of organic carbon pools in soil aggregates. Soil Biol. Biochem. 119, 22–31 (2018).
Jiang, Y. et al. Nematode grazing promotes bacterial community dynamics in soil at the aggregate level. ISME J. 11, 2705–2717 (2017).
De Mesel, I. et al. Top-down impact of bacterivorous nematodes on the bacterial community structure: a microcosm study. Environ. Microbiol. 6, 733–744 (2004).
Kreuzer, K. et al. Grazing of a common species of soil protozoa (Acanthamoeba castellanii) affects rhizosphere bacterial community composition and root architecture of rice (Oryza sativa L.). Soil Biol. Biochem. 38, 1665–1672 (2006).
Xiao, H.-F., Li, G., Li, D.-M., Hu, F. & Li, H.-X. Effect of different bacterial-feeding nematode species on soil bacterial numbers, activity, and community composition. Pedosphere 24, 116–124 (2014).
Jin, X., Wang, Z., Wu, F., Li, X. & Zhou, X. Litter mixing alters microbial decomposer community to accelerate tomato root litter decomposition. Microbiol. Spectr. 10, e0018622 (2022).
Mielke, L. et al. Nematode grazing increases the allocation of plant-derived carbon to soil bacteria and saprophytic fungi, and activates bacterial species of the rhizosphere. Pedobiologia (Jena.). 90, 150787 (2022).
Rashidi, G. & Ostrowski, E. A. Phagocyte chase behaviours: discrimination between Gram-negative and Gram-positive bacteria by amoebae. Biol. Lett. 15, 20180607 (2019).
Rønn, R., McCaig, A. E., Griffiths, B. S. & Prosser, J. I. Impact of protozoan grazing on bacterial community structure in soil microcosms. Appl. Environ. Microbiol. 68, 6094–6105 (2002).
Shu, L. et al. A dormant amoeba species can selectively sense and predate on different soil bacteria. Funct. Ecol. 35, 1708–1721 (2021).
Fanin, N. et al. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 128, 111–114 (2019).
Kramer, C. & Gleixner, G. Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol. Biochem. 38, 3267–3278 (2006).
Balasooriya, W. K., Denef, K., Huygens, D. & Boeckx, P. Translocation and turnover of rhizodeposit carbon within soil microbial communities of an extensive grassland ecosystem. Plant Soil 376, 61–73 (2014).
Buckeridge, K. M. et al. Sticky dead microbes: rapid abiotic retention of microbial necromass in soil. Soil Biol. Biochem. 149, 107929 (2020).
Bonkowski, M., Cheng, W., Griffiths, B. S., Alphei, J. & Scheu, S. Microbial-faunal interactions in the rhizosphere and effects on plant growth§1Paper presented at the 16th World Congress of Soil Science, 20–26 August 1998, Montpellier, France. Eur. J. Soil Biol. 36, 135–147 (2000).
Bonkowski, M. & Brandt, F. Do soil protozoa enhance plant growth by hormonal effects? Soil Biol. Biochem. 34, 1709–1715 (2002).
Frey, S. D., Gupta, V. V. S. R., Elliott, E. T. & Paustian, K. Protozoan grazing affects estimates of carbon utilization efficiency of the soil microbial community. Soil Biol. Biochem. 33, 1759–1768 (2001).
Miltner, A., Bombach, P., Schmidt-Brücken, B. & Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 111, 41–55 (2012).
Erktan, A., Rillig, M. C., Carminati, A., Jousset, A. & Scheu, S. Protists and collembolans alter microbial community composition, C dynamics and soil aggregation in simplified consumer–prey systems. Biogeosciences 17, 4961–4980 (2020).
Koukol, O., Mourek, J., Janovský, Z. & Černá, K. Do oribatid mites (Acari: Oribatida) show a higher preference for ubiquitous vs. specialized saprotrophic fungi from pine litter? Soil Biol. Biochem. 41, 1124–1131 (2009).
Pollierer, M. M. & Scheu, S. Stable isotopes of amino acids indicate that soil decomposer microarthropods predominantly feed on saprotrophic fungi. Ecosphere 12, e03425 (2021).
Schneider, K., Renker, C. & Maraun, M. Oribatid mite (Acari, Oribatida) feeding on ectomycorrhizal fungi. Mycorrhiza 16, 67–72 (2005).
Crowther, T. W. & A’Bear, A. D. Impacts of grazing soil fauna on decomposer fungi are species-specific and density-dependent. Fungal Ecol. 5, 277–281 (2012).
Crowther, T. W., Jones, T. H. & Boddy, L. Species-specific effects of grazing invertebrates on mycelial emergence and growth from woody resources into soil. Fungal Ecol. 4, 333–341 (2011).
A’Bear, A. D., Boddy, L., Raspotnig, G. & Jones, T. H. Non-trophic effects of oribatid mites on cord-forming basidiomycetes in soil microcosms. Ecol. Entomol. 35, 477–484 (2010).
Ek, H., Sjögren, M., Arnebrant, K. & Söderström, B. Extramatrical mycelial growth, biomass allocation and nitrogen uptake in ectomycorrhizal systems in response to collembolan grazing. Appl. Soil Ecol. 1, 155–169 (1994).
Hedlund, K. & Öhrn, M. S. Tritrophic interactions in a soil community enhance decomposition rates. Oikos 88, 585–591 (2000).
Kaneko, N., McLean, M. A. & Parkinson, D. Do mites and Collembola affect pine litter fungal biomass and microbial respiration? Appl. Soil Ecol. 9, 209–213 (1998).
Bonkowski, M., Griffiths, B. S. & Ritz, K. Food preferences of earthworms for soil fungi. Pedobiologia (Jena.) 44, 666–676 (2000).
Crowther, T. W. et al. Top-down control of soil fungal community composition by a globally distributed keystone consumer. Ecology 94, 2518–2528 (2013).
Crowther, T. W., Boddy, L. & Jones, T. H. Outcomes of fungal interactions are determined by soil invertebrate grazers. Ecol. Lett. 14, 1134–1142 (2011).
Domeignoz-Horta, L. A. et al. Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 11, 3684 (2020).
Tao, F. et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 618, 981–985 (2023).
Potapov, A. M. & Tiunov, A. V. Stable isotope composition of mycophagous collembolans versus mycotrophic plants: Do soil invertebrates feed on mycorrhizal fungi? Soil Biol. Biochem. 93, 115–118 (2016).
Tiunov, A. V. & Scheu, S. Arbuscular mycorrhiza and Collembola interact in affecting community composition of saprotrophic microfungi. Oecologia 142, 636–642 (2005).
Frey, S. D. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 50, 237–259 (2019).
Craig, M. E. et al. Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil organic matter. Glob. Chang. Biol. 24, 3317–3330 (2018).
Gadgil, R. L. & Gadgil, P. D. Suppression of litter decomposition by mycorrhizal roots of Pinus radiata. N. Zeal. J. Sci. 5, 33–41 (1975).
Cotrufo, M. F., Ranalli, M. G., Haddix, M. L., Six, J. & Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 12, 989–994 (2019).
Crowther, T. W., Boddy, L. & Hefin Jones, T. Functional and ecological consequences of saprotrophic fungus–grazer interactions. ISME J. 6, 1992–2001 (2012).
Hannula, S. E., Jongen, R. & Morriën, E. Grazing by collembola controls fungal induced soil aggregation. Fungal Ecol. 65, 101284 (2023).
Scheu, S. The soil food web: structure and perspectives. Eur. J. Soil Biol. 38, 11–20 (2002).
Wardle, D. A., Williamson, W. M., Yeates, G. W. & Bonner, K. I. Trickle-down effects of aboveground trophic cascades on the soil food web. Oikos 111, 348–358 (2005).
Santos, P. F. & Whitford, W. G. The effects of microarthropods on litter decomposition in a chihuahuan desert Ecosystem. Ecology 62, 654–663 (1981).
Huhta, V. et al. Response of soil fauna to fertilization and manipulation of pH in coniferous forests. Acta. Fenn. 195, 7641 (1986).
Taylor, A. R., Pflug, A., Schröter, D. & Wolters, V. Impact of microarthropod biomass on the composition of the soil fauna community and ecosystem processes. Eur. J. Soil Biol. 46, 80–86 (2010).
Tao, J. et al. Earthworms reduce the abundance of nematodes and enchytraeids in a soil mesocosm experiment despite abundant food resources. Soil Sci. Soc. Am. J. 75, 1774–1778 (2011).
Wickenbrock, L. & Heisler, C. Influence of earthworm activity on the abundance of collembola in soil. Soil Biol. Biochem. 29, 517–521 (1997).
Boulton, A. M., Jaffee, B. A. & Scow, K. M. Effects of a common harvester ant (Messor andrei) on richness and abundance of soil biota. Appl. Soil Ecol. 23, 257–265 (2003).
Bonkowski, M., Scheu, S. & Schaefer, M. Interactions of earthworms (Octolasion lacteum), millipedes (Glomeris marginata) and plants (Hordelymus europaeus) in a beechwood on a basalt hill: implications for litter decomposition and soil formation. Appl. Soil Ecol. 9, 161–166 (1998).
Korb, J. & Linsenmair, K. E. Evaluation of predation risk in the collectively foraging termite Macrotermes bellicosus. Insectes Soc. 49, 264–269 (2002).
Lenoir, L., Persson, T., Bengtsson, J., Wallander, H. & Wirén, A. Bottom–up or top–down control in forest soil microcosms? Effects of soil fauna on fungal biomass and C/N mineralisation. Biol. Fertil. Soils 43, 281–294 (2007).
Vetter, S., Fox, O., Ekschmitt, K. & Wolters, V. Limitations of faunal effects on soil carbon flow: density dependence, biotic regulation and mutual inhibition. Soil Biol. Biochem. 36, 387–397 (2004).
A’Bear, A. D., Jones, T. H. & Boddy, L. Potential impacts of climate change on interactions among saprotrophic cord-forming fungal mycelia and grazing soil invertebrates. Fungal Ecol. 10, 34–43 (2014).
Pettit, T., Faulkner, K. J., Buchkowski, R. W., Kamath, D. & Lindo, Z. Changes in peatland soil fauna biomass alter food web structure and function under warming and hydrological changes. Eur. J. Soil Biol. 117, 103509 (2023).
Kardol, P., Reynolds, W. N., Norby, R. J. & Classen, A. T. Climate change effects on soil microarthropod abundance and community structure. Appl. Soil Ecol. 47, 37–44 (2011).
Peng, Y. et al. Responses of soil fauna communities to the individual and combined effects of multiple global change factors. Ecol. Lett. 25, 1961–1973 (2022).
Blankinship, J. C., Niklaus, P. A. & Hungate, B. A. A meta-analysis of responses of soil biota to global change. Oecologia 165, 553–565 (2011).
Lindberg, N., Engtsson, J. B. & Persson, T. Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. J. Appl. Ecol. 39, 924–936 (2002).
Meehan, M. L. et al. Response of soil fauna to simulated global change factors depends on ambient climate conditions. Pedobiologia (Jena.). 83, 150672 (2020).
Aupic-Samain, A. et al. Water availability rather than temperature control soil fauna community structure and prey–predator interactions. Funct. Ecol. 35, 1550–1559 (2021).
Vestergård, M., Dyrnum, K., Michelsen, A., Damgaard, C. & Holmstrup, M. Long-term multifactorial climate change impacts on mesofaunal biomass and nitrogen content. Appl. Soil Ecol. 92, 54–63 (2015).
Xu, G.-L., Kuster, T. M., Günthardt-Goerg, M. S., Dobbertin, M. & Li, M.-H. Seasonal exposure to drought and air warming affects soil collembola and mites. PLoS One 7, 1–9 (2012).
Hao, F. et al. Microbial community succession and its environment driving factors during initial fermentation of maotai-flavor Baijiu. Front. Microbiol. 12, 669201 (2021).
Watzinger, A. et al. Functional redundant soil fauna and microbial groups and processes were fairly resistant to drought in an agroecosystem. Biol. Fertil. Soils 59, 629–641 (2023).
Singh, J., Schädler, M., Demetrio, W., Brown, G. G. & Eisenhauer, N. Climate change effects on earthworms - a review. Soil Org. 91, 114–138 (2019).
Makkonen, M. et al. Traits explain the responses of a sub-arctic Collembola community to climate manipulation. Soil Biol. Biochem. 43, 377–384 (2011).
Phillips, H. et al. Global change and their environmental stressors have a significant impact on soil biodiversity – a meta-analysis. Authorea Prepr. https://doi.org/10.22541/au.167655684.49855023/v1 (2023).
Briones, M. J. I., Ostle, N. J., McNamara, N. P. & Poskitt, J. Functional shifts of grassland soil communities in response to soil warming. Soil Biol. Biochem. 41, 315–322 (2009).
Zhang, G. et al. The response of soil nematode fauna to climate drying and warming in Stipa breviflora desert steppe in Inner Mongolia, China. J. Soils Sediment. 20, 2166–2180 (2020).
Singh, J., Cameron, E., Reitz, T., Schädler, M. & Eisenhauer, N. Grassland management effects on earthworm communities under ambient and future climatic conditions. Eur. J. Soil Sci. 72, 343–355 (2021).
Tsiafouli, M. A. et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Chang. Biol. 21, 973–985 (2015).
Vanolli, B. S. et al. Epigeic fauna (with emphasis on ant community) response to land-use change for sugarcane expansion in Brazil. Acta Oecologica 110, 103702 (2021).
Bufebo, B., Elias, E. & Getu, E. Abundance and diversity of soil invertebrate macro-fauna in different land uses at Shenkolla watershed, South Central Ethiopia. J. Basic Appl. Zool. 82, 11 (2021).
Franco, A. L. C. et al. Loss of soil (macro)fauna due to the expansion of Brazilian sugarcane acreage. Sci. Total Environ. 563–564, 160–168 (2016).
Minor, M. A. & Cianciolo, J. M. Diversity of soil mites (Acari: Oribatida, Mesostigmata) along a gradient of land use types in New York. Appl. Soil Ecol. 35, 140–153 (2007).
Ponge, J. F. et al. Collembolan communities as bioindicators of land use intensification. Soil Biol. Biochem. 35, 813–826 (2003).
Kooch, Y., Tavakoli, M. & Akbarinia, M. Tree species could have substantial consequences on topsoil fauna: a feedback of land degradation/restoration. Eur. J. Res. 137, 793–805 (2018).
Marsden, C., Martin-Chave, A., Cortet, J., Hedde, M. & Capowiez, Y. How agroforestry systems influence soil fauna and their functions - a review. Plant Soil 453, 29–44 (2020).
Fründ, H.-C. et al. Using earthworms as model organisms in the laboratory: recommendations for experimental implementations. Pedobiologia (Jena.). 53, 119–125 (2010).
Ganault, P. et al. Earthworms and plants can decrease soil greenhouse gas emissions by modulating soil moisture fluctuations and soil macroporosity in a mesocosm experiment. PLoS One 19, 1–23 (2024).
Crecelius, A. C., Michalzik, B., Potthast, K., Meyer, S. & Schubert, U. S. Tracing the fate and transport of secondary plant metabolites in a laboratory mesocosm experiment by employing mass spectrometric imaging. Anal. Bioanal. Chem. 409, 3807–3820 (2017).
Jílková, V. et al. Macrofauna amplify plant litter decomposition and stabilization in arctic soils in a warming climate. Soil Biol. Biochem. 188, 109245 (2024).
Just, C. et al. A Simple Approach to Isolate Slow and Fast Cycling Organic Carbon Fractions in Central European Soils—Importance of Dispersion Method. Front. Soil Sci. 1, 692583 (2021).
Lavallee, J. M., Soong, J. L. & Cotrufo, M. F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 26, 261–273 (2020).
Yin, R. et al. Climate change does not alter land-use effects on soil fauna communities. Appl. Soil Ecol. 140, 1–10 (2019).
Pollierer, M. M. et al. Compound-specific isotope analysis of amino acids as a new tool to uncover trophic chains in soil food webs. Ecol. Monogr. 89, e01384 (2019).
Glaser, B. Compound-specific stable-isotope (δ13C) analysis in soil science. J. Plant Nutr. Soil Sci. 168, 633–648 (2005).
Nakatsuka, H., Karasawa, T., Ohkura, T. & Wagai, R. Soil faunal effect on plant litter decomposition in mineral soil examined by two in-situ approaches: sequential density-size fractionation and micromorphology. Geoderma 357, 113910 (2020).
Chertov, O. G. Quantification of soil fauna metabolites and dead mass as humification sources in forest soils. Eurasia. Soil Sci. 49, 77–88 (2016).
Cheng, W. et al. Carbon budgeting in plant–soil mesocosms under elevated CO2: locally missing carbon? Glob. Chang. Biol. 6, 99–109 (2000).
Anthony, M. A., Bender, S. F. & van der Heijden, M. G. A. Enumerating soil biodiversity. Proc. Natl Acad. Sci. USA 120, e2304663120 (2023).
FAO, ITPS, GSBI, CBD & EC. State of knowledge of soil biodiversity - Status, challenges and potentialities. https://doi.org/10.4060/cb1928en (2020).
Acknowledgements
This research was funded by the Czech Science Foundation (GAČR, grant no. 24-10574S, to G.A.), the Czech Academy of Sciences (LQ200962401 Soil fauna – the neglected driver of soil carbon formation and stability, to G.A.), and the Deutsche Forschungsgemeinschaft (DFG—German Research Foundation, grant no. AN 1706/2-1, to G.A.; Ei 862/29-1 and Ei 862/31-1, to N.E.). A.P. acknowledges the support of the DFG (project no. 493345801). We gratefully acknowledge the support of iDiv, which is funded by the German Research Foundation (DFG—FZT 118, 202548816).
Author information
Authors and Affiliations
Contributions
G.A. conceived of the idea and designed the conceptual framework of the review together with A.P. G.A. performed the literature review and wrote the first manuscript draft. A.P., F.X.J., Š.A., J.F., P.G., and N.E. critically commented on the manuscript and added and/or revised content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Christina Kaiser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Angst, G., Potapov, A., Joly, FX. et al. Conceptualizing soil fauna effects on labile and stabilized soil organic matter. Nat Commun 15, 5005 (2024). https://doi.org/10.1038/s41467-024-49240-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-49240-x
This article is cited by
-
Soil biodiversity effects on ecosystems
Nature Reviews Biodiversity (2026)
-
Promotion of root Cd enrichment and inhibition of its translocation through integrated strategies
Plant and Soil (2026)
-
Microbial turnover mediates nonlinear response of soil N2O and CH4 fluxes to diversity loss
Soil Ecology Letters (2026)
-
Climate change enhances soil fauna population and biomass in grasslands of the Loess Plateau
Communications Earth & Environment (2025)
-
Un(der)explored links between plant diversity and particulate and mineral-associated organic matter in soil
Nature Communications (2025)