Abstract
In situ exploration of the dynamic structure evolution of catalysts plays a key role in revealing reaction mechanisms and designing efficient catalysts. In this work, PtCu/MgO catalysts, synthesized via the co-impregnation method, outperforms monometallic Pt/MgO and Cu/MgO. Utilizing quasi/in-situ characterization techniques, it is discovered that there is an obvious structural evolution over PtCu/MgO from PtxCuyOz oxide cluster to PtCu alloy with surface CuOx species under different redox and CO oxidation reaction conditions. The synergistic effect between PtCu alloy and CuOx species enables good CO oxidation activity through the regulation of CO adsorption and O2 dissociation. At low temperatures, CO oxidation is predominantly catalyzed by surface CuOx species via the Mars-van Krevelen mechanism, in which CuOx can provide abundant active oxygen species. As the reaction temperature increases, both surface CuOx species and PtCu alloy collaborate to activate gaseous oxygen, facilitating CO oxidation mainly through the Langmuir-Hinshelwood mechanism.
Similar content being viewed by others
Introduction
CO oxidation is a significant reaction in heterogeneous catalysis due to its practical applications in the purification of exhaust gases from motor vehicles, such as CO, NO, and hydrocarbons and as a typical model reaction for the fundamental catalysis study1,2,3. Supported platinum-based catalysts have attracted extensive attention recently due to their outstanding CO oxidation activity and stability at a relatively high temperature (>150 °C)4,5. However, the strong and oversaturated adsorption of CO on metallic Pt sites frequently inhibits CO oxidation at low temperatures because of CO poisoning and its poor ability to activate O26. With the increasingly stringent requirements of emission regulations, especially now the new standard for cold start emissions of vehicles, which requires catalysts to achieve CO oxidation conversion at low temperatures (<150 °C).
In the CO oxidation reaction, there are a lot of methods to improve the catalytic performance of Pt-based catalysts, such as constructing alloy components, building metal-support interaction7, metal-oxide8, and metal-metal hydroxide9 active sites. Among these methods, alloying Pt with another metal to form bimetallic alloy nanoparticles has been proven to be a promising pathway to improve the catalytic properties, such as Pt-Pd10, Pt-Rh11, Pt-Au12, Pt-Ag13, Pt-Fe14, Pt-Co15, Pt-Ni16, and Pt-Cu17,18,19,20 alloys. It is well known that elements with similar electronegativity are more likely to form alloys because of their easy share of electrons. Copper (Cu), as a representative transition metal, has received wide research due to its various valence states and rich surface oxygen species21,22. From the perspective of the alloy, Cu is a suitable element with a relatively narrow electronegativity gap and atomic radius difference with Pt atom, contributing to the substitution in the lattice and avoiding significant lattice distortion23. Furthermore, PtCu alloys are also revealed as promising catalysts in CO oxidation due to low cost, special geometric, electronic, and multifunctional effects. Komatsu et al.24 found that PtCu/SiO2 alloy catalysts exhibited better CO oxidation activity because of electron transferring from Pt to Cu to promote the activation of O2. Furthermore, Liu et al.25 exploited that metallic Pt and Cu+ in PtCu nanocage alloy could provide dual active sites for the adsorption of CO and O2. In addition, PtCu alloy samples were employed as high-performance catalysts in other oxidation reactions, such as methanol oxidation26, selective catalytic oxidation of ammonia27, and polyhydric alcohol oxidation28. Although some detailed and profound researches on PtCu alloy have been investigated, there are still some debatable and unsolved problems for PtCu alloy in CO oxidation. Firstly, can the PtCu alloy be maintained during the whole CO oxidation? Secondly, what is the precise dynamic structural evolution of the PtCu alloy in the oxidation atmosphere, such as the oxidation level and the distinct role of Cu and Pt species in consideration of the instability and oxygen sensitivity of metallic Cu species? Thirdly, the lack of advanced in-situ characterization methods to explore what is the dominant mechanism (MvK or LH) in CO oxidation reaction for PtCu alloy catalysts with various active sites (alloy and oxide species)? The above existed problems matter the exploration and determination of active site and the “structure-activity” relationship in CO oxidation.
For supported catalysts, the choice of support frequently makes an influence on the catalytic performance through the formation of interaction between metal and support to improve the dispersion or regulate the electronic structure of the active site. MgO nanosheet offers a combination of high surface area, thermal stability, and cost-effectiveness, making it a favorable catalyst carrier for CO oxidation reaction29,30. Thus, in this work, MgO nanosheet was chosen as a good carrier to prepare platinum copper bimetallic catalysts by co-impregnation method, which displayed good performance compared to monometallic Pt/MgO or Cu/MgO catalysts. The in-situ XAFS results unravel the dynamic structural evolution of active site from platinum-copper oxide cluster to PtCu alloy-CuOx interface undergoing reductive and oxidized conditions. In situ DRIFTS/CO-TPR and isotope labeling experiments indicated that CO oxidation can be motivated at ~50 °C on surface CuOx species through M-vK mechanism, in which CuOx can provide abundant active oxygen species. As the reaction temperature increases, a moderate CO adsorption on PtCu alloy guarantees enough sites for the activation of gas oxygen into active oxygen species to promote CO oxidation by L-H mechanism.
Results
Structural characterization of PtCu/MgO catalysts
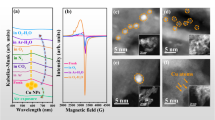
MgO-supported platinum-copper catalysts were prepared by a co-incipient wetness impregnation method, denoted as PtCu/MgO, in which Pt/MgO and Cu/MgO catalysts were synthesized by the same method as reference. According to previous reports, the ratio of metal in bimetallic catalysts plays a key role in the catalytic performance in various reactions31. Therefore, the ratio of Pt and Cu is optimized by setting the content of platinum (0.5 wt.%) and regulating the amount of copper to acquire the best CO oxidation activity (Supplementary Fig. S1). It was found that when the designed content for copper was 6 wt.%, the best catalytic activity can be achieved. The ICP-AES results indicated that the actual content of platinum and copper (Pt: 0.48 and 0.54 wt.% for Pt/MgO and PtCu/MgO, respectively; Cu: 6.0, and 5.3 wt.% for Cu/MgO and PtCu/MgO, respectively) was well consistent with the designed value (Supplementary Table S1). In order to detect the basic structure information, the XRD experiment (Fig. 1a) was carried out. A typical MgO crystal (JCPDS 75-1525) can be observed for all samples, in which the diffraction peaks at 36.9°, 42.9°, 62.3°, 74.7°, and 78.6° were attributed to the (111), (200), (220), (311), and (222) planes of MgO, respectively. Moreover, no obvious diffraction peaks of Pt/PtO/PtO2 and Cu/Cu2O/CuO were detected, presumably due to the highly dispersed platinum and copper species32. The morphology of the MgO support is nanosheets (Supplementary Fig. S2). In order to provide more microscopic data and clearly observe the precise distribution of Pt and Cu elements in PtCu/MgO, HAADF-STEM images and the related mapping were supplied in Fig. 1b–d. It can be seen that Pt and Cu elements were distributed uniformly at the cluster level within the same areas on the surface of MgO. For further confirmation about the elemental composition of clusters in PtCu/MgO, the corresponding line-profile analysis (Fig. 1d) manifest that Pt and Cu are homogeneously dispersed throughout the PtxCuyOz binary oxide clusters. In addition, because of the high loading of Cu species about 6 wt.%, a part of Cu species existed in isolation without forming interaction with Pt species according to the reduction peak at 209 °C (CuOx cluster) in H2-TPR profile of PtCu/MgO (Fig. 1e). Therefore, CuOx and PtxCuyOz clusters coexisted in PtCu/MgO. Because of the high dispersion and low contrast between Cu (Z = 29) and Mg (Z = 12), it is difficult to visually observe CuOx clusters over Cu/MgO in STEM image (Supplementary Fig. S3). The observed cluster in PtCu/MgO (Fig. 1b) can be attributed to PtxCuyOz binary oxide clusters with an average particle size of 1.4 ± 0.3 nm. More importantly, the H2-TPR results (Fig. 1e) also uncovered the strong interaction between platinum and copper in PtCu/MgO. A strong reduction peak at 187 °C assigned to Pt-[O]x-Cu structure was detected in PtCu/MgO33, which was different from the reduction peaks of PtOx (213 °C) and CuOx (209 °C) species. It indicated that the interaction between platinum and copper could significantly enhance the reducibility of catalysts. The XAFS spectra further provide more precise electronic and coordination information about copper and platinum. The Pt L3-edge XANES profiles in Fig. 1f showed that the white line intensity of Pt/MgO was higher than that of PtCu/MgO, in which the average oxidation state of Pt for Pt/MgO and PtCu/MgO was 4 and 3.8, respectively (Supplementary Table S2). According to the fitting results of EXAFS of PtCu/MgO in Fig. 1g and Supplementary Table S2, the strong Pt-O (R ≈ 1.98 Å and CN ≈ 5.5), Pt-O-Cu (R ≈ 3.10 Å and CN ≈ 2.8), and Pt-O-O shells were acquired, further evidencing the formation of PtxCuyOz component. The absence of Pt-O-Pt shells suggests that there is no isolated PtOx cluster in PtCu/MgO. In addition, the coordination structure of Cu species was also investigated. In Fig. 1h, the Cu K-edge XANES spectra of Cu/MgO and PtCu/MgO are very similar in both peak position and line shape. The average oxidation state of Cu/MgO and PtCu/MgO were both +2. For PtCu/MgO, it is a challenge to fit the Cu-O-Pt coordination shell at Cu K edge, because XAFS is a bulk technique sensitive to all of the forms of an element in a sample. In practical, XAFS experiment, millions of x-rays are absorbed by millions of atoms, and the average information of one element was collected together, such as Cu-O-Cu (CuOx) and Cu-O-Pt (PtxCuyOz) in this system. In this work, the isolated CuOx species is much more abundant than PtxCuyOz clusters, which results in the dominated CuO coordination structure (Fig. 1i). A main Cu-O shell (R ≈ 1.97 Å, CN ≈ 2.0 and R ≈ 1.96 Å, CN ≈ 2.0) plus Cu-O-Mg shell (R ≈ 2.95 Å, CN ≈ 4.7 and R ≈ 2.93 Å, CN ≈ 5.4) can be fitted for both Cu/MgO and PtCu/MgO samples (Supplementary Table S3). A minor Cu-O-Cu shell (R ≈ 2.84 Å, CN ≈ 0.5 and R ≈ 2.81 Å, CN ≈ 0.6) can be fitted for Cu/MgO and PtCu/MgO, which indicates that there are very small clusters of copper oxide on Cu/MgO and PtCu/MgO. Therefore, a combination of TEM, H2-TPR, and XAFS results, it evidenced the formation of PtxCuyOz binary oxide cluster (Fig. 1j) in PtCu/MgO possessing different reducibility and structure from PtOx and CuOx clusters.
a XRD pattern, (b) HAADF-STEM images of PtCu/MgO, (c) EDS mapping results of PtCu/MgO, (d) line-scanning results of PtCu/MgO, (e) H2-TPR profiles, (f) Pt L3-edge XANES profiles, (g) Pt L3-edge EXAFS fitting results (the data are k2-weighted and not phase-corrected), (h) Cu K-edge XANES profiles, (i) Cu K-edge EXAFS fitting results in R space (the data are k3-weighted and not phase-corrected), (j) Schematic demonstration of platinum-copper oxide cluster.
Catalytic performance of PtCu/MgO catalysts in CO oxidation
CO oxidation has been widely used as a model reaction for the fundamental study of reaction mechanisms and the surface properties of catalysts. According to previous reports, the pretreatment conditions have a profound effect on the catalytic performance via regulating the oxidation state or the local coordination structure of active site in various bimetallic catalysis systems31. In this work, when the catalysts were pretreated under O2 atmosphere, all samples exhibited bad CO oxidation activity with a complete conversion temperature of CO oxidation >200 °C (Fig. 2a), presumably due to the poor adsorption of CO molecules on active site, according to the only existence of gas CO bands in in-situ DRIFTS results (Supplementary Fig. S4). However, there is a dramatic improvement in activity for all samples with hydrogen reduction at 500 °C. PtCu/MgO exhibited good CO oxidation activity with 100% CO conversion at 130 °C, even can motivate this reaction at ~50 °C (Fig. 2b). For monometallic Pt and Cu samples, the CO complete conversion can only be achieved at 154 °C and 248 °C with few activity below 100 °C. It demonstrated that the hydrogen reduction may not only reduce the oxidation state of platinum and copper species, but also regulate the coordination structure of active site. In order to verify our assumption, the related kinetic data was acquired (Fig. 2c). The apparent activation energy (Ea) of the PtCu/MgO catalyst is around 42 kJ⋅mol−1, which is much lower than that of Pt/MgO (82 kJ⋅mol−1), Cu/MgO (90 kJ⋅mol−1) and other reported Ea values in Supplementary Table S4 (from 45 to 98 kJ⋅mol−1) of Pt-based34,35,36 or Cu-based catalysts22,37, indicating the difference in active structure or reaction pathway. Besides, a long-term catalytic evaluation at 150 °C for PtCu/MgO under a simulative vehicle exhaust condition (GHSV:190,000 ml h−1 gcat−1) showed 94% conversion without any deactivation within 20 h (Fig. 2d). Meanwhile, pure Pt catalyst also showed good stability at 150 °C with a poor conversion efficiency of about 20% under the same test conditions. In addition, PtCu/MgO also exhibited good catalytic stability under water vapor condition (Supplementary Fig. S5). Moreover, PtCu/MgO showed better catalytic activity compared to other platinum-based catalyst in Supplementary Table S5.
Structural evolution of PtCu/MgO after hydrogen reduction
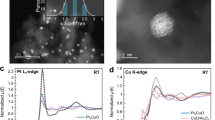
According to our previous research, the enhancement of hydrogen reduction on catalytic activity is frequently accompanied by the evolution of active structure31. Therefore, in order to acquire more precise structural information under reduction conditions, the catalysts were deeply characterized. After hydrogen reduction, the size of the active site in PtCu/MgO was maintained at ~1.8 nm (Fig. 3a), in which the particle of PtCu/MgO in Fig. 3b can be assigned to PtCu alloy (CuPt JCPDS 48-1549) with a clear lattice spacing of 0.22 nm for the (111) plane. Meanwhile, the related EDS mapping results also evidenced the space consistency of Pt and Cu element distribution (Fig. 3c). Zhang et al. also prepared ultrasmall Pt-Cu alloy clusters on the surface of TiO2 NBs after hydrogen reduction at 400 °C for 2 h38. In order to further confirm the formation of PtCu alloy, quasi-situ synchrotron XRD and in-situ XAFS were conducted. The XRD pattern for PtCu/MgO after hydrogen reduction exhibited two additional peaks positioned at 40.1°, 50.4° (Fig. 3e), which match with the (111) planes of PtCu alloy and the (200) planes of metallic Cu, respectively. The in-situ XANES in Supplementary Fig. S6, and Fig. S7 show that the average oxidation states of Cu and Pt were both 0 valence in PtCu/MgO after hydrogen reduction. The in-situ EXAFS of Pt L3-edge for PtCu/MgO(H2) exhibits one prominent peak at ~2.60 Å (Fig. 3f) with a coordination number of ~11.8 (Supplementary Table S6), which is significantly different from the Pt foil, and is in accordance with the standard path of Pt-Cu from CuPt (mp-644311 in The Materials Project)39. The in-situ EXAFS of Cu K-edge for PtCu/MgO(H2) in Fig. 3g exhibits one prominent peak of Cu-Cu shell at ~2.59 Å (CN ≈ 6.3) (Supplementary Table S7 and Supplementary Fig. S15), which indicates that copper oxide clusters were reduced into metallic Cu particles after hydrogen pretreatment. To further strengthen the formation of PtCu alloy, wavelet transforms (WT) analysis of Pt EXAFS oscillations was conducted. The WT contour plots of Pt foil (Fig. 3h) and PtO2 (Supplementary Fig. S8) demonstrate that the intensity maxima at ~10 Å−1 and ~5 Å−1 are attributed to the Pt-Pt and Pt-O contributions, respectively. However, for the WT contour plot of PtCu/MgO (H2) (Fig. 3i), just one intensity maximum at about 7 Å−1 is shown, which is attributed to the Pt-Cu contribution40. Therefore, the results of HAADF-STEM, quasi in-situ synchrotron XRD and in situ XAFS characterizations verify the formation of PtCu alloy in PtCu/MgO after H2 reduction (Fig. 3d).
a HAADF-STEM images, (b) HRTEM, (c) EDS mapping results of PtCu/MgO after hydrogen pretreatment, (d) Schematic illustration of PtCu Alloy, (e) synchrotron XRD graph, (f) in-situ Pt L3-edge EXAFS profiles (the data are k2-weighted and not phase-corrected), (g) in-situ Cu K-edge EXAFS profiles (the data are k3-weighted and not phase-corrected), and (h, i) WT-EXAFS contour plot of Pt L3-edge signals for Pt foil and the PtCu/MgO after hydrogen reduction.
Structural evolution during CO oxidation
Due to the oxidation atmosphere during CO oxidation reaction, the coordination structure of the active site, especially the oxidation state, may undergo further evolution. After CO oxidation, the aberration-corrected HAADF-STEM images showed that the average particle sizes of the active site for PtCu/MgO (used) (Fig. 4a), Pt/MgO (used) (Supplementary Fig. S9) were 1.9 ± 0.6 nm, 1.7 ± 0.4 nm, respectively. The high-resolution TEM (HRTEM) images of PtCu/MgO used (Supplementary Fig. S9) display a typical lattice fringe of Pt-Cu alloy with d-spacing of 0.22 nm from (111) plane. The corresponding inverse Fourier transfer (IFFT) pattern and the line intensity profile also show an interplanar space of 0.22 nm. Meanwhile, the line scan results of PtCu alloy particles in Fig. 4b obviously evidenced the element distribution of Pt and Cu in PtCu alloy. It demonstrated that PtCu alloy structure is stable during the whole CO oxidation reaction. The quasi in-situ synchrotron XRD pattern of PtCu/MgO(used) in Fig. 4c also shows the diffraction signal at 40.1° from (111) crystal plane of PtCu alloy, confirming the existence of PtCu alloy in PtCu/MgO after CO oxidation reaction. For MgO-supported Pt sample (Supplementary Fig. S9), the main active site is metallic platinum with 0.23 nm for Pt (111) plane. Furthermore, the structure-sensitive in-situ XAFS technique was employed to determine the precise structural evolution of active site for PtCu/MgO in Fig. 4d–i. During the CO oxidation reaction at 90 °C, the Pt-Cu shell remained stable and without obvious the formation of Pt-O shell (Fig. 4e and Supplementary Table S6). Figure 4d and Supplementary Fig. S7a show that the white line peak gradually increased with the increase in reaction temperature, indicating the slight oxidation of platinum species during CO oxidation reaction with +0.8 and +1.8 state for Pt species at 150 °C and 270 °C respectively (Fig. 4f and Supplementary Fig. S7b). Meanwhile, the XPS results in Supplementary Fig. S10 also evidenced that the platinum species underwent oxidation after CO oxidation. However, the main PtCu alloy was still stabilized during CO oxidation reaction at <150 °C (Fig. 4e and Supplementary Table S6). For Pt/MgO catalysts after CO oxidation reaction, a major metallic Pt-Pt shell (R ≈ 2.77 Å, CN ≈ 6.0) plus Pt-O shell (R ≈ 2.02 Å, CN ≈ 1.7) can be fitted (Supplementary Fig S11 and Table S2), indicating the existence of PtOx species was not the dominant factor in promoted CO oxidation activity. For Cu species in PtCu/MgO, it can be reduced to metallic Cu species with zero valence after hydrogen reduction at 500 °C. When it switched to CO oxidation condition at 90 °C, Cu-O and Cu-O-Cu shells began to appear, indicating the formation of CuOx species (Fig. 4h). During the CO oxidation reaction at 150 °C, the metallic Cu-Cu shell completely disappeared with an oxidation state of +0.4, indicating that the reoxidation and redispersion of isolated Cu species and Cu species on the surface of PtCu alloy. For comparison, the XAFS data for Cu/MgO were also acquired in Supplementary Fig. S12. The isolated CuOx species can be reduced to metallic Cu component after hydrogen reduction and remained at metallic state (Cu0) during the CO oxidation at 90 °C. It indicated that in PtCu/MgO sample after hydrogen activation, when the reaction gas was switched into the reactor, the Cu atoms in PtCu alloy could rapidly disassociate O2 gas into oxygen species, together with the formation of CuOx species on the surface of PtCu alloy. Furthermore, the metallic Cu particles can be seen in Cu/MgO (used) sample by the aberration-corrected HAADF-STEM (Supplementary Fig. S9), and there is still small Cu-Cu shell (CN ≈ 0.7) can be fitted in Cu/MgO (used) (Supplementary Fig. S13 and Table S3), indicating that the complete dispersion of the isolated metallic Cu particles into small CuOx clusters requires a higher temperature in Cu/MgO. Tomita et al. also reported that the formation of the interface between metallic Pt nanoparticles and FeOx promoted the oxidation of CO at low temperatures14. Furthermore, the aberration-corrected HAADF-STEM images of PtCu/MgO could also display some clusters at subnanometer on the surface of PtCu alloy, which may be assigned to CuOx cluster on the basis of element contrast (Supplementary Fig. S9c). Therefore, it can be concluded that the main active site for PtCu/MgO during CO oxidation is PtCu alloy with surface CuOx species (Fig. 4j). In addition, we found that there is a slight deactivation after multiple cycles of CO oxidation (Supplementary Fig. S14), which may be due to the degradation of PtCu alloy under long time operation in oxidation atmosphere. However, the activity can be recovered to initial state by H2 reduction.
a aberration-corrected HAADF-STEM images of PtCu/MgO after catalytic CO oxidation, (b) the line scan results of PtCu/MgO after catalytic CO oxidation, (c) synchrotron XRD pattern, (d) in-situ Pt L3-edge XANES profiles, (e) in-situ Pt L3-edge EXAFS profiles (the data are k2-weighted and not phase-corrected), (f) the average oxidation state of Pt in PtCu/MgO catalysts from XANES spectra, (g) in-situ Cu K-edge XANES profiles, (h) in-situ Cu K-edge EXAFS profiles (the data are k3-weighted and not phase-corrected), (i) the average oxidation state of Cu in PtCu/MgO catalysts from XANES spectra, and (j) Schematic illustration of CuOx /PtCu Alloy.
The reducibility and active oxygen for PtCu/MgO catalysts
We also carried out the in-situ H2-TPR experiments (Fig. 5a) for the samples after CO oxidation without air exposure to detect the reducibility of catalysts. No visual peak can be found for used Pt/MgO due to the vast majority of platinum species is reduced to Pt0 during H2 pretreatment and the oxidation state of platinum is insensitive to oxidative atmosphere. However, for Cu/MgO and PtCu/MgO, there is an obvious reduction peak at 170 or 155 °C, which was attributed to the reduction of well dispersed copper oxide species. It indicated that there were abundant CuOx species on PtCu alloy, which is more reducible than the conventional CuOx species in Cu/MgO. Meanwhile, the XANES at Cu K edge (Supplementary Fig. S6) and XPS results (Fig. 5b) uncovered that the average oxidation state of Cu on PtCu/MgO catalyst surface was +2 after CO oxidation. Moreover, the in-situ CO-TPR results in Fig. 5c also uncovered the difference in surface active oxygen species between PtCu/MgO and Cu/MgO. The surface oxygen in PtCu/MgO can react with CO molecules at ~50 °C, well consistent with the CO oxidation “light off” temperature (Fig. 2b). The CuOx species on the surface of PtCu alloy may motivate initial CO oxidation (~50 °C) through Mars-van Krevelen (M-vK) mechanism31, promoting the CO oxidation activity. Nan et al. also found super active oxygen species in Pt-based catalysts to exhibit excellent CO oxidation activity31. However, the formation of CO2 was only detected over Pt/MgO and Cu/MgO at much higher temperatures (>150 °C). It indicated that the isolated CuOx species make little contribution to CO oxidation activity at low temperatures. Moreover, the latter peak in the range 230–350 °C is attributed to the water-gas shift reaction of surface hydroxyl groups bounded to MgO. In addition, the EPR results in Fig. 5d exhibited symmetric signals at g of about 2.003, attributed to electrons trapped on the Ov41. It was found that PtCu/MgO possessed the most abundant oxygen defects possibly stemming from the CuOx species on the surface of PtCu alloy.
The ability of oxygen activation
O2-TPD experiment was performed to characterize the active oxygen species on the catalyst surface. According to O2-TPD results (Supplementary Fig. S16), a distinct desorption peak at the range of 100–200 °C can be ascribed to the surface-adsorbed oxygen species. Surface-adsorbed oxygen species desorbed at low temperatures are known as the active species for catalytic oxidation. The desorption temperature for PtCu/MgO is lower than that of Pt/MgO and Cu/MgO, indicating that the adsorbed oxygen is easier to be activated in PtCu/MgO than in Pt/MgO and Cu/MgO. In addition, we designed an O2 pulse experiment to test the ability of the oxygen decomposition of each catalyst in Fig. 6. At 70 °C, PtCu/MgO can activate O2 to generate CO2, while no obvious CO2 signal can be seen for Pt/MgO and Cu/MgO due to their poor ability to dissociate oxygen gas. As the experiment temperature increased to 130 °C, the integral area of CO2 signal for PtCu/MgO catalyst was larger than that for Pt/MgO and Cu/MgO, indicating a better decomposition capacity of O2 for PtCu/MgO. In addition, it was found that there was a CO2 shoulder peak in PtCu/MgO, which was not consistent with the variation tendency of oxygen gas. It can be attributed to the consumption of oxygen species in CuOx species with good recyclability on the surface of PtCu alloy. For Pt/MgO and Cu/MgO, the generated CO2 signal of Pt/MgO was much better than that of Cu/MgO, well consistent with the trend of apparent activation energy. When the O2 pulse experiment is conducted at 170 °C, there is also additional CO2 generation for Cu/MgO. However, the peak area of Cu/MgO was smaller than that of PtCu/MgO, indicating that CuOx on the surface of PtCu alloy has better oxygen activation ability than isolated Cu species.
CO adsorption on PtCu/MgO catalysts
The characterization results from synchrotron XRD, STEM, in situ-XAFS, and TPR revealed the exact structure of active sites for Pt/MgO (metallic Pt), Cu/MgO (CuOx), and PtCu/MgO (PtCu alloy with surface CuOx). Furthermore, the adsorption behaviors of reaction molecule also play a key role in catalytic performance. Thus, an in-situ DRIFTS experiment was carried out to investigate the specific adsorption and desorption patterns. As shown in Fig. 7a, the CO adsorption bands at 2174 and 2115 cm−1 were attributed to gaseous CO31,42. In addition, the peaks in Pt/MgO (Supplementary Fig. S17) at 2064 and 2082 cm−1 were attributed to linear CO adsorbed on Pt0, Ptδ+ sites, respectively43,44. The CO adsorption peaks in Cu/MgO (Supplementary Fig. S17) at 2086 cm−1, 2103 cm−1, and 2134 cm−1 were attributed to linear CO adsorbed on Cu0, Cu+, and Cu2+ sites, respectively2,45. According to the in-situ EXAFS fitting results, PtCu alloy with surface CuOx species is the main active site for PtCu/MgO. Meanwhile, the CO adsorption bands at 2086, 2106, 2134 cm−1 (CO-Cu0, CO-Cu+1 and CO-Cu2+) and 2064 cm−1 (CO-Pt0) were clearly observed in the spectra of PtCu/MgO, confirming the reliability of structural characterization. During CO adsorption process for PtCu/MgO, CO molecules adsorbed at Pt0 site (2064 cm−1) quickly reached saturation at 120 s, the CO adsorption on the surface CuOx cluster (2106 cm−1) also quickly reached saturation at 120 s and the peak intensity is the highest, indicating the rich CuOx cluster on the surface of PtCu alloy. In addition, there was a tiny band at 2013 cm−1, which was attributed to the CO adsorption on PtCu alloy15,46. Later, when 2 vol.% O2/N2 was introduced into the cell, it can be seen that the bands at 2106 and 2134 cm−1 (CO-Cu1+ and CO-Cu2+) rapidly decreased and the peaks at 2064 cm−1 remained at the beginning stage with gradual diminution after 200 s. It indicated that the CuOx species on the surface of PtCu alloy was good at the dissociation of O2 into reactive O radicals to convert CO to CO2. However, the strong adsorption on Pt0 species resulted in not enough sites to efficiently activate O2 gas and motivate further CO oxidation. In Fig. 2b, the light off profile of PtCu/MgO also exhibited the good CO oxidation activity at low temperatures (<100 °C) for two reasons: the abundant active oxygen species in CuOx and the better ability to dissociate O2 molecules. Therefore, in order to detect the role of PtCu alloy in CO oxidation activity, we conducted further DRIFTS experiment at relatively high temperatures (200 °C) in Fig. 7c. It can be seen that there was a visible variation for the CO adsorption behavior of PtCu/MgO. The adsorption peak strength of CO on Cu+ (2016 cm−1) and Pt0 (2064 cm−1) decreased, while the adsorption peak strength of CO on PtCu alloy (2013 cm−1) significantly increased, indicating that CO molecules prefer to adsorb on PtCu alloy rather than CuOx species and Pt0 sites at relatively high temperatures. More importantly, the CO adsorbed at the Pt sites saturated quickly (40 s), while the CO adsorbed slowly on the PtCu alloy with unsaturation until 1600 s. Thus, the slow adsorption of CO at the PtCu alloy site provides enough sites for the activation of gas oxygen into active oxygen species. When the gas O2 was introduced into PtCu/MgO system (Fig. 7d), the peaks at 2106 and 2013 cm−1 simultaneously and rapidly vanished at 160 s, which indicates that the high temperature can provide sufficient energy for both CuOx and PtCu alloy to activate the gas oxygen to participate in CO oxidation.
a–d In-situ DRIFTS spectra for used PtCu/MgO catalysts tested at 100 °C and 200 °C (The catalysts were pretreated at 500 °C for 30 min under H2 flow and underwent CO oxidation reaction from 30 to 300 °C in the DRIFTS reaction cell before data collection) and (e, f) 18O2 isotope-labeling experiments.
Reaction mechanism of PtCu/MgO catalysts
According to in-situ DRIFTS results, CuOx species and PtCu alloy play a different role in CO oxidation activity at different temperature ranges. The CO oxidation can be motivated by CuOx species because of their abundant active oxygen species at low temperatures (<100 °C). As the reaction temperature increased, PtCu alloy efficiently adsorbed CO molecules and dissociated gas O2 to promote the conversion of CO. For the CO oxidation reaction, two possible mechanisms, e.g., the LH and M-vK mechanisms, have been proposed. According to the 18O2 isotope-labeling results over PtCu/MgO (Fig. 7e, f), the M-vK and LH reaction mechanisms played a predominant role in different temperature ranges, respectively. It can be seen that when the reaction temperature is 70 °C, surface CuOx species could provide active oxygen species to react with CO molecules and generate C16O2. Meanwhile, the Ov in CuOx could dissociate gaseous 18O2 to produce active O18 radicals and promote the further generation of C16O18O. On the basis of MS signal of CO2, the M-vK mechanism occupied a dominate position (∼63%) in CO oxidation at 70 °C. When the reaction temperature is 150 °C, with the increment of CO adsorption at the PtCu alloy site and the easier dissociation of gaseous O2 at this site, the catalyst can quickly convert CO molecules and 18O2 into C16O18O through the LH mechanism. At this temperature, the CO oxidation was mainly promoted by LH mechanism (∼60%) in Fig. 7f. In addition, the related Ea of PtCu/MgO at low temperatures (38 kJ⋅mol−1) is also slightly lower than that (42 kJ mol−1) at high temperatures (Supplementary Fig. S18) because of the different roles of CuOx species and PtCu alloy in CO oxidation. Thus, it can be seen that the CO molecules prefer to adsorb on surface CuOx species to drive the occurrence of CO oxidation with the affluent and active oxygen species provided by CuOx component at low temperatures. As the reaction temperature increases, it can supply enough energy to meet the energy requirement of PtCu alloy to participate in CO oxidation, plus the strong and slow CO adsorption and the efficient activation of O2 over PtCu alloy (Fig. 8b).
a Schematic illustration of structural evolution for the PtCu/MgO catalyst during H2 pretreatment and CO oxidation reaction, (b) Graphical representation of proposed reaction mechanisms for CO oxidation over Pt at low temperatures, Pt at higher temperature, CuOx/PtCu alloy at low temperatures, and CuOx/PtCu alloy at higher temperature.
Discussion
To sum up, MgO-supported Pt-Cu bimetallic catalysts were prepared by the co-impregnation method, and displayed good activity compared to the related monometallic Pt/MgO or Cu/MgO catalysts. The apparent activation energy (Ea) of the PtCu/MgO catalyst is around 42 kJ⋅mol−1, which is lower than that of Pt/MgO (82 kJ mol−1) and Cu/MgO (90 kJ⋅mol−1). Combined with various characterization methods including in-situ XAFS, quasi in-situ synchrotron XRD, and STEM, we reveal an obvious structural evolution over PtCu/MgO catalysts (Fig. 8a), in which from PtxCuyOz binary oxide cluster (air calcination) to PtCu alloy (H2 activation) and PtCu alloy with surface CuOx species (main active site in CO oxidation). The simultaneous existence of the PtCu alloy and CuOx species enables a synergy for catalyzing CO oxidation. It is discovered that PtCu alloy can activate O2 gas to form surface CuOx species and provide oxygen species to motivate CO oxidation by M-vK mechanism at low temperatures. At high temperatures, both CuOx and PtCu alloy work together to activate gas oxygen to participate in CO oxidation. Around 60% of the acquired CO2 is produced by O atoms from the introduced O2 gas adsorbed on PtCu alloy and CuOx (L-H mechanism).
Methods
Catalyst preparation
All of the chemicals were obtained from Sinopharm Chemical Reagent Co. Ltd. and used without additional purification. MgO is synthesized by thermal decomposition. Firstly, 0.04 mol Mg(CH3COO)2·4H2O and 0.06 mol H2C2O4·2H2O were dissolved in 50 ml and 200 ml Millipore (>18 MΩ) water, respectively. Under constant agitation, the dissolved magnesium acetate solution was dropped into oxalic acid solution. At this time, precipitation was not immediately generated. After 12 h aging at room temperature, the final pH value of the solution was 2. Then, the precipitates were washed by Millipore (>18 MΩ) water for three times. The MgO was obtained by drying the as-washed product under a vacuum at 80 °C for 12 h and further calcined in air at 600 °C for 10 h (heating rate: 2 °C min−1). Deposition of platinum, and copper onto the MgO support was carried out by a co-incipient wetness impregnation. Firstly, a solution of Cu (NO3)2·3H2O (0.24 g) and Pt (NH3)4(NO3)2 (0.01 g) in Millipore (>18 MΩ) water (5 ml) was added dropwise onto MgO power (1 g) under manually stirring. Subsequently, the sample was dried at 80 °C for 12 h, and calcined at 400 °C for 4 h (ramping rate: 2 °C min−1).
Catalytic activity tests
The CO oxidation activities of MgO-supported platinum-copper catalysts were conducted in a fixed-bed flow reactor. 30 mg of catalysts were first pretreated under 5 vol.% H2/He (30 mL·min−1) at 500 °C for 30 min. After cooling down to room temperature under H2 atmosphere, the atmosphere was switched to 1 vol.% CO/20 vol.% O2/He (60 mL·min−1). Afterward, the temperature was ramped to 300 °C at 5 °C min−1. Nondispersive IR spectroscopy (Gasboard-3000, Hubei Ruiyi Company, China) was used to continuously monitor the compositions of CO and CO2 in the output gases. The conversion of CO was evaluated as follows: COconv. (%) = (COin−COout)/COin × 100%.
Materials characterization
Inductively coupled plasma optical emission spectroscopy (ICP‐OES, Optima 8000) was used to determine the metal content for all sample. The powder X-ray diffraction (XRD) patterns were collected on a Bruker D8 Advance diffractometer (40 kV and 40 mA) using the Cu Ka radiation (k = 1.5418 Å) from 10 to 90°. N2 adsorption/desorption isotherms were measured on an ASAP2020-HD88 analyzer (Micromeritics Co., Ltd.) at 77 K. The samples were degassed at 250 °C for 4 h. The BET specific surface areas (SBET) were calculated using data between 0.05 and 0.20 relative pressure. The transmission electron microscopy (TEM) and scanning transmission electron microscopy-energy dispersive spectrometer (STEM-EDS) elemental mapping results were obtained from a FEI Tecnai G2 F20 S-TWIN microscope and transmission electron microscopy of cold field emission (JEOL JEM-F200) operating at 200 kV. The scanning electron microscope (SEM) images were taken on a field emission scanning electron microscope (Zeiss, G300). The aberration-corrected HAADF-STEM images and EDS mappings were obtained using a probe aberration-corrected Hitachi HF5000 equipped with a secondary detector, operated at 200 kV. X-ray photoelectron spectroscopy (XPS) was performed by PHI 5000 VersaProbe III with a monochromatic Al Kα X-ray source. Rectify the binding energy for all the spectra using the C 1 s peak at 284.8 eV as an internal standard. The quasi in-situ synchrotron XRD pattern were recorded at BL17B of the Shanghai Synchrotron Radiation Facility (SSRF) at a wavelength of 0.68883 Å. The catalysts were pretreated in a stainless reactor with two air valves on each side. After the sample is cooled to room temperature, the stainless reaction tube with catalyst is transferred to a glove box under N2 atmosphere to prepare sample for the test. Pilatus3 S-2M detector was used to record the diffraction Pattern. LaB6 standard was used for wavelength calibration. The 2θ Angle was converted to the corresponding value of the Cu Ka radiation (k = 1.5418 Å) by Scherrer formula.
In-situ/ex-situ XAFS experiments
The X-ray absorption fine structure (XAFS) spectroscopy at the Pt L3 (E0 = 11564 eV) edge and Cu K (E0 = 8979 eV) edge were operated at the BL14W and 16U1 beamline of Shanghai Synchrotron Radiation Facility (SSRF) which operates at 3.5 GeV with a current of 200 mA. To investigate chemical coordination environment of Pt species and Cu species for PtCu/MgO catalysts after H2 activation, during, and after the CO oxidation reaction, the in-situ XAFS experiment were carried out. The 50 mg of catalyst was placed on a sample holder in the center of a high-temperature high-pressure gas-solid catalytic in-situ device. Firstly, the sample was pretreated with H2 at 500 °C for 30 min, followed by cooling to 30 °C under H2. Then, the XAFS spectra of Pt L3 edge and Cu K edge were collected under H2 atmosphere. Subsequently, 1vol.%CO/20%O2/79%He was introduced into the in‑situ device, when the reaction temperature reached 90 °C, 150 °C, 210 °C, and 270 °C, the XAFS spectra were collected. For the quasi in-situ XAFS experiment, firstly, the catalysts were pretreated in a stainless reactor with two air valves on each side under H2 flows at 500 °C for 30 min. After the sample is cooled to room temperature, the stainless reaction tube with catalyst is transferred to a glove box under N2 atmosphere to prepare sample for XAFS test. The XAFS spectra of Pt L3 edge and Cu K edge were collected in fluorescence mode using an Ar-filled Lytle detector. The X-ray energy was calibrated with the absorption edge of Pt foil and Cu foil. The data were analyzed using the Demeter software package (including Athena and Artemis software)47. Athena was used for data normalization data preprocessing and Artemis was used for EXAFS fitting.
In-situ DRIFTS
In situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS) were continuously collected using a Thermo Nicolet iS10 spectrometer with a mercury-cadmium-telluride (MCT) detector cooled with liquid nitrogen. The 6 mg of catalyst was placed on a sample holder in the center of diffuse reflectance cell (Harrick system). Prior to the testing, the sample was pretreated with H2 at 500 °C for 30 min, followed by cooling to 30 °C under purging with He gas. Then, the sample is heated and kept at 100 °C. The background spectra were acquired under He purging. Subsequently, 5 vol.% CO/He (20 mL min−1) was introduced into the in‑situ cell for CO adsorption for 30 min. This was followed by He gas purging and CO re-adsorption for 30 min. Afterward, 5 vol.% O2/He was introduced.
Reducible property and surface oxygen
Hydrogen temperature program reduction (H2-TPR) experiments were performed on Micromeritics Autochem II 2920 instrument equipped with a thermal conductivity detector TCD detector. 50 mg of the catalysts was placed in a U-type quartz tube. The catalysts were pretreated at 300 °C for 30 min under O2 (5 vol.%O2/He) flow before the analysis, and then cooled to room temperature. Subsequently, 5 vol.% H2/Ar was introduced for analysis. After stabilizing the TCD signal in H2 flow (50 mL min−1) at room temperature, the reduction temperature was raised from room temperature to 700 °C at rate of 10 °C min−1.
CO‑temperature programmed reduction (CO‑TPR) experiment was conducted in a fixed-bed flow reactor connected to online mass spectrometry (LC‑D200M, TILON). 50 mg of catalysts were first pretreated under 5 vol.% H2/He (30 mL min−1) at 500 °C for 30 min. Afte the sample was purged by He and then cooled down to room temperature, the atmosphere was switched to 2.2 vol.% CO/He (60 mL min−1). Afterward, the temperature was ramped to 400 °C at 10 °C min−1. During the heating process, the signals of H2 (m/z = 2), He (m/z = 4) and CO2 (m/z = 44) were recorded.
Electron paramagnetic resonance (EPR) measurements were performed at the X-band using a Bruker Emxplus spectrometer. The center field was 3505.00 G, the microwave frequency was 9.843 GHz, the microwave power was 3.170 mW.
O2 pulse experiment
O2 pulse experiments were performed in a fixed-bed flow reactor connected to online mass spectrometry (LC‑D200M, TILON). The catalysts were firstly treated under 5%H2/He atmosphere at 500 °C for 30 min. Afterward, the catalyst is then cooled to room temperature in H2 atmosphere. Then the sample was heated from room temperature to 70 °C in He atmosphere, 2%CO/He (30 mL min−1) was then introduced to purging until the baseline is stable. Subsequently, O2 (20 vol.% O2/He) pulses of 7.5 mL were injected. The signals of CO2 (m/z = 44), O2 (m/z = 32) were detected. Because of the total reduction of Pt and Cu after H2 activation, the production of CO2 is from the decomposition of oxygen gas.
18O2 isotope-labeling experiments
18O2 isotope-labeling experiments were performed in a fixed-bed flow reactor connected to online mass spectrometry (LC‑D200M, TILON). 30 mg of catalysts were first activated in 5 vol.% H2/He (30 mL min−1) at 500 °C for 30 min. Afterward, the catalyst is then cooled to room temperature in N2 atmosphere. Then the sample was heated from room temperature to 70 °C, 18O2 was then introduced to purging until the baseline is stable. Subsequently, CO (2.2 vol.% CO/He) pulses of 15 cm3 were injected at 100 s intervals. The signals of C16O2 (m/z = 44), C16O18O (m/z = 46) were detected.
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information. The data generated in this study are provided in the Source Data file. Source data are provided with this paper.
References
Chen, J. et al. Reverse oxygen spillover triggered by CO adsorption on Sn-doped Pt/TiO2 for low-temperature CO oxidation. Nat. Commun. 14, 3477 (2023).
May, Y. A., Wang, W.-W., Yan, H., Wei, S. & Jia, C.-J. Insights into facet-dependent reactivity of CuO-CeO2 nanocubes and nanorods as catalysts for CO oxidation reaction. Chin. J. Catal. 41, 1017–1027 (2020).
Abdel-Mageed, A. M. et al. Unveiling the CO oxidation mechanism over a molecularly defined copper single-atom catalyst supported on a metal-organic framework. Angew. Chem. Int. Ed. Engl. 62, e202301920 (2023).
Xie, S. et al. Pt atomic single-layer catalyst embedded in defect-enriched ceria for efficient CO oxidation. J. Am. Chem. Soc. 144, 21255–21266 (2022).
Zhou, Y., Doronkin, D. E., Chen, M., Wei, S. & Grunwaldt, J.-D. Interplay of Pt and crystal facets of TiO2: CO oxidation activity and operando XAS/DRIFTS studies. ACS Catal. 6, 7799–7809 (2016).
Ganzler, A. M. et al. Unravelling the different reaction pathways for low temperature CO oxidation on Pt/CeO2 and Pt/Al2O3 by spatially resolved structure-activity correlations. J. Phys. Chem. Lett. 10, 7698–7705 (2019).
Kim, H. Y., Lee, H. M. & Henkelman, G. CO oxidation mechanism on CeO2-supported Au nanoparticles. J. Am. Chem. Soc. 134, 1560–1570 (2012).
Liu, X. et al. Structural changes of Au–Cu bimetallic catalysts in CO oxidation: In situ XRD, EPR, XANES, and FT-IR characterizations. J. Catal. 278, 288–296 (2011).
Cao, L. et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2. Nature 565, 631–635 (2019).
Martin, N. M. et al. CO oxidation and site speciation for alloyed palladium–platinum model catalysts studied by in situ FTIR spectroscopy. J. Phys. Chem. C 121, 26321–26329 (2017).
Van Den Bosch-Driebergen, A. G. CO oxidation over silica supported Pt-Rh alloy catalysts. Catal. Lett. 2, 73–80 (1989).
Li, J.-J. et al. Enhanced CO catalytic oxidation over an Au–Pt alloy supported on TiO2 nanotubes: investigation of the hydroxyl and Au/Pt ratio influences. Catal. Sci. Technol. 8, 6109–6122 (2018).
Mo, S. et al. Immobilizing ultrafine bimetallic PtAg alloy onto uniform MnO2 microsphere as a highly active catalyst for CO oxidation. Chin. Chem. Lett. 32, 2057–2060 (2021).
Zhu, H. et al. Constructing hierarchical interfaces: TiO2-supported PtFe–FeOx nanowires for room temperature CO oxidation. J. Am. Chem. Soc. 137, 10156–10159 (2015).
Pan, Y. et al. Identification of active sites in Pt-Co Bimetallic catalysts for CO oxidation. ACS Appl. Energy Mater. 4, 11151–11161 (2021).
Tripathi, A., Hareesh, C., Sinthika, S., Andersson, G. & Thapa, R. CO oxidation on Pt based binary and ternary alloy nanocatalysts: reaction pathways and electronic descriptor. Appl. Surf. Sci. 528, 146964 (2020).
Wang, Q. et al. Co-promotion of two-type active sites: PtCu single-atom alloy and copper-ceria interface for preferential oxidation of CO. Appl. Catal. B Environ. 306, 121117 (2022).
Amaya Suárez, J., Plata, J. J., Márquez, A. M. & Fdez. Sanz, J. Catalytic activity of PtCu intermetallic compound for CO oxidation: a theoretical insight. Catal. Today 383, 339–344 (2022).
Saravanan, G., Khobragade, R., Chand Nagar, L. & Labhsetwar, N. Ordered intermetallic Pt–Cu nanoparticles for the catalytic CO oxidation reaction. RSC Adv. 6, 85634–85642 (2016).
Komatsu, T., Takasaki, M., Ozawa, K., Furukawa, S. & Muramatsu, A. PtCu intermetallic compound supported on alumina active for preferential oxidation of CO in hydrogen. J. Phys. Chem. C 117, 10483–10491 (2013).
Si, R. et al. Structure sensitivity of the low-temperature water-gas shift reaction on Cu-CeO2 catalysts. Catal. Today 180, 68–80 (2012).
DeSario, P. A. et al. Low-temperature CO oxidation at persistent low-valent Cu nanoparticles on TiO2 aerogels. Appl. Catal. B Environ. 252, 205–213 (2019).
Tantardini, C. & Oganov, A. R. Thermochemical electronegativities of the elements. Nat. Commun. 12, 2087 (2021).
Komatsu, T. & Tamura, A. Pt3Co and PtCu intermetallic compounds: promising catalysts for preferential oxidation of CO in excess hydrogen. J. Catal. 258, 306–314 (2008).
Liu, Y. et al. Dual active sites of CO adsorption and activation over PtCu alloy nanocages for preferential oxidation of carbon monoxide. ACS Appl. Energy Mater. 5, 604–614 (2021).
Zhao, Y., Liu, J., Liu, C., Wang, F. & Song, Y. Amorphous CuPt alloy nanotubes induced by Na2S2O3 as efficient catalysts for the methanol oxidation reaction. ACS Catal. 6, 4127–4134 (2016).
Liu, Y. et al. Unraveling the lattice O assisted internal selective catalytic reduction mechanism on high N2 selectivity of CuOx/PtCu catalysts in NH3-SCO. ACS Catal. 13, 7178–7188 (2023).
Huang, X.-Y., Wang, A.-J., Zhang, X.-F., Zhang, L. & Feng, J.-J. One-step synthesis of PtCu alloyed nanocages with highly open structures as bifunctional electrocatalysts for oxygen reduction and polyhydric alcohol oxidation. ACS Appl. Energy Mater. 10, 5779–5786 (2018).
Chen, Y. et al. Atomically dispersed platinum in surface and subsurface sites on MgO have contrasting catalytic properties for CO oxidation. J. Phys. Chem. Lett. 13, 3896–3903 (2022).
Rastegarpanah, A. et al. Influence of group VIB metals on activity of the Ni/MgO catalysts for methane decomposition. Appl. Catal. B Environ. 248, 515–525 (2019).
Nan, B. et al. Unique structure of active platinum-bismuth site for oxidation of carbon monoxide. Nat. Commun. 12, 3342 (2021).
Tang, C. et al. A stable nanocobalt catalyst with highly dispersed CoNxactive sites for the selective dehydrogenation of formic acid. Angew. Chem. Int. Ed. Engl. 129, 16843–16847 (2017).
Wang, S. et al. Pt-O-Cu anchored on Fe2O3 boosting electrochemical water-gas shift reaction for highly efficient H2 generation. J. Catal. 417, 98–108 (2023).
Zhang, Z. et al. Memory-dictated dynamics of single-atom Pt on CeO2 for CO oxidation. Nat. Commun. 14, 2664 (2023).
Lee, S. et al. Manganese oxide overlayers promote CO oxidation on Pt. ACS Catal. 11, 13935–13946 (2021).
Almana, N. et al. Design of a core–shell Pt–SiO2 catalyst in a reverse microemulsion system: Distinctive kinetics on CO oxidation at low temperature. J. Catal. 340, 368–375 (2016).
Ahasan, M. R., Wang, Y. & Wang, R. In situ DRIFTS and CO-TPD studies of CeO2 and SiO2 supported CuOx catalysts for CO oxidation. Mol. Catal. 518, 112085 (2022).
Zhang, L. et al. Hot hole enhanced synergistic catalytic oxidation on Pt-Cu alloy clusters. Adv. Sci. 4, 1600448 (2017).
Johansson, C. H. & Linde, J. O. Gitterstruktur und elektrisches leitvermoegen der mischkristallreihen Au-Cu, Pd-Cu und Pt-Cu. Ann. Phys. (Leipz.) 82, 449–478 (1927).
Zhang, X. et al. Platinum–copper single atom alloy catalysts with high performance towards glycerol hydrogenolysis. Nat. Commun. 10, 5812 (2019).
Wang, G. et al. Surface-layer bromine doping enhanced generation of surface oxygen vacancies in bismuth molybdate for efficient photocatalytic nitrogen fixation. Appl. Catal. B Environ. 310, 121319 (2022).
Nan, B. et al. Hydrogen-controlled structural reconstruction of palladium-bismuth oxide cluster to single atom alloy for low-temperature CO oxidation. Appl. Catal. B Environ. 334, 122818 (2023).
Tankov, I., Cassinelli, W. H., Bueno, J. M. C., Arishtirova, K. & Damyanova, S. DRIFTS study of CO adsorption on praseodymium modified Pt/Al2O3. Appl. Surf. Sci. 259, 831–839 (2012).
Chen, J. et al. Spotlight on Pt/γ-Al2O3 with high catalytic performance induced by Barium: synergistic effect of electron-rich Ptδ- single-atoms and available oxygen species. Chem. Eng. J. 474, 145574 (2023).
Yu, W. Z. et al. Construction of active site in a sintered copper-ceria nanorod catalyst. J. Am. Chem. Soc. 141, 17548–17557 (2019).
Sun, G. et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 9, 4454 (2018).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Acknowledgements
This work was supported by “Photon Science Research Center for Carbon Dioxide”, Science and Technology Innovation Plan of Shanghai Science and Technology Commission (23YF1453700 received by B.N.), “Shanghai Science and Technology Innovation Action Plan” (22JC1403800 received by L.N.L), “2022 Self Deployed Instrument Design Project of Shanghai Advanced Research Institute” and “National Natural Science Foundation of China”(12105351 received by B.N.). We appreciate the assistance of TILON Group Technology Limited (Division of China) and In-situ Center for Physical Sciences, Shanghai Jiao Tong University in characterization of catalysts. Additionally, the User Experiment Assist System of SSRF, CAS-Shanghai Science Research Center, and 14 W, 13SSW, 06B, 17B and 16U1 beamline of SSRF provided support for data collecting for this work.
Author information
Authors and Affiliations
Contributions
L.N.L. supervised the work and procured funds. Y.N.L., L.L.G., L.Z.J., and B.N. designed the experiments and analyzed the results. M.D. assisted in-situ DRIFTS data analysis. C.T. and Z.Y.L. carried out XRD and XPS experiments. G.Z. and X.L. performed the aberration-corrected HAADF-STEM measurements. L.L.G. carried out the TEM/HRTEM measurements. Y.N.L. and L.Z.J. performed the 18O2 isotope-labeling experiments. Y.N.L., Z.W.L., and K.M.H. carried out XAS measurements and analyzed the data. J.X.C. assisted with the TPR test. Y.N.L. and B.N. wrote the manuscript. All authors discussed the results and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Huiyuan Zhu, Daiqi Ye and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Guo, L., Du, M. et al. Unraveling distinct effects between CuOx and PtCu alloy sites in Pt−Cu bimetallic catalysts for CO oxidation at different temperatures. Nat Commun 15, 5598 (2024). https://doi.org/10.1038/s41467-024-49968-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-49968-6
This article is cited by
-
Tender energy spectroscopy beamline at the Shanghai Synchrotron Radiation Facility
Nuclear Science and Techniques (2026)
-
Applications of Environmentally Friendly Metal Oxides as PEM Fuel Cell Cathode Catalysts
International Journal of Precision Engineering and Manufacturing-Green Technology (2026)
-
Advances for in situ characterization techniques applied to gas-solid heterogeneous catalysis under reaction conditions
Frontiers of Chemical Science and Engineering (2025)