Abstract
Pesticides may have serious negative impacts on bee populations. The pesticide exposure of bees could depend on the surrounding landscapes in which they forage. In this study, we assess pesticide exposure across various land-use categories, while targeting the Japanese honey bee, Apis cerana japonica, a native subspecies of the eastern honey bee. In a project involving public participation, we measured the concentrations of major pesticides in honey and beeswax collected from 175 Japanese honey bee colonies across Japan and quantitatively analyzed the relationships between pesticide presence/absence or pesticide concentration and land-use categories around the colonies. Our findings revealed that the surrounding environment in which bees live strongly influences pesticide residues in beehive materials, whether the pesticides are systemic or not, with a clear trend for each land-use category. Agricultural lands, particularly paddy fields and orchards, and urban areas resulted in higher pesticide exposure, whereas forests presented a lower risk of exposure. To effectively control pesticide exposure levels in bees, it is essential to understand pesticide usage patterns and to develop appropriate regulatory systems in non-agricultural lands, similar to those in agricultural lands.
Similar content being viewed by others
Introduction
Pollination plays a crucial role in human well-being by maintaining biodiversity and ensuring ecosystem stability1. Approximately 85% of wild flowering plants rely on animal pollinators2,3. Among these, bees are the key pollinators, contributing to reproductive success in many wild plants and cultivated crops4,5. In recent years, however, there has been growing concern about declining populations of both wild and managed bees in many parts of the world, particularly in the Northern Hemisphere6,7.
The decline in bee populations appears to be caused by various factors, with pesticides considered one significant contributor8,9,10. Many experimental studies showed that insecticides, herbicides, and fungicides can disrupt various aspects of bee health by impairing their behaviors or the establishment of gut microbiota or by directly influence their mortality11,12,13. Neonicotinoid insecticides, in particular, have attracted much attention due to their systemic nature and their contamination of pollen and nectar, and a number of ecological impact assessments have been published14,15. Pesticides are sprayed directly on plants or applied to the seeds or soil to be systemically distributed throughout the plants. When bees visit these plants, they can transfer the pesticides to their colonies. Surveys conducted in Europe16,17,18, North America19, and China20, among others, have detected a wide variety of pesticides from hive materials including honey, pollen, and beeswax and the bees themselves.
The types and quantity of pesticide residues to which bees are exposed could be influenced by the land use(s) around their nests, because bees forage within a certain range from their nest (e.g., the western honey bee, Apis mellifera, has an average home range radius of 5.5 km; ref. 21). In agricultural lands, where a large quantity of pesticides is used, the influence of the proportion and structure of agricultural lands on pesticide exposure has been reported in recent years22,23,24,25. On the other hand, the possible risk of exposure to pesticides in non-agricultural lands has also been raised. In urban areas, several neonicotinoids were detected in honey more frequently than in agricultural lands, suggesting that exposure to these pesticides may occur due to urban human activities26. In forests, although pesticide usage is significantly less than for agricultural crop production27, herbicides are employed during reforestation for weed control and insecticides and fungicides are used for controlling insects and pathogens28,29. The European Food Safety Authority has devised a standard index to assess exposure risk in a single treated field30, but the actual situation involves broader contamination at the landscape level, as described above. Therefore, we require a comprehensive means of evaluating pesticide exposure across diverse landscapes, encompassing agricultural lands, urban environments, forests, and various other land uses. To conduct a comprehensive survey, numerous samples are needed to compensate for lower detection rates resulting from increased landscape diversity. In practice, to accurately assess bees’ pesticide exposure in their natural environments, it would be appropriate to analyze pesticide residues from wild bee nests, which do not receive any human intervention. However, locating wild bee nests and acquiring a substantial number of samples can be exceedingly challenging.
Here, we conducted a comprehensive exposure assessment for various land-use categories by using the Japanese honey bee, Apis cerana japonica, which is a subspecies of Asian honey bee. Although it is a wild species, numerous beekeepers across the country tend the Japanese honey bee, making it possible to perform extensive and large-scale sampling. In addition, most beekeepers maintain the traditional practice of providing empty spaces (box or trunk hives), which are essentially equivalent to nesting conditions in the wild. We quantitatively evaluated honey and beeswax samples for neonicotinoids, other mode-of-action class insecticides, a herbicide, and a fungicide, which were selected because they represent various pesticide classes and are shipped at such large volumes that the compounds would likely be detected in the environment. We then analyzed the relationship between pesticide presence and/or concentration and the proportion of each land-use category surrounding the sampling locations to assess the effects of landscape structure on pesticide exposure.
Results
Basic information on pesticide residues
The summary of detected pesticides is presented in Table 1, and the full data set can be found in Supplementary Data 1. The seven neonicotinoids detected in the honey samples were dinotefuran (52.0% of samples), acetamiprid (43.4%), clothianidin (26.3%), nitenpyram (14.3%), thiamethoxam (13.1%), thiacloprid (5.7%), and imidacloprid (2.9%), with median concentration ranging from 0.02 to 0.12 ng/g. Glyphosate was detected in 23.4% of the honey samples, with a median concentration of 22.00 ng/g, whereas its metabolite aminomethylphosphonic acid (AMPA) was not detected. In the beeswax samples, we detected chlorantraniliprole (25.7%), diazinon (22.9%), fipronil (22.9%), fenobucarb (19.4%), and etofenprox (10.9%), with a median concentration ranging from 0.03 to 0.16 ng/g. Fenitrothion was detected in two cases and chlorothalonil in only one case. The histograms of concentrations are similar to an exponential distribution with a long tail, indicating that many colonies were exposed to relatively low concentrations and a few to high concentrations (Supplementary Fig. S1).
Land-use status within a 1000-m radius around the locations of all colonies
For the 175 locations, agricultural lands (total of paddy fields, orchards, tea plantations, and farmlands), forests (total of natural and plantation forests), and urban areas occur around almost all of them (>96%), with the mean proportions in the order of forests (38.6%), agricultural lands (28.1%), and urban areas (25.9%) (Table 2). Considering that forests account for 67.6% of Japan’s total land area, agricultural lands for 14.6%, and urban areas for 8.3%, the 175 locations we analyzed in this study have a lower proportion of forests but higher proportion of agricultural lands and urban areas, suggesting that we studied more “human-inhabited” environments. The maximum proportion of agricultural lands was 83.7% (standard deviation, 19.2%) and the land-use categories within agricultural lands were also diverse (Supplementary Fig. S2). The other land-use categories also varied.
Association between land use and presence/absence of pesticides
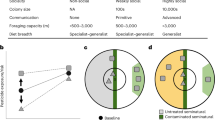
The relationship between pesticide presence in honey samples and land use (Figs. 1, 2, Supplementary Fig. S3) showed different trends for nitenpyram and other pesticides. One-to-one analyses indicated that pesticides, excluding nitenpyram, were more frequently detected in orchards and urban areas and less prevalent in natural and plantation forests (Fig. 1). In contrast, nitenpyram tended to be more readily detected in forests. The correlation between the dissimilarity matrix of pesticide presence/absence in honey and the dissimilarity matrix of land use was not significant (Mantel test, r = 0.04, p = 0.07); the overall canonical correlation analysis (CCA) was significant (residual degrees of freedom = 131, χ2 = 0.23, F = 1.37, p < 0.05) with no significant axis. Natural forests were significant (χ2 = 0.06, F = 5.00, p < 0.01) for environmental variables, with a contribution of 0.514 for CCA1, with higher scores for natural forests (0.639) and urban areas (–0.608) for environmental variables and higher scores for pesticide data for nitenpyram (0.923) and thiamethoxam (–0.593) (Fig. 2).
The tested sample is honey (left: acetamiprid to glyphosate) or beeswax (right: diazinon to fipronil). Generalized linear models (GLMs) were employed with pesticide presence/absence as the response variable and land-use proportions as the explanatory variable. The models utilized a binomial distribution and a logistic link function. Statistical analysis was conducted using a two-sided Wald test without adjustments for multiple comparisons. The association was categorized as follows: positive: odds ratio > 1 and p < 0.05; negative: odds ratio <1 and p < 0.05; non-significant: others. Each GLM result is shown in Supplementary Fig. S3. Summary statistics are provided as a Source Data file.
The tested sample is honey (n = 145, left) or beeswax (n = 109, right). The proportion variables for each land-use category sum to 1, and one category was dropped from the plot because of degrees-of-freedom constraints; we decided not to show coastal vegetation. Pesticides: ACE: acetamiprid; CLO: clothianidin; DIN: dinotefuran; NIT: nitenpyram; THM: thiamethoxam; GLY: glyphosate in honey (left); DZN: diazinon; FBC: fenobucarb; EFP: etofenprox; CPL: chlorantraniliprole; FIP: fipronil in beeswax (right). Land-use categories: pad: paddy fields; orc: orchards; tea: tea plantations; far: farmlands; nat: natural forests; pla: plantation forests; bam: bamboo; urb: urban areas; lar: large parks; gra: grasslands; bar: bare lands; wet: wetlands; wat: water bodies. Summary statistics are provided as a Source Data file.
In one-to-one analyses of pesticide presence in beeswax samples versus each land use (Fig. 1, Supplementary Fig. S3), diazinon and chlorantraniliprole were more likely to be detected in paddy fields and less likely to be detected in plantation forests. Etofenprox was more likely to be detected in orchards. Conversely, fenobucarb tended to be more frequently detected in plantation forests. There was a significant correlation between the dissimilarity matrix of pesticide presence/absence in beeswax and the dissimilarity matrix of land use (Mantel test, r = 0.08, p < 0.01); the overall CCA was significant (residual degrees of freedom = 95, χ2 = 0.35, F = 1.50, p < 0.05), and only axis CCA1 was significant (χ2 = 0.21, F = 13.17, p < 0.01). Environmental variables were significant for farmlands (χ2 = 0.07, F = 4.12, p < 0.01) and plantation forests (χ2 = 0.09, F = 4.84, p < 0.01). The contribution of CCA1 was 0.619, and environmental variables scored higher for plantation forests (0.794) and farmlands (–0.491); pesticide data scored higher for fenobucarb (0.730) and diazinon (–0.509) (Fig. 2).
Association between land use and pesticide concentrations
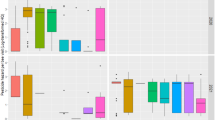
In the one-to-one analyses of pesticide concentrations in honey and land use, it was difficult to find clear trends for each land-use category when compared to pesticide presence/absence (binary values) (Fig. 3, Supplementary Fig. S4). Nevertheless, characteristic results were that acetamiprid concentrations were positively related to proportions of tea plantations and urban areas, and nitenpyram concentrations were positively related to orchards, natural forests, and bamboo. There was a significant correlation between the Euclidean matrix of pesticide concentrations in honey and the dissimilarity matrix of land use (Mantel test, r = 0.14, p < 0.01). The overall redundancy analysis (RDA) of honey was significant (residual degrees of freedom = 131, variance = 1.02, F = 2.07, p < 0.01), and only the RDA1 axis was significant (variance = 0.62, F = 17.08, p < 0.01). Environmental variables were significant for natural forests (variance = 0.25, F = 6.67, p < 0.01), orchards (variance = 0.23, F = 6.01, p < 0.01), and bamboo (variance = 0.11, F = 2.81, p < 0.05). The contribution of RDA1 was 0.603, and environmental variables scored higher for natural forests (–0.586) and orchards (0.506), whereas pesticide data scores were high for dinotefuran (1.096) and clothianidin (0.874).
The tested sample for pesticides is honey (left: acetamiprid to glyphosate) or beeswax (right: diazinon to fipronil). Generalized linear models (GLMs) were employed with pesticide concentration (log) as the response variable and land-use proportions as the explanatory variable. The models utilized a Gaussian distribution and an identity link function. Statistical analysis was conducted using a two-sided Wald test without adjustments for multiple comparisons. The association was categorized as follows: positive: odds ratio > 1 and p < 0.05; negative: odds ratio <1 and p < 0.05; non-significant: others. Each GLM result is shown in Supplementary Fig. S4. Summary statistics are provided as a Source Data file.
The one-to-one analyses of pesticide concentrations in beeswax and land use also showed significant positive relationships in some combinations, as in honey samples. However, no overall trend was found (Fig. 3, Supplementary Fig. S4). The correlation between the Euclidean distance matrix of beeswax pesticide concentrations and the land-use dissimilarity matrix was insignificant (Mantel test, r = 0.07, p = 0.07). The overall RDA regarding beeswax samples was significant (residual degrees of freedom = 95, variance = 0.90, F = 1.60, p < 0.05), and the RDA1 axis was significant (variance = 0.46, F = 11.59, p < 0.05). Environmental variables were significant for paddy fields (variance = 0.17, F = 4.02, p < 0.01), farmlands (variance = 0.22, F = 5.16, p < 0.01), and wetlands (variance = 0.14, F = 3.17, p < 0.05) (Fig. 4), with RDA1 contributing 0.513 and environmental variables scoring higher for paddy fields (–0.593) and farmlands (–0.569); pesticide data scores for diazinon (–0.969) and chlorantraniliprole (–0.768) were higher. Although concentrations of certain pesticides were sporadically identified as significantly associated with a certain land use, in most cases the presence of these pesticides showed no association with the land use. In such cases, the data were insufficient to support that those land-use categories had a higher risk of exposure.
The tested sample for pesticides is honey (n = 145, left) or beeswax (n = 109, right). The proportion variables for each land-use category sum to 1, and one category was dropped from the plot due to degrees-of-freedom constraints; we decided not to show coastal vegetation. Pesticides: ACE: acetamiprid; CLO: clothianidin; DIN: dinotefuran; NIT: nitenpyram; THM: thiamethoxam; GLY: glyphosate in honey (left); DZN: diazinon; FBC: fenobucarb; EFP: etofenprox; CPL: chlorantraniliprole; FIP: fipronil in beeswax (right). Land-use categories: pad: paddy fields; orc: orchards; tea: tea plantations; far: farmlands; nat: natural forests; pla: plantation forests; bam: bamboo; urb: urban areas; lar: large parks; gra: grasslands; bar: bare lands; wet: wetlands; wat: water bodies. Summary statistics are provided as a Source Data file.
Association between land use and exposure to multiple neonicotinoids
At least one neonicotinoid was detected in 81.7% of the honey samples and two or more in 48.6% (Fig. 5). Regarding the association between each land-use category and the presence of multiple neonicotinoids, the rate of increase in the number of neonicotinoids for orchards, urban areas, and paddy fields (in that order) was higher with increasing land-use coverage. In contrast, the number of neonicotinoids for natural and plantation forests decreased with increasing area (Fig. 6, Supplementary Fig. S5).
This figure specifically highlights primary land-use categories, whereas the comprehensive results for all land-use categories can be found in Supplementary Fig. S5. Agricultural lands encompass paddy fields, orchards, tea plantations, and farmlands and forests include natural forests and plantation forests. These models use a binomial distribution and a logistic link function. The dashed lines represent the predicted linear regressions, and the gray ribbons represent the 95% confidence intervals. Statistical analysis was conducted using a two-sided Wald test without adjustments for multiple comparisons (n = 175). Summary statistics are provided as a Source Data file.
Discussion
There was a clear trend for each land-use category, with a higher risk of exposure to pesticides in agricultural lands, especially orchards and paddy fields, and in urban areas and a lower exposure risk in forests. For some pesticides, however, the opposite trend was observed, with the higher risk of exposure in forests. Other land uses showed no significant trend. Associations of some kind with land use were found not only for the systemic neonicotinoids but also for organophosphorus, carbamate, pyrethroid, and diamide pesticides and a herbicide. In this study, the glyphosate metabolite, AMPA, was not detected in any of the samples, but a previous study reported frequent detection and a high concentration of AMPA in honey31. We hypothesize that the immediate storage of honey samples in a dark and frozen condition after collection may have prevented degradation. Therefore, the risk of honey bees being exposed to AMPA through nectar or honey might be low.
In many previous studies, the risks of exposure to pesticides were evident in agricultural lands or in specific crop fields. However, our results have revealed differences in the strength of pesticide exposure depending on the land-use categories in agricultural lands. Specifically, diazinon and chlorantraniliprole were significantly detected in paddy fields, while acetamiprid, dinotefuran, and glyphosate were significant in orchards. Both land uses showed significantly higher risks of exposure to multiple neonicotinoids. On the other hand, tea plantations and farmlands did not present high exposure risks. Thus, when predicting or evaluating pesticide exposure in agricultural lands, we need to take into account the types of land uses. Since the flowering periods of rice and fruit trees are very limited, persistent pesticide exposure from non-crop flowering plants within and around agricultural lands could be a significant factor contributing to high pesticide exposure probabilities in these fields32,33,34,35, although these non-crop plants are considered crucial resources supporting bee diversity36,37. Therefore, it is necessary to consider not only exposure through crop plants but also via surrounding wild plants within and outside agricultural lands. Incidentally, our farmland data showed no high risks of pesticide exposure, whereas higher risks for certain crops have been reported overseas24,38. The agricultural practice of pesticide seed coating and sowing the seeds with large machinery, which is a cause of pesticide drift overseas (especially in Europe and North America), is not common in Japan. Moreover, considering the characteristic feature of small-scale management of agriculture in Japan and the wide variety of crops grown across farmlands, the exposure amount of each pesticide within the bees’ foraging range might not be very large.
In urban areas, our quantitative assessment clearly revealed exposure associated with three neonicotinoids—clothianidin, dinotefuran, and thiamethoxam—along with detection of multiple neonicotinoids. Some previous investigations were not based on a quantitative comparison of land uses26,39, and our understanding of the impact of urban area coverage on pesticide exposure was insufficient. In addition, because glyphosate is one of the most widely used herbicides, its contamination of streams in urban areas is a serious problem40. In our study, glyphosate was detected in more than 20% of the honey samples, showing a positive relationship with urban areas as well as orchards. These neonicotinoids and glyphosate are contained not only in agricultural products but also in a variety of pesticides used for urban residential, sports, and recreational areas26 and in ornamental plants considered bee-friendly41, resulting in frequent exposure to bees even in urban areas. Unfortunately, while strict standards regarding usage amounts and timing were established in Japan by the Pesticide Control Law for agricultural pesticides, regulations for pesticide use in urban areas are not thoroughly enforced. In a sense, the pesticide exposure in urban areas has become “a black box” in Japan; this may be a global issue as well. With the continued expansion of urban and residential land development, pesticide use in green spaces is predicted to increase, highlighting the need to both continue assessing the exposure to bees as an endpoint of environmental residue and to establish regulations.
The proportion of natural and plantation forests had a negative association with more than half of the tested pesticides, and multivariate analyses (CCA) supported this trend. Assessing the food resource value of forests for bees is complex in part because the value varies among tree types and forest ages42,43, but forests are considered vital foraging grounds for the Japanese honey bee. Many forests are either not contaminated by pesticides or contain them only at low frequency, thus reducing pesticide residues in bee hives. In addition, broadleaf forests (natural forests) can provide nesting spaces for honey bees in the later stages of tree growth42,43, underscoring the significance of forests for honey bee conservation.
Our data indicated that some pesticides not expected for use in certain land-use categories showed positive associations. For example, nitenpyram was positively associated with natural forests and fenobucarb with plantation forests; we lack a satisfactory explanation for these findings. Furthermore, the results showed a positive relationship between diazinon and paddy fields, but diazinon is not currently applied in paddy fields in Japan. These unexpected results implied drift and contamination across land uses, long-term residues in soil, and little information about the actual status of how the pesticides are being used, highlighting the challenges of uniformly evaluating all pesticides or creating a single index for all pesticides in the field.
Although clear relationships with specific land uses were observed for the presence/absence of certain pesticides, the correlations with concentrations remained unclear. Teasing out the relationship between pesticide concentration and exposure is difficult because pesticides are used simultaneously among multiple land uses and there might be variations in all exposure processes, including subsequent contamination, foraging, and decomposition. Nonetheless, it is evident that the existence of each surrounding land use reflects the actual exposure situation, and understanding the usage practices in each land-use category, which serves as the primary sources of exposure, will remain an important task in controlling bees’ exposure to pesticides, while considering variations.
Our honey survey results showed mean concentrations of each neonicotinoid on the order of 0.01 or 0.1 ng/g, which is one order of magnitude lower than those reported in global surveys of residues in honey44. One possible reason for this discrepancy is differences in the foraging preferences among honey bee species. Compared to the Japanese honey bee, the western honey bee—the primary species examined in previous studies—is likely to be at higher risk of neonicotinoid exposure, given that herbaceous plants, especially around agricultural lands, are a likely source of neonicotinoids22 and the western honey bee’s preference for herbaceous over woody plants45. Although the level of pesticide exposure of Japanese honey bees is thought to be relatively low, that may not be the case. Our present research is an observational study that sampled live bee colonies, making it difficult to determine whether few colonies are actually exposed to high concentrations of a given pesticide or whether exposed colonies have died off and are therefore unobservable. Near paddy fields in Japan, incidents of mass honey bee death occurred during and after rice flowering (July–August)46, prior to the sampling season of our study. Moreover, the Japanese honey bee is approximately 10 times more sensitive to insecticides than is the western honey bee47. Even though glyphosate concentrations in honey from the Japanese honey bee have been detected at levels comparable to those from the western honey bee in other regions31,48,49, the neonicotinoid concentrations are low. These facts may support the latter pessimistic scenario of bee colonies dying before they could be sampled. On the other hand, the concentrations detected in this study are certainly much lower than those suggested to have colony-level impacts on western honey bees in fields23,50. However, the Japanese honey bee’s high susceptibility to pesticides makes it difficult to extrapolate the results of previous studies of western honey bees to our study. The impacts of concentrations of all pesticides detected in this study must be evaluated over longer periods and will be clarified by following up on the sampled colonies.
In conclusion, our findings indicate that the surrounding environment in which bees live strongly influences pesticide residues in honey and beeswax. This applies not only to neonicotinoids, which are transferred systemically to pollen and nectar, but also to non-systemic pesticides. Agricultural lands should be categorized accordingly, with high pesticide exposure associated with paddy fields and orchards, each exhibiting distinct pesticide patterns. Urban areas also should be recognized as high-risk areas, while forests should be considered pesticide mitigation areas. Pesticide exposure from agricultural lands typically yielded anticipated results consistent with applying pesticides on crops and fruit trees. Conversely, the exposure from non-agricultural lands (urban areas and forests) influenced residues within beehives as endpoints, but there is a complete lack of knowledge about the actual pesticide usage practices in these areas. While pesticide use in agricultural lands is tightly regulated with strict guidelines regarding timing and frequency, non-agricultural lands are largely unregulated—and these problems in Japan are likely similar to those worldwide. To effectively control the pesticide exposure levels of bees, it is essential to understand pesticide usage patterns and develop appropriate regulatory systems in non-agricultural lands as exist for agricultural lands.
Methods
Sampling
From May to July 2021, we invited A. cerana japonica beekeepers from across Japan to participate in this study, and more than 2000 colonies were registered, along with information on the number of colonies kept and their respective conditions. From among them, we requested samples from colonies that met two criteria: (1) newly nesting in 2021, and (2) not artificially fed at all prior to sampling. These criteria ensured that the sampled honey and beeswax would be those produced in that year.
Beekeepers, who understood and agreed to the purpose and methods of this study, collected honey and beeswax between August and November 2021. A minimum of 20 g of honey or beeswax was sealed in a 50-mL light-shielded centrifuge tube, and the tubes were stored in a household freezer until shipping. Samples were sent to the analytical laboratory of Heisei Riken by frozen delivery maintained at –18 °C or lower. At the laboratory, the samples were stored at –20 °C or lower until the analysis.
Samples were collected from 186 colonies that met the study criteria, but the distance between some colonies was less than 1000 m. In such cases, samples from the more southern colony of the pair were excluded, and we analyzed samples from a total of 175 colonies, all separated by more than 1000 m.
Tested pesticides
In principle, we prioritized the analysis of pesticides that raised concerns about bee toxicity and/or had large shipping volumes (Table S1). We examined 15 pesticides and 1 metabolite. We tested honey samples for 7 neonicotinoids (acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, thiamethoxam, and thiacloprid) and the herbicide glyphosate and its metabolite AMPA. Beeswax samples were tested for the phenylpyrazole fipronil, the organophosphates diazinon and fenitrothion, the carbamate fenobucarb, the pyrethroid etofenprox, the diamide chlorantraniliprole, and the fungicide chlorothalonil. Water-soluble pesticides tend to be more easily detected in honey, whereas lipid-soluble pesticides are more likely to be found in beeswax. Through preliminary testing, we identified whether each pesticide was more likely to be detected from honey or beeswax and then only analyzed each pesticide in the most likely sample type (see Table 1 for details).
Sample analysis
For the analysis of neonicotinoids in honey, 2 g of honey was removed from each sample. For the analysis of diazinon, fenobucarb, etofenprox, chlorantraniliprole, fipronil, fenitrothion, and chlorothalonil, 1 g of beeswax was removed. A mixture of 2 ml acetonitrile and acetic acid (99:1) and 2 ml of purified water was added, and crushing extraction was performed in a Nippi BioMasher. Then, 1.6 g of magnesium sulfate and 0.7 g of sodium acetate were added, and the extraction was performed with a reciprocating shaker at 180 rpm for 10 min. After extraction, samples were centrifuged at 4769 × g for 10 min. The supernatant (organic layer) was transferred into a 10-ml centrifuge tube, 0.5 g of magnesium sulfate and 160 mg of Bondesil-PSA (ethylenediamine-N-propyl) were added, and the tubes were shaken in a reciprocating shaker at 180 rpm for 10 min. The supernatant was separated and filtered through a centrifugal filtration unit (Millipore Ultrafree 0.20 μm) to prepare the test solution for analysis.
For the analysis of glyphosate and AMPA in honey samples, 20 ml of a mixture of water and methanol (1:1) was added to 1 g of the sample, and the sample was sonicated for 10 min. The extract was centrifuged at 4769 × g for 10 min, and 18 ml of the upper layer was used as the sample solution for the column treatment. An InertSep-SCX (benzene sulfonyl propyl) mini-column and an InertSep-PSA (ethylenediamine-N-propyl) mini-column were connected below the InertSep-HLB (styrene-divinylbenzene and a nitrogen-containing vinyl monomer) mini-column and washed sequentially with 10 ml of methanol and 20 ml of water (using a vacuum manifold at a flow rate of 2–3 ml/min). The sample solution was passed through the top of the column and drained. The column was then washed with 10 ml of a mixture of water and methanol (1:1). Only the PSA mini-column was removed and eluted with 2 ml of sodium borate solution (pH = 9.5). The eluate was filtered through a centrifugal filtration unit (Millipore Ultrafree 0.20 μm) and used as the test solution for analysis.
Calibration curves were prepared by adding a standard sample to honey or beeswax that did not contain the test pesticide and then performing the same sample preparation process. The extract after percolation (Millipore Ultrafree 0.20 μm) was analyzed by liquid chromatography-tandem mass spectrometry (LC/MSMS, Xevo-TQ) or gas chromatography-tandem mass spectrometry (GC/MSMS, GCMS‐TQ; GC‐2010 Plus). A series of parallel spiked‐recovery tests was conducted to evaluate the recovery rate of each pesticide using simulated samples (honey and beeswax) with seven repetitions for each sample type. The mean recovery rate for each pesticide from simulated sample types was 100% (with a minimum of 82.6% and a maximum of 115.1%, as shown in Supporting Information, Table S2). Spike recovery tests were also conducted for seven concentrations of all target pesticides under the same conditions as our samples to produce a calibration curve, where the concentrations and analytical values showed high R2 values (above 0.995). The analyses were divided into two parts due to budget allocations: those conducted in 2021 and those in 2022.
Land-use analysis
Latitude and longitude for each bee colony location were collected from each beekeeper, either by extracting it from an online map or by marking it on a printed map. The locations of the 175 colonies are shown in Fig. 7.
Each dot represents a location, with positions shifted up to 0.1° to maintain privacy. Map data: GADM.
The proportion of each land use within a 1000-m radius of the locations was calculated using the raster and sf packages version 1.0-16 in R. The 1000-m radius range is based on the mean foraging range of Japanese honey bees, which is considerably less than that of A. mellifera51. Data from the 6th and 7th National Survey on the Natural Environment by Ministry of the Environment, Japan (http://gis.biodic.go.jp/webgis/sc-006.html) were used to determine land-use status. For one colony located in an area lacking data, the information from the 5th National Survey was utilized. The original vegetation map had approximately 520 categories, but for this study these were aggregated into 14 categories: paddy fields, orchards, tea plantations, farmlands, natural forests, plantation forests, bamboo, urban areas, large parks, grasslands, bare lands, wetlands, coastal vegetation, and water bodies. We also considered two higher level categories: (1) agricultural lands, which include paddy fields, orchards, tea plantations, and farmlands (i.e., croplands not included in the first three categories); and (2) forests, which include natural forests and plantation forests.
Statistical analysis
Pesticide data were treated as separate groups for honey and beeswax samples, as the accumulation process and physicochemical properties of pesticides, such as water and lipid solubility, were different for honey and beeswax samples. The analyses were conducted in three sections: (1) assessing the individual association between each land-use category and each pesticide by using a generalized linear model (GLM); (2) assessing the association between land use and the pesticide community (multivariate analyses); and (3) assessing the association between each land use and the presence of multiple neonicotinoids (GLM). In the multivariate analyses, 14 categories of land use were used, not including the high-level categories of agricultural lands and forests. For the presence/absence of pesticide data, two values were used: 1 for above the lower limit of quantification (LOQ) and 0 for below the LOQ. All statistical analyses were performed in R version 4.2.1 using the vegan package version 2.6-4.
Assessing the association between each land-use category and each pesticide by GLM
In evaluating the association between each land use and colony exposure to each pesticide, causality was assumed to be unidirectional, from land use to pesticide exposure. First, we concentrated on establishing a one-to-one relationship between each land-use category and each pesticide. Pesticides detected in less than 10% of sampled colonies were excluded from the analysis due to limited positive data. GLMs with binomial distribution and logit link function were conducted using pesticide presence/absence as the response variable and land-use proportion as the explanatory variable. Based on the GLM results, we classified the results as positive if p < 0.05 and odds ratio > 1, negative if p < 0.05 and odds ratio <1, and non-significant otherwise. The GLM was conducted using only the positive data (i.e., exceeding the LOQ), with the log-transformed pesticide concentration as the response variable, the land-use proportions as the explanatory variable, a Gaussian distribution, and the identity link function. The results of the GLM were classified as positive if p < 0.05 and coefficient >0, negative if p < 0.05 and coefficient <0, and non-significant otherwise.
Assessing the association between land use and the pesticide community by multivariate analyses
Multivariate analyses were conducted to analyze not only the relationship between pesticides and land-use categories but also the relationship among pesticides and among land-use categories. Pesticides detected in less than 10% of sampled colonies were excluded from the analysis due to limited positive data, and data from colonies where no pesticides were detected were not used in this analysis. The presence/absence of pesticides and land-use proportions were transformed into the Raup–Crick dissimilarity index matrix and the Bray–Curtis dissimilarity index matrix, respectively, and a Mantel test with Spearman correlation was performed (10,000 permutations). We then used canonical correspondence analysis (CCA) to examine the relationship between the presence/absence of pesticides and land-use proportions, with pesticides as species data and land-use proportions as environmental variables.
Pesticide concentrations and land-use proportions were transformed into the Euclidean distance matrix and the Bray–Curtis dissimilarity matrix, respectively, and a Mantel test with Spearman correlation was performed (10,000 permutations). We subsequently employed redundancy analysis (RDA) to investigate the relationship between pesticide concentrations and land-use proportions. For pesticide concentrations below the LOQ, we substituted the LOQ, then log-transformed and standardized the data to have a mean of 0 and a variance of 1. In this analysis, pesticide concentrations were treated as species data, and land-use proportions were considered as environmental variables.
Assessing the association between each land use and the presence of multiple neonicotinoids by GLM
To assess the risk of exposure to multiple neonicotinoids by land use, we used a GLM with a binomial distribution and logit link function, with the number of neonicotinoids detected in a honey sample as the response variable and land-use proportion as the explanatory variable. Data for all seven neonicotinoids were used in these analyses.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The pesticide concentrations detected in this study, as well as the proportions of each land-use category surrounding the sampling locations, have been deposited on Code Ocean (https://doi.org/10.24433/CO.4722550.v1). The data are also available in a Source Data file. Source data are provided in this paper.
Code availability
The analyses and result figures in this study have been deposited on Code Ocean (https://doi.org/10.24433/CO.4722550.v1).
References
Potts, S. G. et al. Safeguarding pollinators and their values to human well-being. Nature 540, 220–229 (2016).
Friedman, J. & Barrett, S. C. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann. Bot. 103, 1515–1527 (2009).
Ollerton, J., Winfree, R. & Tarrant, S. How many flowering plants are pollinated by animals? Oikos 120, 321–326 (2011).
Klein, A. M. et al. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274, 303–313 (2007).
Rollin, O. et al. Differences of floral resource use between honey bees and wild bees in an intensive farming system. Agriculture Ecosyst. Environ. 179, 78–86 (2013).
Potts, S. G. et al. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353 (2010).
Biesmeijer, J. C. et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354 (2006).
Woodcock, B. A. et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 356, 1393–1395 (2017).
Woodcock, B. A. et al. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 7, 12459 (2016).
Sánchez-Bayo, F. & Wyckhuys, K. A. G. Worldwide decline of the entomofauna: a review of its drivers. Biol. Conserv. 232, 8–27 (2019).
El Hassani, A. K., Dacher, M., Gauthier, M. & Armengaud, C. Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera). Pharmacol. Biochem. Behav. 82, 30–39 (2005).
Thompson, H. M. Behavioural effects of pesticides in bees - Their potential for use in risk assessment. Ecotoxicology 12, 317–330 (2003).
Wu, T. et al. Chlorothalonil alters the gut microbiota and reduces the survival of immature honey bees reared in vitro. Pest Manag Sci. 78, 1976–1981 (2022).
Lu, C., Hung, Y.-T. & Cheng, Q. A review of sub-lethal neonicotinoid insecticides exposure and effects on pollinators. Curr. Pollut. Rep. 6, 137–151 (2020).
Blacquière, T., Smagghe, G., van Gestel, C. A. M. & Mommaerts, V. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21, 973–992 (2012).
Chauzat, M. P. et al. A survey of pesticide residues in pollen loads collected by honey bees in France. J. Econ. Entomol. 99, 253–262 (2006).
Chauzat, M. P. & Faucon, J. P. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest Manag Sci. 63, 1100–1106 (2007).
Bernal, J. et al. Overview of pesticide residues in stored pollen and their potential effect on bee colony (Apis mellifera) losses in Spain. J. Econ. Entomol. 103, 1964–1971 (2010).
Mullin, C. A. et al. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5, e9754 (2010).
Xiao, J. et al. Analysis of honey bee exposure to multiple pesticide residues in the hive environment. Sci. Total Environ. 805, 150292 (2022).
Beekman, M. & Ratnieks, F. L. W. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 14, 490–496 (2000).
Wood, T. J., Kaplan, I., Zhang, Y. & Szendrei, Z. Honeybee dietary neonicotinoid exposure is associated with pollen collection from agricultural weeds. Proc. Biol. Sci. 286, 20190989 (2019).
Tsvetkov, N. et al. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 356, 1395–1397 (2017).
Bednarska, A. J. et al. Effects of agricultural landscape structure, insecticide residues, and pollen diversity on the life-history traits of the red mason bee Osmia bicornis. Sci. Total Environ. 809, 151142 (2022).
Nicholson, C. C. et al. Pesticide use negatively affects bumble bees across European landscapes. Nature 628, 355–358 (2024).
Kavanagh, S., Henry, M., Stout, J. C. & White, B. Neonicotinoid residues in honey from urban and rural environments. Environ. Sci. Pollut. Res. Int. 28, 28179–28190 (2021).
Thompson, D. G. Ecological impacts of major forest-use pesticides. In: Ecological Impacts of Toxic Chemicals (eds Sánchez-Bayo F., Brink P. J., van den Mann R. M.). Bentham Science Publishers Ltd. (2012).
Wang, L. et al. Current and future control of the wood-boring pest Anoplophora glabripennis. Insect Sci. 30, 1534–1551 (2023).
Eatough Jones, M. et al. Evaluations of insecticides and fungicides for reducing attack rates of a new invasive ambrosia beetle (Euwallacea sp., Coleoptera: Curculionidae: Scolytinae.) in infested landscape trees in California. J. Econ. Entomol. 110, 1611–1618 (2017).
European Food Safety Authority. Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 11, 3295 (2013).
Thompson, T. S., van den Heever, J. P. & Limanowka, R. E. Determination of glyphosate, AMPA, and glufosinate in honey by online solid-phase extraction-liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 36, 434–446 (2019).
Suzuki, T., Ikegami, M., Goka, K. & Sakamoto, Y. Insecticide residues associated with apple orchard treatments in the mason bee, Osmia cornifrons, and their nests. Environ. Toxicol. Chem. 42, 1564–1574 (2023).
Botías, C. et al. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 49, 12731–12740 (2015).
Stewart, S. D. et al. Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the mid-southern United States. Environ. Sci. Technol. 48, 9762–9769 (2014).
Favaro, R. et al. Botanical origin of pesticide residues in pollen loads collected by honeybees during and after apple bloom. Front. Physiol. 10, 1069 (2019).
Stopes, C., Measures, M., Smith, C. & Foster, L. Hedgerow management in organic farming. In: Biodiversity and Land Use, The Role of Organic Farming Multitext, Barcelona, Spain (eds Isart J., Llerena J. J.) (1995).
Tscharntke, T., Steffan-Dewenter, I., Kruess, A. & Thies, C. Contribution of small habitat fragments to conservation of insect communities of grassland–cropland landscapes. Ecol. Appl. 12, 354–363 (2002).
Smodis Skerl, M. I., Velikonja Bolta, S., Basa Cesnik, H. & Gregorc, A. Residues of pesticides in honeybee (Apis mellifera carnica) bee bread and in pollen loads from treated apple orchards. Bull. Environ. Contam. Toxicol. 83, 374–377 (2009).
David, A. et al. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 88, 169–178 (2016).
Okada, E., Allinson, M., Barral, M. P., Clarke, B. & Allinson, G. Glyphosate and aminomethylphosphonic acid (AMPA) are commonly found in urban streams and wetlands of Melbourne, Australia. Water Res. 168, 115139 (2020).
Lentola, A. et al. Ornamental plants on sale to the public are a significant source of pesticide residues with implications for the health of pollinating insects. Environ. Pollut. 228, 297–304 (2017).
Taki, H., Yamaura, Y., Okabe, K. & Maeto, K. Plantation vs. natural forest: Matrix quality determines pollinator abundance in crop fields. Sci. Rep. 1, 132 (2011).
Taki, H. et al. Succession influences wild bees in a temperate forest landscape: the value of early successional stages in naturally regenerated and planted forests. PLoS ONE 8, e56678 (2013).
Mitchell, E. A. D. et al. A worldwide survey of neonicotinoids in honey. Science 358, 109–111 (2017).
Nagamitsu, T. & Inoue, T. Differences in pollen sources of Apis cerana and Apis mellifera at a primary beech forest in central Japan. J. Apic. Res. 38, 71–78 (1999).
Kimura, K., Yoshiyama, M., Saito, K., Nirasawa, K. & Ishizaka, M. Examination of mass honey bee death at the entrance to hives in a paddy rice production district in Japan: the influence of insecticides sprayed on nearby rice fields. J. Apic. Res. 53, 599–606 (2015).
Yasuda, M., Sakamoto, Y., Goka, K., Nagamitsu, T. & Taki, H. Insecticide susceptibility in Asian honey bees (Apis cerana (Hymenoptera: Apidae)) and implications for wild honey bees in Asia. J. Econ. Entomol. 110, 447–452 (2017).
de Souza, A. P. F., Rodrigues, N. R. & Reyes, F. G. R. Glyphosate and aminomethylphosphonic acid (AMPA) residues in Brazilian honey. Food Addit. Contam Part B Surveill. 14, 40–47 (2021).
Zoller, O., Rhyn, P., Rupp, H., Zarn, J. A. & Geiser, C. Glyphosate residues in Swiss market foods: monitoring and risk evaluation. Food Addit. Contam Part B Surveill. 11, 83–91 (2018).
Dively, G. P., Embrey, M. S., Kamel, A., Hawthorne, D. J. & Pettis, J. S. Assessment of chronic sublethal effects of imidacloprid on honey bee colony health. PLoS One 10, e0118748 (2015).
Sasaki, M., Takahashi, H. & Sato, Y. Comparison of the dance dialect and foraging range between Apis mellifera and northern most subspecies of A. cerana in Japan. Honeybee Sci. 14, 49–54 (1993). (in Japanese).
Acknowledgements
We appreciate the following beekeepers, whose contributions of honey and beeswax samples along with details of their hive management were indispensable for this study: K. Adachi, H. Akita, T. Akiyama, S. Amano, H. Asanuma, S. Eto, J. Fujii, M. Fujii, T. Fujii, K. Fujimoto, T. Fukaya, K. Hamano, S. Hara, T. Hara, Y. Hasegawa, I. Hashimoto, N. Hashimoto, Y. Hashimoto, T. Higuchi, T. Hirano, Y. Ibe, K. Ichikawa, H. Ikeda, M. Ishigaki, T. Ishitsuka, H. Ishizuka, G. Isobe, M. Isomi, T. Ito, K. Iwanami, S. Iwase, I. Iwashita, K. Iwata, T. Jinguji, C. Jukai, T. Kakuma, H. Kamakura, T. Kamijo, N. Kamimura, Y. Kamimura, K. Kanehara, R. Kanzaki, H. Kasai, T. Kato, S. Kawamura, T. Kimura, S. Kishinami, Y. Kitafuji, I. Kobayashi, T. Kobayashi, T. Komorita, G. Kondo, K. Kondo, R. Kondo, J. Kosaka, A. Kotaka, M. Kumabe, A. Kumagai, A. Kurogi, T. Maeda, T. Matsuchi, M. Matsuda, T. Matsui, T. Matsuo, S. Matsuoka, K. Minegishi, S. Miura, M. Miyake, A. Miyazawa, O. Mizuno, H. Mogaki, H. Mori, T. Morishita, M. Morita, N. Motozawa, K. Murakami, M. Murakami, K. Murayama, M. Nagao, T. Nagao, K. Nakagawa, S. Nakamura, K. Nakano, M. Nakata, M. Nakayama, M. Nishimura, K. Noda, K. Numajiri, S. Ogasawara, Y. Ogasawara, T. Ogawa, Y. Ogino, S. Okumura, C. Omura, T. Osaka, H. Oshiro, T. Osugi, M. Otagiri, H. Saburi, T. Saika, K. Saito, F. Sakamoto, K. Sakuta, B. Sasaki, A. Sato, K. Sato, R. Sato, S. Seike, T. Seki, T. Shibata, I. Shiga, H. Shiina, M. Shinobe, T. Shinohara, A. Shinomiya, T. Sumita, K. Tabata, H. Tahara, H. Takahashi, Y. Takahashi, A. Takeda, M. Tanaka, S. Tanaka, T. Tanioka, F. Terada, Y. Tokunaga, K. Ueda, K. Ueda, S. Uemura, H. Ueno, M. Watai, R. Yagi, S. Yamamoto, T. Yamamoto, M. Yamamuro, H. Yamana, A. Yamasaki, J. Yamaura, A. Yanai, T. Yano, Y. Yari, N. Yokota, S. Yokota, K. Yoshida, K. Yoshida, W. Yoshida, and S. Yoshioka. We also thank T. Maeda, S. Sasaki, N. Abe, K. Ando, A. Fujiwara, S. Fujiwara, Y. Fujiwara, and K. Ishida for their help in recruiting the collaborators, and other beekeepers who provided information about their colonies in advance (Supplementary Note). We deeply value their expertise and commitment to this project. We are also grateful to N. Hanahara, T. Hirata, K. Yuzawa, and other lab members for arranging samples from beekeepers and for their assistance in organizing data, as well as the members of HeiseiRiken Co., Ltd., including N. Kakinuma and K. Sakamoto, for analyzing the samples and providing useful advice. This work was financially supported by the Ministry of the Environment of Japan through the Environmental Impact Study of Pesticides on Wild Bees project (Y.S., the representative) and JSPS KAKENHI grant no. JP20H00425 (Y.S., the representative).
Author information
Authors and Affiliations
Contributions
This study was conceptualized and supervised by Y.S. and methodology was established through discussions among all authors. M.I. organized the vegetation data. S.H. performed the statistical analysis and modeling with support from M.I. and Y.S. S.H., and M.I. visualized the results. Y.S. wrote the manuscript with significant input from K.G., M.I., and S.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hisamoto, S., Ikegami, M., Goka, K. et al. The impact of landscape structure on pesticide exposure to honey bees. Nat Commun 15, 8999 (2024). https://doi.org/10.1038/s41467-024-52421-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-52421-3
This article is cited by
-
Poor air quality raises mortality in honey bees, a concern for all pollinators
Communications Earth & Environment (2025)
-
Crop pollination by native honey bees (Apis cerana) at risk due to agricultural intensification
Arthropod-Plant Interactions (2025)