Abstract
Discovering antigen-reactive T cell receptors (TCRs) is central to developing effective engineered T cell immunotherapies. However, the conventional technologies for isolating antigen-reactive TCRs (i.e., major histocompatibility complex (MHC) multimer staining) focus on high-affinity interactions between the TCR and MHC-antigen complex, and may fail to identify TCRs with high efficacy for activating T cells. Here, we develop a microfluidic single-cell screening method for antigen-reactive T cells named ATLAS-seq (Aptamer-based T Lymphocyte Activity Screening and SEQuencing). This technology isolates and characterizes activated T cells via an aptamer-based fluorescent molecular sensor, which monitors the cytotoxic cytokine IFNγ secretion from single T cells upon antigen stimulation, followed by single-cell RNA and single-cell TCR sequencing. We use ATLAS-seq to screen TCRs reactive to cytomegalovirus (CMV) or prostate specific antigen (PSA) from peripheral blood mononuclear cells (PBMCs). ATLAS-seq identifies distinct TCR clonotype populations with higher T cell activation levels compared to TCRs recovered by MHC multimer staining. Select TCR clonotypes from ATLAS-seq are more efficient in target cell killing than those from MHC multimer staining. Collectively, ATLAS-seq provides an efficient and broadly applicable technology to screen antigen-reactive TCRs for engineered T cell immunotherapy.
Similar content being viewed by others
Introduction
Cancer immunotherapy is a promising option for chemotherapy-resistant cancers and cancers with poor salvage rates for recurrent disease1. Cancer immunotherapy strategies can be broadly categorized into those that use immune checkpoint inhibitors to augment the suppressed immune response2, and adoptive cell therapy (ACT) strategies that direct or engineer the immune response against tumor-specific antigens. General ACTs include chimeric antigen receptor T cell (CAR-T) therapies, and engineered T cell receptor (TCR) therapies. CAR-T therapies are designed to directly target tumor- or lineage-specific cell-surface proteins by using artificial receptors introduced into immune effector cells3. In contrast, TCR therapies use naturally occurring TCRs to target tumor-specific intracellular proteins that have been processed and presented on the tumor-cell surface via the major histocompatibility complex (MHC) class I antigen presentation pathway. Because there are far more tumor-specific protein sequences within a cell that can be presented by the MHC than there are tumor-specific proteins on the cell surface4, TCR therapies have a potentially broader applicability, especially in the treatment of solid tumors4,5.
In TCR therapies, TCR repertoire profiling and TCR selection are central to treatment development6,7,8. Multiple affinity-based TCR selection technologies are currently available; however, they have significant limitations. For example, MHC multimer technologies use fluorescently-labeled oligomeric MHC molecules formed through a tetrameric agent or dextran backbone to isolate and characterize antigen-specific T cell populations9,10. These MHC multimer assays focus on high-affinity physical/molecular interactions between the TCR and MHC-antigen (peptide-MHC/ pMHC) complex. However, T cell affinity in these assays is not equivalent to T cell efficacy (i.e., how effectively the TCR-pMHC interaction activates T cells)11,12. As TCR efficacy is a crucial criterion for clinical applications of TCR therapy, there is a critical unmet need to develop TCR selection technologies that identify TCRs based on their ability to activate T cells.
Cytokine secretion is an important marker of T cell development and cytotoxic activities13,14,15. New methods are being developed to directly measure the cytokine production capacity of T cells through intracellular cytokine staining16,17. However, these methods use fixed cells to measure intracellular RNA or protein products of cytokines rather than the secretion of cytokines from living cells. Additionally, T cells communicate with surrounding cells in conventional bulk activation assays, which could alter the behavior of specific T cells in such assays18,19 or result in artificial immunosuppression of T cells20. A droplet-based T cell screening technology could potentially provide an enclosed environment for the accurate measurement of each individual T cell activation event through cytokine secretion and prevent communication among neighboring T cells21.
In this work, to address these challenges, we develop aptamer-based T-lymphocyte activity screening and sequencing (ATLAS-seq), which cost-effectively isolates and characterizes activated T cells, using an aptamer-based fluorescent molecular sensor to monitor cytokine secretion from T cells upon antigen stimulation, followed by single-cell sequencing. This microfluidic-based technology uses water-in-oil emulsions to provide a high-throughput platform to examine a single T cell’s activation (assayed by cytokine release) after it interacts with antigen-loaded artificial antigen-presenting cells (aAPCs) in droplets. Cholesterol-modified aptamers22 can be used directly on living cells without loss of viability or need for cellular gene manipulations. ATLAS-seq can efficiently and cost-effectively identify activated antigen-reactive T cells and provide fine-grained identification of TCRs with high-activation potential for immunotherapies.
Results
An IFNγ-specific aptamer beacon for antigen-specific T cell activation
We constructed a membrane-anchored IFNγ aptamer beacon as per a previous report22. Briefly, an aptamer IFNγ recognition unit is extended by a 3’-end 9mer DNA sequence complementary to the 5’-end of the aptamer, which can form a stem-loop structure through self-hybridization. The aptamer is modified with a 5’-Cy3 fluorophore and a 3’-TAO quencher, which is further connected to a cholesterol modification by an iSP18 linker sequence (Fig. 1A). This aptamer beacon can anchor onto the cell membrane through the hydrophobic interaction between cholesterol and phospholipid22. In the absence of IFNγ, the stem-loop structure brings Cy3 and the TAO quencher in close proximity, quenching the fluorescence. Upon binding with IFNγ, the aptamer beacon adopts a tertiary structure that separates Cy3 from the TAO quencher, allowing fluorescence signal emission.
A Stem-loop structure of IFNγ-specific aptamer beacon. The 5’ end of the aptamer is decorated with the Cy3 fluorophore. The 3’ end of the aptamer is decorated with the TAO quencher, iSP18 linker and cholesterol. B MART1 or NY-ESO-1 peptide-loaded aAPCs were added to 5 × 105 cells/well DMF5 Jurkat cells or control (CTRL) Jurkat cells decorated with the IFNγ-specific aptamer beacons (1nmol/106 cells) at 2:1 ratio in 96-well plate. Top: Representative images of control or DMF5 Jurkat cells treated with MART1 aAPCs on day 2. Bottom: Average cellular fluorescence intensities of the aptamer beacons were measured to detect IFNγ secretion due to MART1 antigen-specific DMF5 Jurkat T cell activation. mean ± s.d. of n = 3 independent biological replicates. Arb. Units: arbitrary units. C Control (CTRL) Jurkat and DMF5 Jurkat cells were decorated with IFNγ-specific aptamer beacons (1nmol/106 cells) and co-encapsulated in droplets with MART1 peptide-loaded aAPCs or empty aAPCs for 2 days at 37 °C, 5% CO2. Fluorescence intensities of aptamer beacons were measured by flow cytometry to analyze the IFNγ secretion from MART1 antigen-specific activated single Jurkat cells in droplets. Aptamer beacon-decorated control Jurkat cells: with empty aAPCs (green); with MART1 peptide-loaded aAPCs (orange); with aptamer cDNA (red). Aptamer beacon-decorated DMF5 Jurkat cells: with empty aAPCs (gray); with MART1 peptide-loaded aAPCs (dark blue); with aptamer cDNA (light blue). Aptamer cDNA: DNA oligo with a complementary sequence to the aptamer stem region. D ATLAS-seq workflow. A single aptamer-labeled T cell is co-encapsulated with antigen peptide-loaded aAPC beads within uniformly-sized water-in-oil microdroplets using a cross junction channel in a microfluidic chip. Droplets are incubated off-chip to accumulate IFNγ secreted from the encapsulated single T cell. Secreted IFNγ will switch on the Cy3 fluorescence signal of the aptamer beacon on the T cell surface. After breaking the emulsion, recovered T cells are analyzed and sorted by flow cytometry based on the intensity of Cy3 fluorescence signal. CD8+/Cy3high T cells are used for preparing both single-cell TCR-seq and single-cell RNA-seq libraries using 10X Genomics Chromium Controller. Source data are provided as a Source Data file.
To evaluate the cytotoxicity of the aptamer used in ATLAS-seq, we performed MTS assays23 on Jurkat and the TCR DMF5 expressing Jurkat cells24,25 decorated with different amounts of IFNγ aptamer beacon. MTS assay is a colorimetric method for determining the number of viable cells in culture, which is proportional to the conversion of the tetrazolium compound MTS to formazan as measured by 490 nm absorbance23. The results showed that varying amounts of IFNγ aptamer beacon (0.5, 1 and 2 nmol/106 cells) do not reduce the viability of either Jurkat or DMF5 Jurkat cells (Fig. S1A), indicating no cytotoxicity or cytostatic effect of IFNγ aptamer beacon on the decorated cells up to 2 nmol/106 cells.
To test the specificity of the aptamer, we used artificial antigen-presenting cell beads (aAPCs: superparamagnetic epoxy beads coupled with human HLA-A2-Ig dimer and anti-CD28Ab26,27) and Jurkat cells as a model system. Melanoma-specific MART1 peptide (ELAGIGILTV) was presented by aAPCs and can be specifically recognized by the TCR DMF524. DMF5 expressing Jurkat cells with or without IFNG gene knockout (IFNGKO) were decorated with the IFNγ-specific aptamer beacon and co-incubated with MART1 peptide-loaded aAPCs (MART1 aAPC) for 2 days. Only wild-type DMF5 Jurkat cells exhibited fluorescence signal increase from the aptamer beacon upon activation by MART1 aAPC, while IFNGKO DMF5 Jurkat cells showed no detectable release of IFNγ or significant fluorescence signal increase from the aptamer beacon upon activation by MART1 aAPC (Fig. S1B), indicating the aptamer beacon is specific to IFNγ.
To test the sensitivity of the aptamer, we treated IFNγ aptamer beacon decorated IFNGKO Jurkat cells with different concentrations of IFNγ. The IFNγ-specific aptamer can emit detectable fluorescence with as low as ~10 nM IFNγ (Fig. S1C), which is consistent with previously reported results22,28.
To test the reporter function of the IFNγ-specific aptamer in antigen-specific T cell activation, Jurkat cells with or without DMF5 expression were decorated with the IFNγ-specific aptamer beacon and co-incubated with MART1 or NY-ESO-1 peptide-loaded aAPCs. The negative control NY-ESO-1 peptide (SLLMWITQC, JPT Peptide Technologies) is a widely used HLA-A*02:01 restricted cancer-testis antigen (CTA) that does not trigger the activation of DMF5 expressing T cells. Only MART1 aAPCs can elicit significant increase in fluorescence on IFNγ aptamer decorated DMF5 Jurkat cells (Fig. 1B, bottom right, blue line). In contrast, aptamer decorated DMF5 Jurkat cells challenged with NY-ESO-1 aAPC only emit low baseline fluorescence (Fig. 1B, bottom right, orange line), which is comparable to those on the control Jurkat cells that cannot be activated by either MART1 or NY-ESO-1 aAPCs (Fig. 1B, bottom left). When using antigen-presenting T2 cells29,30 to present MART1 peptides to Jurkat cells with or without DMF5 expression, significantly higher fluorescence signal was also observed on DMF5 Jurkat cells than the baseline fluorescence signal detected on control Jurkat cells (Fig. S1D). These results suggest that the IFNγ-specific aptamer beacon can be used to efficiently and specifically detect IFNγ secretion from antigen-specific T cells activated with either antigen-presenting aAPC beads or T2 cells.
Microfluidic co-encapsulation of T cells and antigen-presenting cells
We hypothesized that IFNγ specific aptamer beacon could be used to distinguish the antigen-activated T cells from non-activated T cells when single T cells are co-encapsulated with antigen-presenting aAPCs or T2 cells in water-in-oil droplets. Co-encapsulation spatially confines the IFNγ secreted from each T cell and prevents cross-binding of IFNγ to the aptamers located on non-activated T cells.
We designed and optimized the single T cell encapsulation procedure based on COMSOL multiphysics simulations. Droplets generated from the single T cell encapsulation procedure were examined using a machine vision system based on a FLIR high-speed camera (Fig. S2A left and middle). With optimized settings, among all generated droplets, 19% contained a single T cell, 2% contained multiple T cells, and 79% were empty (Fig. S2A right).
We chose to use a two-day co-incubation for T cell activation with antigen-presenting cells, based on results in Fig. 1B. For single-cell sequencing and sample preparation efficiency, a minimum of 70% T cell viability is required. Encapsulated T cells show reduced viability due to oxygen deprivation when incubated in a thick layer of water-in-oil droplets. To maximize encapsulated T cell viability after a two-day incubation, we compared T cell viabilities after incubation in different thicknesses of the T cell-containing droplet layer. Droplet layer thicknesses of 3 mm or less resulted in >75% T cell viability (Fig. S2B). Thus, 3 mm was chosen as the maximum thickness for the collected droplet layer for incubation.
To compare the performances of T2 cells and aAPCs in the encapsulation process, we simulated their droplet encapsulation statistics in cell culture media. Because the density of a cell is between 1 to 1.3 g/mL and similar to the cell culture media (~1 g/mL), cells are distributed randomly in culture media suspension, and the number of cells per droplet follows Poisson statistics31. According to the simulation, the number of encapsulated T2 cells in each droplet ranges from 0 to 8 cells per droplet, depending on the input T2 cell concentrations (Fig. S2C left) and a large proportion of the droplets (10–50%) will contain no T2 cells (Fig. S2C left). In contrast, the density of an aAPC bead is ~2 g/mL and the diameter (4.5μm) is much smaller than a T2 cell (12μm). aAPC beads are organized as close-packed spheres before entering the site of droplet formation, which allows the number of beads per droplet to be proportional to the Gaussian distributed droplet size32. According to the simulation, the number of encapsulated aAPC beads in each droplet is approximately equal and much higher than the number of T2 cells when using the same input concentration (Fig. S2C right). These results suggest aAPC beads are more efficient than T2 cells in the droplet co-encapsulation process. Also, using magnetic aAPC beads, rather than T2 cells, will make the separation of selected T cells in downstream applications more efficient.
To co-encapsulate single CD8+ T cell with aAPC beads, we designed a microfluidic chip for droplet generation (Fig. S2D). In this chip, two inputs are simultaneously injected into the chip with the same flow rate: (1) aptamer beacon-decorated CD8+ T cells in media suspension without IL-2; and (2), antigen peptide-loaded aAPC beads in media suspension with IL-2. The two aqueous streams are merged and cut into ~120μm diameter droplets by injected oil flow.
High-throughput selection of activated antigen-specific T cells using ATLAS-seq
We tested the utility of our optimized set-up by using an IFNγ-specific aptamer beacon to identify single T cell activation in droplets. Jurkat cells with or without DMF5 expression were decorated with IFNγ-specific aptamer beacon and co-encapsulated, in single-cell droplets, with aAPC beads loaded either with or without MART1 peptides. DNA oligos complementary to the IFNγ-specific aptamer beacon stem region (5′-AGGGGTTGGACACAACACCCAACACAACCAACCCCT-3′; referred to as “cDNA” in Fig. 1C) were used to treat the aptamer beacon-decorated Jurkat cells as a reference for the range of maximum fluorescence signal that can be emitted from the aptamer beacons on these cells. After a two-day incubation, the Jurkat cells were released from the droplets and the fluorescence signal on the surface of each cell was measured by flow cytometry. The fluorescence signals on DMF5 Jurkat cells cocultured with MART1 aAPCs (Fig. 1C, dark blue) were significantly higher than on those cocultured with empty aAPCs (Fig. 1C, gray), and closer to the maximum fluorescence signal range indicated by the “cDNA” treatments (Fig. 1C, light blue). In contrast, the fluorescence signals on control Jurkat cells with or without MART1 aAPCs remained low, at around baseline levels (Fig. 1C, orange and green).
We established a high-throughput aptamer-based T-lymphocyte activity screening and sequencing (ATLAS-seq) workflow to efficiently select highly reactive antigen-specific TCRs (Fig. 1D). Briefly, we used the microfluidic chip to generate droplets co-encapsulating aptamer decorated single CD8+ T cells and antigen-loaded aAPCs. Generated droplets were collected and incubated for 2 days to allow T cell activation by antigen-loaded aAPCs. An activated T cell containing the cognate TCR for the target antigen pMHC may release IFNγ molecules into the same droplet which, in turn, bind to the IFNγ-specific aptamer beacons on the same T cell surface and emit Cy3 fluorescence signals. The droplet emulsions were then broken to release aptamer-labeled CD8+ T cells for fluorescent sorting using flow cytometry. CD8+Cy3high T cells were then collected and subject to single-cell TCR and RNA sequencing (scTCR-seq and scRNA-seq), prepared using a 10X Genomics single-cell sequencing system.
ATLAS-seq is an efficient method to screen for antigen-specific T cells
To evaluate the efficiency of ATLAS-seq for screening antigen-specific T cells, we measured the recovery rate and the accuracy of antigen-specific T cell selection in spike-in samples with different known abundances of antigen-specific T cells. We generated GFP expressing DMF5 Jurkat cells to be spiked into non-GFP control Jurkat cells at ratios of 1:10, 1:100, 1:1,000 and 1:10,000. GFP-positive cell fractions represent the actual abundance of DMF5 Jurkat cells in the input populations. After being decorated with the IFNγ-specific aptamer beacons, the spike-in cell mixtures were used in ATLAS-seq to be co-encapsulated with MART1 aAPC beads, and the GFP+/Cy3high cell fraction was selected (Fig. S3A, middle). As a comparison, the same spike-in cell mixtures were also subject to Dextramer staining using MART1 Dextramer and the GFP+/PE+ cell fraction was selected (Fig S3B, bottom). The selection recovery rate is defined as the selected DMF5 Jurkat cells in all DMF5 Jurkat cells, and the selection accuracy is defined as the fraction of selected DMF5 Jurkat cells in all selected cells. Both ATLAS-seq and Dextramer staining showed >95% selection recovery rate and >95% selection accuracy at a spike-in abundance as low as 1: 1,000. However, at 1:10,000, both methods showed slightly reduced recovery rate and the accuracies dropped to ~80% (Fig. S3C and S3D). In addition, spike-in samples were decorated with IFNγ specific aptamer beacons and co-incubated, in bulk, with MART1 aAPCs for 2 days and the GFP+/Cy3high cell fraction was selected. (Fig. S3A, bottom). Compared to the results of single-cell droplet-based ATLAS-seq, the bulk assays showed significantly lower recovery rate (50% − 80%, Fig. S3C) and drastically reduced accuracy as spike-in abundance decreases, from 38% at 1:10 spike-in abundance, down to 0.3% at 1:10,000 spike-in abundance (Fig. S3D). This result shows that the bulk assay format is not suitable for using aptamer beacon to screen antigen-specific T cells.
ATLAS-seq recovers a distinct CMV pp65 antigen-specific TCR population from the Dextramer assay
As a proof-of-principle study, we used ATLAS-seq to screen for CMV pp65 antigen-specific T cells. CMV pp65 peptide NLVPMVATV is an HLA-A*02:01 restricted antigen peptide. We isolated CD8+ T cells from the PBMCs of a CMV-positive donor (Donor# 402, Male, Cellular Technology Limited) and expanded them with CD3/CD28 beads. After removing CD3/CD28 beads and resting, these expanded CD8+ T cells were used as the input for either Dextramer staining or ATLAS-seq, followed by TCR clonotype profiling (Fig. 2A and S5A). Here, in ATLAS-seq, the input CD8+ T cells were decorated with the IFNγ-specific aptamer beacon and co-encapsulated with CMV pp65 peptide (NLVPMVATV)-loaded aAPCs in single-cell droplets for two days before fluorescence-activated cell sorting (FACS); parallelly in Dextramer staining, input CD8+ T cells were also cocultured with the same antigen peptide-loaded aAPCs in bulk for two days before CMV pp65 MHC dextramer staining and cell sorting. Here, the “two-day incubation” is a protocol modified from the conventional long-term 2-week antigen stimulation protocol33,34 (Fig. S8C). The two-day protocol efficiently prevented the potential bias introduced by CMV immunodominance35 (Fig. S7). Two independent replicates were performed for each method. As negative controls, we also performed two addition ATLAS-seq experiments, using 1) CD8+ T cell from CMV-negative donor (Donor# 571, female, Cellular Technology Limited) and CMV aAPC (Fig. S5C) 2) CD8+ T cell from CMV-positive donor (Donor# 513, Female, Cellular Technology Limited) and PSA-aAPC (Fig. S5D). Here PSA aAPC is used as a negative control peptide that will not elicit CMV pp65-specific activation of the CD8+ T cells, nor will it elicit any PSA-specific response as these CD8+ T cells were from a healthy female donor. Both control screens resulted in minimum number of CD8+/Cy3high cells that are not enough to be collected for downstream analyses.
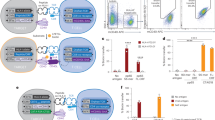
A Flow cytometry analysis for selecting CMV antigen-specific CD8+ T cells using Dextramer staining or ATLAS-seq. T cells from the same CMV+ donor human PBMCs were stimulated with aAPCs carrying the cognate peptide CMV pp65 (NLVPMVATV)/HLA-A2 complex and subjected to either Dextramer staining or ATLAS-seq. CD8-Pacific Blue/PE (Dextramer staining) or CD8-Pacific Blue/Cy3 (ATLAS-seq) double-positive cells were selected using each method for 10X Genomics single-cell sequencing. Input: unstimulated T cells from the same donor PBMCs. Gating strategies are demonstrated in supplementary information (Input: Fig. S4A, Dextramer: Fig. S4B, ATLAS-seq: Fig. S4C). B Average proportions of the flow cytometry-selected TCR clonotypes with at least two cells from the two replicates of Dextramer staining or ATLAS-seq. Remaining panels (C)–(G) are based on these flow cytometry-selected TCR clonotypes with at least two cells from the two replicates of Dextramer staining or ATLAS-seq. C Overlaps of the TCRα or TCRβ clonotypes from the two methods. D Chord diagrams of Vα-Jα and Vβ-Jβ combinations from the TCR clonotypes. The upper and lower parts of each semicircle represent V and J gene segments, respectively. Top 10 abundant V-J combinations are labeled. E Overlaps of the TCRα or TCRβ CDR3 amino-acid sequences from the two methods. F Amino-acid length distributions of TCRα or TCRβ CDR3s. P-values of CDR3 length distributions from the two methods were calculated using two-tailed Mann–Whitney U test. G Amino acid sequence motifs of TCRα or TCRβ CDR3s identified by GLAM2. CMV+ donor: Donor# 402, male, Cellular Technology Limited. Source data are provided as a Source Data file.
scTCR profiling following Dextramer staining detected 1547 clonotypes from 2035 T cells in replicate 1 and 1474 clonotypes from 1938 T cells in replicate 2. On average, 19.5 ± 0.21% of the clonotypes in each replicate had at least two cells (Fig. 2B). scTCR profiling using ATLAS-seq detected 1412 clonotypes from 2070 T cells in replicate 1 and 622 clonotypes from 939 T cells in replicate 2. On average, 24 ± 0.28 % of the clonotypes in each replicate had at least two cells (Fig. 2B). The TCRα and TCRβ genes identified using the two methods were largely different, with only 17.2% of TCRα genes (86/500) and 18.5% of TCRβ genes (92/498) from the ATLAS-seq analysis being shared with TCRs from Dextramer staining (Fig. 2C).
Analyses of TCRα and TCRβ V, J segment usage in clonotypes detected by Dextramer staining and ATLAS-seq revealed they shared similar usage. For example, among the top 10 TRAVs from each method, 8 are overlapped: TRAV19, TRAV21, TRAV12-2, TRAV13-1, TRAV3, TRAV14/DV4, TRAV29/DV5, and TRAV17. The top-5 TRBVs from each method were the same: TRBV27, TRBV9, TRBV7-9, TRBV28, and TRBV6-5. The top TRBJ in each method is also the same: TRBJ2-7 (Fig. 2D). Similar V, J segment usages in CMV-specific TCR clonotypes have been reported in previous research36,37,38, where the above V, J segments were the major segments detected in CMV-specific CD8+ T cells from donors of different ages and genders.
The TCRα and TCRβ CDR3 amino-acid (AA) sequences detected by Dextramer staining and ATLAS-seq were distinct, with only 17.6% of TCRα CDR3s (88/500) and 18.7% of TCRβ CDR3s (93/498) from ATLAS-seq being shared with TCRs from Dextramer staining (Fig. 2E). The AA length distributions from the two methods were similar (p-value > 0.05 by two-tailed Mann-Whitney U test) with most being 14 and 15 AAs in length (Fig. 2F), consistent with the TCRβ AA length distribution from patients with repeated CMV reactivation reported in previous research39. We used GLAM240 to identify the consensus AA sequence of TCRα and TCRβ 15 AA-long CDR3s from each method (Fig. 2G). For Dextramer staining, we identified N-terminal “CAVS” and C-terminal “KLI/TF” motifs in TCRα CDR3, and N-terminal “CASS” and C-terminal “N/YEQYF” motifs in TCRβ CDR3, which have also previously been reported in CMV-specific TCR CDR3 sequences36,39,41. The AA sequences identified using ATLAS-seq had largely distinct central regions, while possessing similar terminal sequences to those identified using Dextramer staining.
In addition, we also performed the ATLAS-seq and Dextramer assay on the CD8+ T cells from the PBMCs of a second CMV+ donor (Donor# 513, Female, Cellular Technology Limited) (Fig. S5B and S6A). Similar to the first CMV+ donor, these results also showed largely different clonotypes detected by Dextramer staining and ATLAS-seq (Fig. S6B), while clonotypes detected by two methods shared similar TCRα and TCRβ V, J segment usages (Fig. S6C) and TCRα and TCRβ CDR3 AA sequence lengths (Fig. S6D). The AA sequences identified using ATLAS-seq also had largely distinct central regions, while possessing similar terminal sequences to those identified using Dextramer staining (Fig. S6E).
In summary, Dextramer staining and ATLAS-seq selected distinct CMV pp65 antigen-specific TCR populations. The differences between CMV-specific TCR clonotypes selected by the two methods mainly result from different V-J combinations and the central regions of the CDR3 sequences, while V, J segment usages, CDR3 lengths, and the terminal regions of the CDR3 sequences were similar.
ATLAS-seq selects CMV pp65-specific CD8+ T cells with high activation levels
We also prepared libraries for scRNA-seq from the CD8+ T cells used for scTCR profiling and analyzed the data using 10X Genomics Cell ranger42 and Seurat43. In the Dextramer staining (n = 2 replicates) and ATLAS-seq (n = 2 replicates) inputs, we grouped all individual CD8+ T cells into distinct clusters, based on gene expression, through dimensionality-reduction UMAP (Fig. 3A). Clusters (Fig. 3A, gray) lacking TCR information are mainly cells with a high proportion of mitochondrial gene transcripts (filtered with Seurat, indicating low quality cells43). We also projected the TCR clonotype status (presence/absence) of each individual cell onto these cell clusters (Fig. 3B). Only cells with TCR information were used for downstream analyses (Fig. 3B, blue).
Five experiments were analyzed: input, Dextramer-staining selected (2 replicates) and ATLAS-seq-selected (2 replicates). A UMAP maps showing identified cell clusters. The major cell clusters (C1-C5) are colored. Pie charts: the proportions (%) of different cell clusters. B TCR clonotypes status (presence/absence) from scTCR-seq are projected onto the corresponding scRNA-seq UMAP maps. Cells with TCR clonotype information are colored blue. Pie charts: the proportions (%) of cells with TCR clonotype information. C Normalized average expression level of T cell marker genes for the 5 major cell clusters in (A). D Volcano plot shows the differential activities of the immune regulation pathways in the major CD8+ T cell clusters in (A). Green: pathways with activities significantly higher in ATLAS-seq hits than in Dextramer staining hits (fold change >10 and adj p-value < 0.01). Red: pathways with activities significantly lower in ATLAS-seq hits than in Dextramer staining hits (fold change < −10 and adj p-value < 0.01). Adj p-values are calculated by two-tailed t test with Benjamini–Hochberg correction. The table: immune regulation pathways with highly differential activities in TCRs selected using the two methods. CMV+ donor: Donor# 402, male, Cellular Technology Limited. Source data are provided as a Source Data file.
We used 12 gene markers of T cell activation, differentiation, and exhaustion14,44,45,46,47 and calculated their normalized average expression level48 to evaluate the CD8+ T cell phenotypes from the major clusters of different samples (Cluster 1-5, Fig. 3A, C). All clusters had negligible expression of PDCD1, which is responsible for both T cell exhaustion and negative regulation of the activation of antigen-specific T cells49,50. Expression of IL7R (CD127), a marker of T cells with lower exhaustion status49,50, was significantly higher in both Dextramer staining (Cluster 2 and 3) and ATLAS-seq (Cluster 4 and 5) selected CD8+ T cells, compared to input (Cluster 1). Selected CD8+ T cells from both Dextramer staining and ATLAS-seq showed lower expression levels of IL2RA (CD25) and CCR7, compared to input, consistent with previous reports that IL2RA (CD25) and CCR7 are down-regulated in activated effector T cells14,44,51. HLA-DRA, a marker of activated CD8+ T cells, showed higher expression in the selected CD8+ T cells14,44 (Fig. 3C). Thus, T cells selected by both methods appear to be activated without signs of exhaustion.
To compare the antigen-specific activation status between Dextramer staining and ATLAS-seq selected CD8+ T cells, we used Seurat43 to integrate scRNA-seq data from both methods, then analyzed the differential immune regulation pathway activities with the SCPA package52. A total of 117 immune regulation pathways were identified from Gene Ontology53, Reactome Pathway54, Human Molecular Signatures55, WikiPathways56, Pathway Interaction57, and KEGG databases58. Thirty-four pathways had significantly higher activities and 32 pathways had significantly lower activities in ATLAS-seq selected CD8+ T cells compared to Dextramer-staining selected CD8+ T cells (Fig. 3D). In particular, the activities of several pathways that positively regulate cytokine production and secretion (especially IFNγ) were significantly higher in ATLAS-seq selected CD8+ T cells (pathway #1, 3, 4, 5). These cells also exhibited higher activity for pathways that positively regulate antigen responses (pathway #2) and cell killing (pathway #6) (Fig. 3D). In contrast, several pathways that negatively regulate cytokine production (pathway #9) and T cell activation (pathway #11) had significantly higher activities in CD8+ T cells selected using Dextramer staining (Fig. 3D). Thus, based on single-cell RNA expression profiles, ATLAS-seq was able to select specific CD8+ T cells with higher reactivities to an antigen CMV pp65 peptide-MHC stimulation compared to the CD8+ T cells selected using Dextramer staining.
ATLASs-seq selected TCR clonotypes are more efficient for CMV positive target cell killing
We compared CMV-specific TCR clonotypes selected by Dextramer staining and ATLAS-seq to further evaluate their target cell killing potentials. In these analyses we only used CD8+ T cells belonging to one of the major clusters (Fig. 3A) with complete α, β paired TCR information (Fig. 3B). Only clonotypes with at least 2 cells in at least one replicate of each method were kept for further analysis. A total of 358 TCR clonotypes were selected using Dextramer staining, and a total of 256 TCR clonotypes were selected using ATLAS-seq. We defined “high-priority” CMV-specific TCR clonotypes as those with at least a total of 4 cells in the two replicates of each method. We identified a total of 80 high-priority clonotypes using Dextramer staining and 45 high-priority clonotypes using ATLAS-seq (Fig. 4A). Consistent with the earlier observation that the TCRα and TCRβ genes identified using the two methods were largely different (Fig. 2C), these “high-priority clonotypes” selected by ATLAS-seq only has a total of 9 clonotypes shared with those selected by Dextramer staining (~11%, Supplementary Data 3 and 4). We focused our analyses on these “high-priority” clonotypes to avoid the potential noise introduced by low abundance clonotypes.
High-priority CMV-specific TCR clonotypes: with at least a total of 4 cells in the two replicates of each method. A Proportions of high-priority CMV-specific TCR clonotypes of each method. CMV-specific TCR clonotypes with at least 2 cells in at least one replicate were used as background. B Violin plots (median, interquartile range) showing the following comparisons of high-priority CMV-specific TCR clonotypes of each method indicated in (A): clonotype proportions, cytotoxic cytokine index (IFNγ, TNF, and LTα), TCR-pMHC binding score, cytotoxic protein index (GZMB and PRF1), cytotoxic chemokine index (CCL3, CCL4 and CCL5) and 4-1BB/4-1BBL index (4-1BB: aka CD137/TNFRSF9; 4-1BBL: aka CD137L/TNFSF9). Box plots feature median (center line), first and third quantiles (box boundaries), and minimum and maximum excluding outlier data points beyond 1.5x interquartile range from the first and third quartiles (whiskers). n = 80 for Dextramer staining and n = 45 for ATLAS-seq. P-values were calculated by 2-tailed t test. CMV+ donor: Donor# 402, male, Cellular Technology Limited. Source data are provided as a Source Data file.
We defined four “cytotoxicity indexes”. The “cytotoxic cytokine index” is the normalized average RNA expression level of three cytotoxic cytokines: IFNγ, TNF, and LTα59,60,61. The “cytotoxic protein index” is the normalized average RNA expression level of two cytotoxic proteins: GZMB and PRF146,62. The “cytotoxic chemokine index” is the normalized average RNA expression level of three chemokines related to target-specific T cell cytotoxicity: CCL3, CCL4 and CCL546,63,64,65.The “4-1BB/4-1BBL index” is the normalized average RNA expression level of two proteins related to antigen-specific T cell activation signaling: 4-1BB (aka CD137/TNFRSF9) and its ligand 4-1BBL (aka CD137L/TNFSF9)66,67,68. These four cytotoxicity indexes were used as proxies for quantifying the cytotoxicity of T cells with a specific TCR clonotype. We calculated the proportions, cytotoxic cytokine indexes, cytotoxic protein index, cytotoxic chemokine index, 4-1BB/4-1BBL index and TCR-pMHC binding scores69 of the high-priority clonotypes from each method (Fig. 4B, S7A, B). The clonotypes derived from ATLAS-seq showed higher proportions and higher cytotoxicity indexes in the selected CD8+ T cells than those derived from Dextramer staining, whereas there was no significant difference between their TCR-pMHC binding scores. Thus, there is an increased clonotype proportion and enrichment for higher cytotoxicity clonotypes when using ATLAS-seq. This is in concordance with the observation that cytotoxicity-related cytokine production and secretion pathway activities were significantly different between Dextramer-staining and ATLAS-seq selected cells (Fig. 3D). In addition, we also analyzed the cytotoxicity indexes of all TCR clonotypes with at least 2 cells detected by Dextramer staining and ATLAS-seq from a second CMV positive donor. The results also showed an increased clonotype proportion and enrichment for higher cytotoxicity clonotypes when using ATLAS-seq, while no significant difference between TCR-pMHC binding scores from these two different methods (Fig. S6F).
We measured the on-target killing potentials of selected CMV-specific TCR clonotypes, choosing clonotypes with top abundances (base on clonotype proportion) or top cytotoxic cytokine indexes (based on scRNA-seq) from the two methods (Supplementary Data 1). For both methods, we selected the 5 most abundant clonotypes: D1, D2, D3, D4 and D5 for Dextramer staining, and A1, A2, A3, A4 and A5 for ATLAS-seq. We also selected the 5 clonotypes with the highest cytotoxic cytokine indexes: D67, D10, D77, D47 and D79 for Dextramer staining and A41, A3, A2, A14, and A42 for ATLAS-seq (in descending order, Fig. S8A, B). Some clonotypes with the highest cytotoxic cytokine indexes also have the highest clonotype proportions, such as A2 and A3 (Fig. S8B). We used TCR α and β gBlocks™ Gene Fragments to clone the CMV-specific TCR clonotypes into retroviral vectors and overexpressed them in CMV-negative human PBMCs, using RetroNectin transduction.
PBMCs transduced with CMV-specific TCRs were activated using CMV pp65 peptide (NLVPMVATV)-loaded aAPC beads and cocultured for 3 days with a PC3 epithelial prostate cancer cell line expressing GFP, HLA-A*02:01, and CMV pp65 peptide17 (PBMC:PC3 cell ratio = 10:1, Fig. 5A, B). CMV-specific cytotoxicity was visualized by measuring loss of green fluorescence in the GFP-expressing PC3 cells, revealing that all clonotypes except A42 from ATLAS-seq had better killing performances than all clonotypes from Dextramer staining (Fig. 5A). There was no detectable killing observed from un-transduced (UT) PBMCs or TCR-transduced PBMCs co-cultured with an unrelated SK-N-AS neuroblastoma cell line that does not express CMV pp65 peptide (Fig. 5A).
A Target cell killing assays: Activated TCR-transduced PBMCs were cocultured for 72 h with target CMV+ GFP+ PC3 cells (GFP+ PC3 cells expressing HLA-A2 and CMV pp65) or unrelated neuroblastoma SK-N-AS cells (negative for CMV pp65 peptide). Relative viability of CMV+ PC3 cells is measured by GFP fluorescence using the Incucyte system. Each dot: mean of n = 3 independent biological replicates. B Images of GFP-expressing CMV+ PC3 cells after 72-h coculturing with TCR A2, A3, A14, DL and D10 transduced PBMCs or un-transduced (UT) PBMCs. CMV+ GFP+ PC3 target cells are shown in green. Scale bar: 400μm. C Box plots showing comparison of target killing efficiencies (72-h time point) of select CMV-specific clonotypes from Dextramer staining and ATLAS-seq. Box plots feature median (center line), first and third quantiles (box boundaries), and minimum and maximum excluding outlier data points beyond 1.5x interquartile range from the first and third quartiles (whiskers). Each data point represents the target killing efficiency of a TCR-transduced PBMC sample (n = 11 for Dextramer staining clonotypes and n = 8 for ATLAS-seq clonotypes). P-values were calculated by 2-tailed Mann–Whitney U test. **p ≤ 0.01, ***p ≤ 0.001. D Secreted IFNγ and TNF concentrations after the 72-h cell killing assay. Conditioned media in the same wells were measured by IFNγ and TNF ELISA (mean ± s.d. of n = 3 independent biological replicates). Source data are provided as a Source Data file.
For comparison, we also tested the CMV-specific cytotoxicity of a predominant clonotype (DL, 67%, Fig. S8C), selected using Dextramer staining of PBMCs from the same donor. These PBMCs went through a conventional long-term 2-week antigen-stimulation, instead of the two-day antigen-stimulation before the Dextramer staining in all other parts of this study. The killing efficiency of clonotype DL fell on the higher end of the range of killing efficiencies of the clonotypes from Dextramer staining but was still less than most clonotypes from ATLAS-seq (Fig. 5A). TCR clonotypes with significant cytotoxicity show visible T cell clustering70 and less GFP signal from GFP+ PC3 cells (Fig. 5B). The tested clonotypes from ATLAS-seq had significantly higher target killing efficiency (p-value = 3.4E−3) than those from Dextramer staining (Fig. 5C).
We measured the concentrations of secreted IFNγ and TNF in the conditioned media after the 3-day killing assay, using ELISA (Fig. 5D). PBMCs transduced with TCR clonotypes from both methods showed significant secretion of IFNγ and TNF when co-incubated with the CMV+ PC3 cells, which is in contrast to co-incubation with the CMV- SK-N-AS negative control cells. However, no significant differences in secreted IFNγ or TNF concentrations were observed between the clonotypes from the two methods (Fig. S8E). In summary, compared to Dextramer staining, ATLAS-seq was able to enrich CMV pp65-specific TCR clonotypes that elicit more efficient target cell killing.
ATLASs-seq selected PSA-specific TCR clonotypes can efficiently target the PSA-positive LNCaP cells
To further demonstrate the utilities of ATLAS-seq, we used ATLAS-seq to screen Prostate Specific Antigen (PSA)-specific TCRs from healthy donor PBMCs and validated their cancer killing functions by targeting a prostate cancer cell line LNCaP, which is positive for PSA expression71,72.
Specifically, we primed T cells from healthy donor PBMCs (Donor# 500, male, HLA-A*02:01, Cellular Technology Limited) with PSA peptide (VISNDVCAQV) loaded aAPC beads. Then the T cells were expanded with CD3/CD28 beads. After removing CD3/CD28 beads and resting, expanded CD8+ T cells were isolated and used as the input for either Dextramer staining or ATLAS-seq. Due to low target cell capture (0.053% from the input cell population), Dextramer assay failed to collect sufficient CD8+ T cells for downstream single-cell TCR-seq and did not identify any PSA-specific TCR clonotype (Fig. S5E). In contrast, ATLAS-seq resulted in 3.19% of CD8+/Cy3high T cells, from which we identified 51 PSA-specific TCR clonotypes. The clonotype features are shown in Fig. S9.
We then cloned the top 5 most abundant clonotypes (P1, P2, P3, P4 and P5) and the top 5 clonotypes with the highest cytotoxic cytokine indexes (P17, P40, P14, P38 and P30) (Fig. 6A, Supplementary Data 5). We tested the target killing abilities of these clonotypes using LNCaP as HLA-A*02:01 PSA positive target cell line, as well as HLA-A*02:01 expressing PC3 cell line as a PSA negative control cell line. All 10 clonotypes, regardless the inclusion criteria (clonotype proportion vs cytotoxic cytokine indexes), exhibited high PSA+ LNCaP target cell killing activities (85–93%, Fig. 6B, C). These clonotypes also showed PSA-specific cytotoxic cytokine secretion (Fig. 6D) when compared to the coculture with PSA-negative HLA-A*02:01 expressing PC3 cells. These results highlighted the flexibility, adaptability, and performance of ATLAS-seq in identifying cancer antigen-specific TCRs.
A ATLAS-seq selected clonotypes sorted according to clonotype proportions (top) and cytotoxic cytokine indexes (bottom). PSA-specific TCRs used for target cell killing assay are highlighted and labeled. B Target cell killing assays: TCR-transduced PBMCs were activated and then cocultured for 72 h with GFP expressing HLA-A*02:01 cell lines LNCaP (PSA positive target) or PC3 cells (PSA negative control). Relative viability of target cells was measured by GFP fluorescence using the Incucyte system. Each dot: mean of n = 3 independent biological replicates. C Images of representative GFP/ HLA-A*02:01 -expressing LNCaP and PC3 cells after 72-h coculture with TCR P1 and P40 transduced PBMCs or un-transduced (UT) PBMCs. Target cells are shown in green. Scale bar: 400μm. D Secreted IFNγ and TNF concentration after the 72-h cell killing assay. Conditioned media in the same wells were measured by IFNγ and TNF ELISA (mean ± s.d. of n = 3 independent biological replicates). +PC3: The mean measurement of cytokines in the conditioned media of cocultures of PC3 cells (PSA negative control) with all 11 tested clonotypes. Source data are provided as a Source Data file.
Discussion
In this study, we have developed a microfluidic-based antigen-specific single T cell activation screening and TCR clonotype profiling workflow, which uses IFNγ secretion levels as an indicator of T cell activation for selection. Single T cell droplets generated by microfluidics provided an encapsulated environment, preventing communications (e.g., diffusion of secreted cytokines) and cross-reactions between T cells during their response to antigen stimulation when done in a bulk assay, allowing the efficient and accurate identification of the activated T cell population (Fig. S3A, C and D). Isolated droplets can also provide a model system to mimic tumor microenvironments73.
Cell surface markers such as CD137 (4-1BB) were previously reported to be used, in bulk staining assays, as proxies for the intracellular effector molecules that respond to T cell activations74,75. However, such cell surface markers are expressed on up to 0.5% of CD8+ T cells in the absence of TCR stimulation, thus resulting in false positive signals and low accuracy in isolating antigen specific CD8+ T cells17.
Although several droplet-based microfluidic T cell screening methods were reported76,77,78,79, these methods had various limitations. Two methods using microgel76,77 were developed for detecting only antigen-independent T cell activation. Additionally, microgel formula posed a challenge for retrieving live cells for subsequent analyses such as single-cell sequencing. In contrast, ATLAS-seq screens for antigen-reactive CD8+ T cells and can retrieve live cells for single-cell sequencing as well as cell culture. Two other methods78,79 used T cells expressing the NFAT-GFP reporter gene for screening, requiring either engineered Jurkat reporter cells or introducing the NFAT-GFP gene into primary CD8+ T cells through viral transduction or electroporation. In contrast, ATLAS-seq uses a membrane-anchored IFNγ aptamer beacon as a simple-to-use and non-invasive reporter for screening patient-derived CD8+ T cells. Finally, activated T cell sorting using laser-based cavitation from microwell plate78 (1 event/15 min) or fluorescence-based dielectrophoresis79 (450 events/s) is slow. ATLAS-seq, on the other hand, uses standard flow cytometry sorting of the live T cells retrieved from the droplets (about 70,000 events/s), resulting in drastic increase in throughput that is suitable for clinical applications. By improving multiple features from these previously reported droplet-based microfluidic T cell screening methods, ATLAS-seq can provide a highly efficient system to screen for patient-derived activated antigen-specific T cells in one simple workflow.
In ATLAS-seq, we used a low-input T cell concentration (0.6 × 106 cells/mL) to achieve isolated single T cells in droplets, resulting in ~0.4 × 106 CD8+ T cells as input in each screen. Using ~0.8 × 106 T cells as input for ATLAS-seq identified 500 CMV-specific TCRα and 498 CMV-specific TCRβ clonotypes. As a reference, Dextramer staining using an input of 2 × 106 CD8+ T cells detected 608 CMV-specific TCRα and 550 CMV-specific TCRβ clonotypes. A previous study using large-scale Dextramer staining41 identified an average of 593 CMV-specific TCRα and 605 CMV-specific TCRβ clonotypes, indicating similar output levels as the current ATLAS-seq workflow. The human CD8+ TCRβ repertoire ranges from 105–108 80,81,82, and the combined projected TCRβ repertoires against NLV (CMV pp65) were estimated to account for up to 3.4% (0.03–3.4%) of the total adult CD8+ TCRβ repertoire41. Thus, ATLAS-seq is not exhaustive, but aims at selecting the most effectively activated CD8+ T cells from a T cell repertoire that has been properly primed with a target antigen, with prior antigen exposure or pre-enriched by other methods.
Our comparison of the features of clonotypes selected by ATLAS-seq and Dextramer staining revealed that ATLAS-seq clonotypes have significantly higher activation levels than Dextramer staining clonotypes, measured by pathway activities (Figs. 3D) and 4 cytotoxicity indexes (Fig. 4B), while there was no significant difference between their TCR-pMHC binding scores (Fig. 4B). This result suggested that high TCR-pMHC binding strength may not always be associated with high TCR activation. TCR-pMHC interaction is a pre-requisite for CD8+ T cells to exit quiescence and initiate antigen-specific T cell clonal expansion83,84. Thus, by selecting TCRs with multiple cell counts in either ATLAS-seq or Dextramer staining, we are already selecting antigen-specific TCRs that bind the pMHC and undergo clonal expansion/proliferation before or during the screening processes. This explains the similar TCR-peptide binding scores between the two methods (Fig. 4B). Previous work has shown that T cell activation levels are maximized at intermediate TCR-pMHC affinities85,86,87, which could be regulated by the fluctuations in TCR-pMHC binding dynamics and TCR phosphorylation signaling88,89,90. Thus, considering the complex relationship between the TCR-pMHC binding and T cell activation level, using T cell activation phenotypes directly (e.g., IFNγ secretion as in ATLAS-seq) could enable more efficient screening for functional TCRs, which can be used in immunotherapy.
To narrow down the CMV-specific TCR clonotypes for cytotoxicity evaluation, we used different clonotype features including clonotype proportions, cytotoxic cytokine indexes, and TCR-pMHC binding scores to rank TCR clonotype hits from the two screening methods (Fig. S7). Here, the clonotype proportions represent the proliferation potentials of activated T cells. Cytotoxic cytokine indexes represent the RNA expression levels of IFNγ, TNF, and LTα. TCR-pMHC binding scores represent the binding strengths between TCRs and CMV pp65 peptide-MHC complex. We chose clonotypes with the highest abundances (base on clonotype proportion) or the highest cytotoxic cytokine indexes (based on scRNA-seq) from the two methods. The target killing efficiencies measured in the target cell killing assays, using PBMC transduced with selected TCRs, showed the highest correlation with clonotype proportions in ATLAS-seq (R2 = 0.43, Fig. S8D, left panel).
This observation could potentially be explained by the proliferation of the highly activated T cell within the droplet during the co-incubation period in ATLAS-seq. If so, we will be sorting the progenies of the activated T cells. Because the cell membrane divides during mitosis, each progeny T cell could also carry aptamers inserted on its surface membrane. The more progeny T cells in one droplet, the higher combined IFNγ concentration in the droplet, the higher fluorescence signal on all progeny T cells, assuming the aptamers are in excess. This fluorescence signal would plateau if the progeny T cells only carry limited number of aptamers. Under this scenario, highly proliferative clonotypes will result in high clonotype proportions and high fluorescence in the selected T cell population, even if such clonotype does not produce high IFNγ. The apparent high fluorescence of the progeny T cells could be the cumulative effect of the IFNγ secreted by all the progeny T cells in the same droplet. This could partially explain the observation that the clonotypes with the highest clonotype proportions in ATLAS-seq show high target cell killing efficiencies (Fig. S8D, left panel, blue dots), despite some clonotypes (A1 and A4) showing lower cytotoxic cytokine index (Fig. S8D, middle panel, blue dots).
This observation was further corroborated by the target cell killing results of the PSA-reactive TCR clonotypes selected by ATLAS-seq. All 10 tested clonotypes, regardless the inclusion criteria (clonotype proportion vs cytotoxic cytokine indexes), exhibited high PSA+ LNCaP target cell killing activities (85–93%, Fig. 6B, C). Based on these results, we speculate that although cytotoxic cytokines expression is widely used as an indicator for T cell activation and cytotoxicity, it may not be sufficient for predicting the potential killing activities of the TCR clonotypes for further testing. In our current ATLAS-seq setting, TCR clonotype abundance, as well as cytotoxic cytokine index, possibly also other cytotoxicity indexes (Fig. S9E), should be considered for choosing TCR clonotypes for further therapeutic performance evaluation.
Our comparison results showed that the clonotypes identified by ATLAS-seq are largely different from the ones identified by Dextramer staining (Fig. 2C, E), having only a small fraction of overlapping clonotypes (~11%, Supplementary Data 3,4). These observations are further corroborated by that the overall abundance of selected antigen-specific CD8+ T cells from ATLAS-seq is much higher than that from Dextramer staining in the CD8+ T cells used. These input CD8+ T cells were from autologous patient PBMCs (CMV) or healthy donor PBMCs primed with target antigen (PSA) aAPCs that have gone through antigen specific T cell expansion before the screen. Specifically, ATLAS-seq can identify ~6% of input CD8+ T cells from the CMV positive donor #1, 3.66% from the CMV positive donor #2, and 3.19% from the PSA primed healthy donor cells, which are all well within the antigen-specific CD8+ T cells abundance range (1:10 to 1:1000) that ATLAS-seq exhibited high recovery rate and high accuracy (Fig. S3). In contrast, the selected antigen-specific CD8+ T cell fraction is much lower in Dextramer assays from the same input cells (0.64%/ 0.44% for CMV and 0.053% for PSA) than in ATLAS-seq (6.02%/3.66% for CMV and 3.19% for PSA) (Fig. 2A, S5E, S6A).
This could be explained by the different mechanisms of ATLAS-seq and Dextramer assay for TCR selection: Dextramer assay relies on the physical interactions between TCR and peptide-MHC complex91. As a result, Dextramer assay might miss the TCRs with low to moderate binding affinity to pMHC, which might still be highly reactive92. In contrast, ATLAS-seq measures T cell efficacy (i.e., how effective the T cell can be activated by the antigen). In our specific ATLAS-seq design, we use the secreted IFNγ as an indicator of the T cell activation. Although both methods have similarly high recovery rate for the TCR DMF5 (Fig. S3), which has high binding affinity to MART1-MHC complex23, Dextramer assay might not be as efficient in retrieving certain lower-binding affinity TCRs as in ATLAS-seq, although the overall TCR-pMHC binding score distribution of the two methods are comparable (Fig.4B, S6F). As a result, the TCRs selected by these two methods could have inherent differences due to their intrinsic characteristics being selected for. It should be noted that IFNγ aptamer decorated DMF5 and control Jurkat cells showed slightly higher proliferation than those without aptamer decoration after a 3-day incubation (Fig. S1A), suggesting that the IFNγ aptamer might also promote the proliferation of T cells. ATLAS-seq uses a two-day incubation, when such effect is insignificant. Whether the potential pro-proliferative property of the IFNγ aptamer partially contributes to the difference between ATLAS-seq and Dextramer assay remains to be determined.
In addition to the interaction between CD8+ T cells and aAPCs in this study, other cell interactions could also be used as the basis to perform phenotype screening with the ATLAS-seq platform. For example, B cell clonotypes with high antigen-specific antibody secretion could be selected based on B cell-APC interactions. Cytotoxic T cells (cytotoxic T lymphocyte, CTLs), with high infiltration abilities, could also be selected based on CTL-tumor-cell interactions. Furthermore, cholesterol-modified aptamer beacons enable stable labeling on cell membranes and direct probing of the extracellular environment22,93. Thus, in addition to IFNγ, a set of aptamer beacons specific to different cytokines or metabolites could be prepared based on previously published aptamers94,95. These could facilitate the monitoring of different combinations of cytokine or metabolite secretions from immune cells96,97 using ATLAS-seq, which would enhance the resolution of the phenotype selection.
In conclusion, ATLAS-seq provides a cost-effective, easy-to-implement, and efficient workflow for screening antigen-reactive TCR clonotypes based on T cell activation signatures, and through direct monitoring of cytotoxic cytokine IFNγ secretion. This would facilitate the discovery of highly functional TCRs for engineered T cell immunotherapies.
Methods
This research complies with all relevant ethical regulations. The Institutional Biosafety Committee (IBC) at the Children’s Hospital of Philadelphia has approved the study protocol. All human PBMCs purchased from Cellular Technology Limited were collected under appropriate ethical permission and consent.
Microfluidic chip fabrication
The photomask of microfluidic chip of ATLAS-seq was printed by Fineline Imaging. The master for microfluidic chip fabrication was generated on a silicon wafer (WaferPro, C04007) by photolithography through ABM 3000HR Mask Aligner (ABM) at Singh Center for Nanotechnology (PA, U.S.A). Polydimethylsiloxane (PDMS) prepolymer and its cross-linking agent (Fisher Scientific, NC9285739) were mixed in 10:1 mass ratio and vacuum degassed, then the mix was poured onto the silicon wafer master to make the PDMS slab at 80 °C for at least 30 min. After the mold was cut and peeled off from the wafer, the inlet/outlet ports were punched. The mold was bonded on a clean glass plate in Tergeo plasma cleaner (PIE Scientific, Tergeo) with 50 W treatment for 1 min, then placed on a hotplate at 60 °C for 1 h to improve the bond. Aquapel (Aquapel Glass Treatment, 47100) treatment was then performed to keep the inside of the channels hydrophobic.
aAPC beads preparation and peptide loading
To prepare aAPC beads, 1ml M-450 Epoxy beads (Invitrogen, 14011) were washed with 1 ml 0.1 M borate buffer, then resuspended in a mixture of 1 ml 0.1 M borate buffer with 20 μg of HLA-A2-Ig dimer (BD Biosciences, 551263) and 20 μg of anti-human CD28 (Clone 9.3, Bio X Cell, BE0248R005MG, Lot 676519J2). The bead mixture was rotated at 4 °C for 24 h and washed twice with 1 ml aAPC wash buffer (950 ml 1X DPBS, 30 ml human AB serum (MilliporeSigma, H4522-100ML), 2 mM EDTA, 0.1 g sodium azide, filter through 0.22μm filter and store at 4 °C). The beads were then incubated in 1 ml aAPC wash buffer and rotated at 4 °C for another 24 h. The prepared aAPC Bead quality was assessed by staining with anti-mouse IgG1-PE (Polyclonal, Invitrogen, P-21129, Lot 1828010) and anti-mouse IgG2a-FITC (Clone R19-15, BD Biosciences, 553390, Lot 9058866) in flow cytometry staining buffer (Invitrogen, 00-4222-26). To load antigen on aAPC beads, 10 μl of 1 mg/ml antigen peptides were mixed with PBS-washed aAPC beads and incubated at 4 °C for 3 days. Antigen-loaded aAPC remained functional for at least 6 months when stored in suspension at 4 °C. Detailed Antibody information are listed in Supplementary Data 7.
T cell isolation, culture, and expansion
Pan T cells were isolated from human PBMCs (Cellular Technology Limited) with human pan T cell isolation kit (Miltenyi, 130096535) and cultured in T cell culture medium (X-VIVO 15 media supplemented with 5% human serum and 300U/ml Interleukin-2 (IL-2)). To expand pan T cells, in each well of a 24-well plate, 1 × 106 pan T cells were resuspended in 1 ml culture medium with 1 × 106 CD3/CD28 beads (Gibco, 11131D) and incubate at 37 °C, 5% CO2 for 14 days. After expansion, CD3/CD28 beads were removed, pan T cells were rested in T cell culture medium without CD3/CD28 beads for 2 days. From these rested pan T cells, CD8+ T cells were isolated with human CD8+ T cell isolation kit (Miltenyi, 130096495). Isolated CD8+ T cells were validated by staining with anti-CD8 antibody (3B5), Pacific Blue (Clone 3B5, ThermoFisher, MHCD0828, Lot 2311799) and FITC anti-human CD3 (Clone UCHT1, Biolegend, 300406, Lot B279208). Isolated CD8+ T cells were used immediately or frozen in 5% DMSO in FBS for cryopreservation in liquid nitrogen. Detailed Antibody information are listed in Supplementary Data 7.
Antigen priming of T cells from healthy donor PBMCs
Pan T cells were isolated from healthy donor PBMCs (Cellular Technology Limited) with human pan T cell isolation kit (Miltenyi, 130096535) and cultured at 1 × 106 cells/ml in PBMC growth medium (X-vivo 15 media supplemented with 10% heat-inactivated FBS, 1x penicillin/streptomycin/L-glutamine and 50U/ml IL-2). To prime pan T cells, antigen peptide loaded-aAPCs were added to pan T cell culture at 1:1 ratio and incubated at 37 °C, 5% CO2 for 14 days. After removing antigen peptide-loaded beads, primed pan T cells were cultured at 1 × 106 cells/ml in PBMC growth medium with 1:1 ratio of CD3/CD28 beads (Gibco, 11131D) for expansion at 37 °C, 5% CO2 for 7 days. After expansion, CD3/CD28 beads were removed, pan T cells were rested in T cell culture medium without CD3/CD28 beads for 2 days before proceeding downstream experiments. From these rested pan T cells, CD8+ T cells were isolated with human CD8+ T cell isolation kit (Miltenyi, 130096495). Isolated CD8+ T cells were used immediately or frozen in 5% DMSO in FBS for cryopreservation in liquid nitrogen.
Aptamer decoration of T cells
IFNγ-specific aptamer beacon (/5Cy3/AGGGGTTGGTTGTGTTGGGTGTTGTGTCCAACCCCT/TAO//iSp18//3CholTEG/) was synthesized by IDT. Three hours before the ATLAS-seq procedure, 1 × 106 CD8+ T cells were resuspended in 50 μl 1X DPBS, transferred to one well of a laminar wash plate (Curiox, U.S.A) and washed 5 times in Laminar Wash HT2000 (Curiox). Then the washed CD8+ T cells were resuspended in the laminar wash plate with 10 μl 100 μM IFNγ aptamer beacon (1nmol beacon/106cells) and 15 μl 1X DPBS, followed by a 30-minute incubation at room temperature in dark. The aptamer-decorated T cells were then washed 5 times in Laminar Wash HT2000 and resuspended in T cell culture medium (no IL-2) with 20% OptiPrep Density Gradient Medium (MilliporeSigma, D1556) to 6 × 105 cell/ml for ATLAS-seq.
Jurkat cell culture and assays
T2 cell culture and antigen loading: 5 × 104 T2 cells were plated in 96-well plate and incubate overnight in medium (IMDM, 20% FBS). 5 μM MART1 peptide (ELAGIGILTV, JPT, SP-MHCI-0006) in 1x PBS was added to wells and incubate for 2 h. T2 cells were washed twice with 1x PBS before being added to Jurkat cells.
MTS assay: Jurkat cells were cultured at 37 °C, 5% CO2 in 200 µl/well medium (RPMI-1640 media supplemented with 10% FBS and 1x penicillin/streptomycin) in a 96-well microtiter plate. 20 µl/well MTS Reagent (Abcam Limited, ab197010) were added at different time points (day 1, day 2 and day 3) during growth and incubated for 4 h at 37 °C, 5% CO2. OD of 490 nm was measured using a plate reader (BioTeK, Synergy H1).
DMF5 expressing IFNGKO Jurkat cell generation: 2 × 107 TU/mL MART1-Specific TCR Lentivirus (Clone DMF5, BPS Bioscience, 78679-1) were used to transduce human IFNG knockout Jurkat cell line (abcam, ab273746) with TransDux MAX transduction reagent (System Biosciences, LV860A-1) following the suspension cell transduction protocol provided by the manufacturer (https://www.systembio.com/wp/wp-content/uploads/2020/10/Manual_LV860A-1-1.pdf). The required virus Multiplicity of Infection (MOI) for Jurkat cells is 10. After 3 days of transduction, cells were cultured in medium (RPMI-1640 media supplemented with 10% FBS and 1x penicillin/streptomycin) supplied with 0.5 μg/mL puromycin dihydrochloride (Gibco, A1113803) for two weeks. MART1-Specific TCR (Clone DMF5) expression was validated by using PE conjugated MART1 peptide (ELAGIGILTV) HLA-A*0201 MHC Dextramer (Immudex, WB02162 PE 50) with flow cytometry.
GFP expressing Jurkat cell generation: GFP lentivirus (CHOP vector core) were used to transduce Jurkat cells with TransDux MAX transduction reagent (System Biosciences, LV860A-1) following the suspension cell transduction protocol provided by the manufacturer (https://www.systembio.com/wp/wp-content/uploads/2020/10/Manual_LV860A-1-1.pdf). The required virus Multiplicity of Infection (MOI) for Jurkat cells is 10. After 3 days of transduction, GFP-expressing cells were sorted by FACS.
ATLAS-seq procedure
Aptamer decorated CD8+ T cells were prepared in 6 × 105 cell/ml suspension in T cell culture medium (no IL-2) with 20% OptiPrep™ Density Gradient Medium and loaded in 1 ml sterile syringe. aAPC beads are prepared in 5 × 107 bead/ml suspension in T cell culture medium with 600U/ml IL-2 and 20% OptiPrep Density Gradient Medium and loaded in 1 ml sterile syringe. HFE7500 oil (3 M, Novec, 7500) with 2% (w/w) surfactant (Ran Biotech, 008-FluoroSurfactant-2wtH-50G) was loaded in Another sterile syringe. All three syringes were connected to the inlet ports of the co-encapsulation microfluidic chip through tubing and were controlled by inDrop pump system (1CellBio, U.S.A). The following flow rates were used: 600 μl/h oil, 200 μl/h T cell suspension and 200 μl/h aAPC suspension. The droplets were collected in a 5 ml round-bottom tube for 20 min per tube, followed by a two-day incubation at 37 °C in 5% CO2. After removing the oil, 500 μl fresh culture medium and 500 μl 20%(vol/vol) 1H,1H,2H,2H-Perfluoro-1-octanol (MilliporeSigma, 370533) in HFE7500 oil were added on top of the emulsion to break the droplets by incubation at room temperature for 2 min. The liquid phase on top was transferred into a new tube and aAPC beads were removed from cell suspension using DynaMag-2 magnet rack (ThermoFisher, 12321D). Recovered CD8+ T cells were prepared for FACS sorting using the following steps: 1) Resuspend the T cells in 50 μl 1X DPBS and wash 5 times in Laminar Wash HT2000. 2) Add 25 μl staining buffer (2% FBS in 1X DPBS) and 5 μl anti-CD8 antibody (3B5), Pacific Blue (Clone 3B5, ThermoFisher, MHCD0828, Lot 2311799) or anti-CD8 antibody (3B5), FITC (Clone 3B5, ThermoFisher, MHCD0801, Lot 2580714) mix and incubate in dark at room temperature for 30 min. 3) Wash cells 5 times in Laminar Wash HT2000. 4) Resuspend the T cells in 500 μl 1X DPBS with 10% FBS for FACS sorting. Detailed Antibody information is listed in Supplementary Data 7. The custom codes98 used for microfluidic parameter simulation, droplet analyses and cell encapsulation estimations are available on Zenodo (https://doi.org/10.5281/zenodo.13905305).
Dextramer Staining
After a two-day co-incubation of CD8+ T cells and aAPC beads in 1:1 ratio in T cell culture medium at 37 °C in 5% CO2, CD8+ T cells were separated from aAPC beads using DynaMag-2 magnet rack and washed twice by PBS. 1 × 106 washed CD8+ T cells were resuspended in 50 μl Dextramer stain buffer (PBS containing 1% human serum and 0.1 g/l Herring sperm DNA (ThermoFisher, 15634017)). Add 2.2 μl antigen-specific Dextramer reagent (0.2 μl 100μM d-Biotin and 2 μl dCODE Dextramer (Immundex)) to the CD8 + T cell suspension and mix thoroughly. Transfer the mix into a well of a laminar wash plate and incubate in dark at room temperature for 10 min, then add 5 μl anti-CD8 antibody (3B5), Pacific Blue (Clone 3B5, ThermoFisher, MHCD0828, Lot 2311799) or anti-CD8 antibody (3B5), FITC (Clone 3B5, ThermoFisher, MHCD0801, Lot 2580714) into the same well of the plate and incubate in dark at room temperature for another 20 min. CD8+ T cells were then washed 5 times in Laminar Wash HT2000 and resuspended in 500 μl 1X DPBS with 10% FBS for FACS sorting. Two independent dextramer staining experiments were done as technical replicates using the CD8+ T cells isolated from the same CMV-positive donor as input. Detailed Antibody information is listed in Supplementary Data 7.
Single-cell TCR and RNA sequencing library preparation and data analysis
After cell sorting, collected T cell suspension was transferred to laminar wash plate (~80 μl in each well) and incubated at room temperature for 30 min, followed by washing 5 times with 1X DPBS in Laminar Wash HT2000. The protocol of 10X Genomics Chromium Next GEM Single Cell 5’ Kits were used to resuspend T cells into a proper concentration to achieve targeted cell recovery and prepare the V(D)J library for single-cell TCR sequencing. Sequencing data was processed by using the pipeline of 10X Genomics Cell ranger42 and Seurat43. The differential immune regulation pathway activities were analyzed by SCPA package52. The TCR clonotypes were analyzed by VDJtools99. Sequencing data are available at Gene Expression Omnibus (GEO) under accession number GSE234359. The TCR-pMHC binding scores of TCR clonotypes were calculated by NetTCR-2.0 based on both TCRα and TCRβ CDR3s69.
TCR cloning and retroviral packaging
Full-length DNA fragments of TCRα and TCRβ were synthesized using gBlocks gene fragments (IDT) with 30 bp overlapping sequences (Supplementary Data 2 and 6). TCR expression vectors were constructed by mixing TCRα and TCRβ g-Blocks with a linearized pMSCV-IRES-mCherry FP (pMIR) expression vector (a gift from Dario Vignali (Addgene plasmid # 52114; http://n2t.net/addgene:52114; RRID:Addgene_52114)), followed by a three-way ligation using Gibson Assembly Cloning (NEB, E2611S) at 50 °C for 15 min and stored on ice. The resulting pTCR-MIR expression vectors encoded TCRα and TCRβ fragments connected by a 2A self-cleaving peptide P2A. During translation, TCRα and TCRβ fragments are separated through P2A-induced ribosomal skipping and form fully functional TCR of interest. Following the protocol of Lipofectamine 3000 (Invitrogen, L3000015), Phoenix retroviral producer cells (ATCC, CRL-3214) were transfected with equal amount of pTCR-MIR and pCMV-VSV-G vectors (Cell Biolabs, RV-110) at 2.5 μg/ 1 × 106 cells concentration and incubated at 37 °C, 5% CO2 for 2 days. Then, from transfected Phoenix retroviral producer cell medium, retroviral particles were precipitated with PEG-it virus precipitation solution (SBI, LV810A-1) at 4 °C overnight followed by spinning at 1,500 xg for 30 min and resuspended in serum-free X-vivo15 medium. Retrovirus-coated plates for PBMC transduction were prepared as follows: RetroNectin (Takara, T100A) coated 6-well plates containing 2 ml/well retroviral particle suspension were centrifuged at 2,000xg for 2 h at 32 °C. Viral supernatant was aspirated and replaced with 2 ml PBMC growth medium (X-vivo 15 media supplemented with 10% heat-inactivated FBS, 1x penicillin/streptomycin/L-glutamine and 50U/ml IL-2).
PBMC transduction
2 × 106 cells/mL healthy PBMCs (Cellular Technology Limited, LP_159, Donor# 131) were stimulated with CD3/CD28 beads in PBMC growth medium at 37 °C, 5% CO2 for 2 days. After removing CD3/CD28 beads, PBMCs were collected with centrifugation at 600xg for 5 min at 25 °C. PBMCs were resuspend in PBMC growth medium to 1.5 × 106 cells/mL and 1 ml cell suspensions were added to each viral-coated well at 1.5 × 106 cells/3 mL/well. Plates were centrifuged at 1,000xg for 10 min at 32 °C, then incubated at 37 °C, 5% CO2 for 2 days. PBMCs were then collected and pelleted at 600xg for 5 min at 25 °C. Transduced PBMCs were resuspended in PBMC growth media with 1% 100x β-mercaptoethanol solution (MilliporeSigma, ES-007-E) and incubated at 37 °C, 5% CO2 for 4 days. Transduced PBMCs can be frozen in FBS with 5% DMSO for cryopreservation in liquid nitrogen.
CMV-specific TCR clonotype cytotoxicity assays
0.2 × 105 cells/well target GFP+ PC3 cells expressing HLA-A2 and CMV pp65 (a gift from Dr. Owen N. Witte) were seeded in 100 μl medium (F-12K media supplemented with 10% FBS and 1x penicillin/streptomycin) at 37 °C, 5% CO2 in 96-well plates overnight. Then, 2 × 105 TCR-transduced PBMCs in 100 μl medium mixed with 6 × 105 CMV pp65 loaded aAPC beads for activation were added to each well for coculturing with target cells. Incucyte S3 (Sartorius, Germany) was used to monitor and measure GFP+ PC3 cell viabilities as GFP fluorescence intensities for 72 h. Relative viabilities of target cells were calculated by normalizing with GFP intensities of non-treated target cell. Target killing efficiencies of clonotypes were calculated as the relative viability decrease after the 72 h assay. Supernatant of each assay was collected for IFNγ and TNF ELISA using IFN gamma Human ELISA Kit (Invitrogen, KHC4021) and TNF alpha Human ELISA Kit (Invitrogen, KAC1751).
PSA-specific TCR clonotype cytotoxicity assays
GFP expressing LNCaP cell generation: LNCaP cells were cultured in medium (RPMI-1640 media supplemented with 10% FBS and 1x penicillin/streptomycin). GFP lentivirus (CHOP vector core) were used to transduce LNCaP cells (ATCC, CRL-1740) with TransDux MAX transduction reagent (System Biosciences, LV860A-1) following the adherent cell transduction protocol provided by the manufacturer (https://www.systembio.com/wp/wp-content/uploads/2020/10/Manual_LV860A-1-1.pdf). The required virus Multiplicity of Infection (MOI) for LNCaP cells is 5. After 3 days of transduction, transduced LNCaP cells with GFP expressing were sorted by FACS.
Killing assay: 0.4 × 105 cells/well target LNCaP cells (ATCC, CRL-1740) expressing GFP were seeded in 100 μl medium (RPMI-1640 media supplemented with 10% Charcoal Dextran Stripped (CDS) FBS (Gemini BioProducts, 100-119-500) and 0.5 mg/500 ml Plasmocin (InvivoGen, ANT-MPT)) at 37 °C, 5% CO2 in 96-well plates overnight. Then, 4 × 105 TCR-transduced PBMCs in 100 μl medium activated with 25 μl/ml CD3/CD28 T cell activator (STEMCELL Technologies, 10971) were added to each well for coculturing with target cells. Incucyte S3 (Sartorius, Germany) was used to monitor and measure LNCaP cell viabilities as GFP fluorescence intensities for 72 h. Relative viabilities of target cells were calculated by normalizing with GFP intensities of non-treated target cell. Target killing efficiencies of clonotypes were calculated as the relative viability decrease after the 72 h assay. Supernatant of each assay was collected for IFNγ and TNF ELISA using IFN gamma Human ELISA Kit (Invitrogen, KHC4021) and TNF alpha Human ELISA Kit (Invitrogen, KAC1751).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are provided in the paper and Supplementary Information. Raw and processed data from Illumina short-read RNA-seq were uploaded to GEO under accession number GSE234359. All other data are available in the article and its Supplementary files or from the corresponding author upon request. Source data are provided with this paper.
Code availability
All analyses were conducted using publicly available software, as detailed in the “Methods” section. The custom codes98 used for microfluidic parameter simulation, droplet analyses and cell encapsulation estimations are available on Zenodo (https://doi.org/10.5281/zenodo.13905305).
References
Wedekind, M. F., Denton, N. L., Chen, C. Y. & Cripe, T. P. Pediatric cancer immunotherapy: opportunities and challenges. Paediatr. Drugs 20, 395–408 (2018).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Hu, Z., Ott, P. A. & Wu, C. J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 18, 168–182 (2018).
Tsimberidou, A. M. et al. T cell receptor-based therapy: an innovative therapeutic approach for solid tumors. J. Hematol. Oncol. 14, 102 (2021).
Marofi, F. et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. 12, 81 (2021).
Hogan, S. A. et al. Peripheral blood TCR repertoire profiling may facilitate patient stratification for immunotherapy against melanoma. Cancer Immunol. Res. 7, 77–85 (2019).
Valpione, S. et al. The T cell receptor repertoire of tumor infiltrating T cells is predictive and prognostic for cancer survival. Nat. Commun. 12, 4098 (2021).
Zhang, Z., Xiong, D., Wang, X., Liu, H. & Wang, T. Mapping the functional landscape of T cell receptor repertoires by single-T cell transcriptomics. Nat. Methods 18, 92–99 (2021).
Bentzen, A. K. et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat. Biotechnol. 34, 1037–1045 (2016).
Chang, J. MHC multimer: a molecular toolbox for immunologists. Mol. Cells 44, 328–334 (2021).
Rius, C. et al. Peptide-MHC class I tetramers can fail to detect relevant functional T cell clonotypes and underestimate antigen-reactive T cell populations. J. Immunol. 200, 2263–2279 (2018).
Stone, J. D. & Kranz, D. M. Role of T cell receptor affinity in the efficacy and specificity of adoptive T cell therapies. Front Immunol. 4, 244 (2013).
Huse, M., Lillemeier, B. F., Kuhns, M. S., Chen, D. S. & Davis, M. M. T cells use two directionally distinct pathways for cytokine secretion. Nat. Immunol. 7, 247–255 (2006).
De Biasi, S. et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 11, 3434 (2020).
Mazet, J. M. et al. IFNgamma signaling in cytotoxic T cells restricts anti-tumor responses by inhibiting the maintenance and diversity of intra-tumoral stem-like T cells. Nat. Commun. 14, 321 (2023).
Nicolet, B. P., Guislain, A. & Wolkers, M. C. Combined single-cell measurement of cytokine mRNA and protein identifies T cells with persistent effector function. J. Immunol. 198, 962–970 (2017).
Nesterenko, P. A. et al. Droplet-based mRNA sequencing of fixed and permeabilized cells by CLInt-seq allows for antigen-specific TCR cloning. Proc. Natl Acad. Sci. USA 118, e2021190118 (2021).
Campbell, J. D. et al. Rapid detection, enrichment and propagation of specific T cell subsets based on cytokine secretion. Clin. Exp. Immunol. 163, 1–10 (2011).
Altan-Bonnet, G. & Mukherjee, R. Cytokine-mediated communication: a quantitative appraisal of immune complexity. Nat. Rev. Immunol. 19, 205–217 (2019).
Gong, Z., Li, Q., Shi, J. & Ren, G. An artifact in intracellular cytokine staining for studying T cell responses and its alleviation. Front. Immunol. 13, 759188 (2022).
Yuan, Y. et al. Droplet encapsulation improves accuracy of immune cell cytokine capture assays. Lab Chip 20, 1513–1520 (2020).
Qiu, L. et al. A membrane-anchored aptamer sensor for probing IFNgamma secretion by single cells. Chem. Commun. (Camb.) 53, 8066–8069 (2017).
Cory, A. H., Owen, T. C., Barltrop, J. A. & Cory, J. G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 3, 207–212 (1991).
Borbulevych, O. Y., Santhanagopolan, S. M., Hossain, M. & Baker, B. M. TCRs used in cancer gene therapy cross-react with MART-1/Melan-A tumor antigens via distinct mechanisms. J. Immunol. 187, 2453–2463 (2011).
Overall, S. A. et al. High throughput pMHC-I tetramer library production using chaperone-mediated peptide exchange. Nat. Commun. 11, 1909 (2020).
Chiu, Y. L., Schneck, J. P. & Oelke, M. HLA-Ig based artificial antigen presenting cells for efficient ex vivo expansion of human CTL. J. Vis. Exp. 50, 2801 (2011).
Neal, L. R. et al. The basics of artificial antigen presenting cells in T cell-based cancer immunotherapies. J. Immunol. Res. Ther. 2, 68–79 (2017).
Tuleuova, N. et al. Development of an aptamer beacon for detection of interferon-gamma. Anal. Chem. 82, 1851–1857 (2010).
Cole, D. J. et al. Characterization of the functional specificity of a cloned T cell receptor heterodimer recognizing the MART-1 melanoma antigen. Cancer Res. 55, 748–752 (1995).
Lyons, G. E. et al. Influence of human CD8 on antigen recognition by T cell receptor-transduced cells. Cancer Res. 66, 11455–11461 (2006).
Collins, D. J., Neild, A., deMello, A., Liu, A. Q. & Ai, Y. The Poisson distribution and beyond: methods for microfluidic droplet production and single cell encapsulation. Lab Chip 15, 3439–3459 (2015).
Peng, F. et al. A droplet-based microfluidics route to temperature-responsive colloidal molecules. J. Phys. Chem. B 123, 9260–9271 (2019).
Yee, C. et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl Acad. Sci. USA 99, 16168–16173 (2002).
Ichikawa, J. et al. Rapid expansion of highly functional antigen-specific T cells from patients with melanoma by nanoscale artificial antigen-presenting cells. Clin. Cancer Res 26, 3384–3396 (2020).
Wolfl, M. & Greenberg, P. D. Antigen-specific activation and cytokine-facilitated expansion of naive, human CD8+ T cells. Nat. Protoc. 9, 950–966 (2014).
Wang, G. C., Dash, P., McCullers, J. A., Doherty, P. C. & Thomas, P. G. T cell receptor alphabeta diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci. Transl. Med. 4, 128ra142 (2012).
Link, C. S. et al. Abundant cytomegalovirus (CMV) reactive clonotypes in the CD8(+) T cell receptor alpha repertoire following allogeneic transplantation. Clin. Exp. Immunol. 184, 389–402 (2016).
Huth, A., Liang, X., Krebs, S., Blum, H. & Moosmann, A. Antigen-specific TCR signatures of cytomegalovirus infection. J. Immunol. 202, 979–990 (2019).
Nakasone, H. et al. Features of repertoire diversity and gene expression in human cytotoxic T cells following allogeneic hematopoietic cell transplantation. Commun. Biol. 4, 1177 (2021).
Frith, M. C., Saunders, N. F., Kobe, B. & Bailey, T. L. Discovering sequence motifs with arbitrary insertions and deletions. PLoS Comput. Biol. 4, e1000071 (2008).
Chen, G. et al. Sequence and structural analyses reveal distinct and highly diverse human CD8(+) TCR repertoires to immunodominant viral antigens. Cell Rep. 19, 569–583 (2017).
Zheng, G. X. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 e3529 (2021).
Penkava, F. et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat. Commun. 11, 4767 (2020).
Schmiedel, B. J. et al. Impact of genetic polymorphisms on human immune cell gene expression. Cell 175, 1701–1715.e1716 (2018).
Szabo, P. A. et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat. Commun. 10, 4706 (2019).
Andreatta, M. et al. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 12, 2965 (2021).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
McLane, L. M., Abdel-Hakeem, M. S. & Wherry, E. J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev. Immunol. 37, 457–495 (2019).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Sheu, B. C., Lin, R. H., Ho, H. N. & Huang, S. C. Down-regulation of CD25 expression on the surface of activated tumor-infiltrating lymphocytes in human cervical carcinoma. Hum. Immunol. 56, 39–48 (1997).
Bibby, J. A. et al. Systematic single-cell pathway analysis to characterize early T cell activation. Cell Rep. 41, 111697 (2022).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet 25, 25–29 (2000).
Fabregat, A. et al. Reactome pathway analysis: a high-performance in-memory approach. BMC Bioinform. 18, 142 (2017).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Martens, M. et al. WikiPathways: connecting communities. Nucleic Acids Res. 49, D613–D621 (2021).
Schaefer, C. F. et al. PID: the Pathway Interaction Database. Nucleic Acids Res. 37, D674–D679 (2009).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Gocher, A. M., Workman, C. J. & Vignali, D. A. A. Interferon-gamma: teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 22, 158–172 (2022).
van Loo, G. & Bertrand, M. J. M. Death by TNF: a road to inflammation. Nat. Rev. Immunol. 23, 289–303 (2023).
Tang, H., Zhu, M., Qiao, J. & Fu, Y. X. Lymphotoxin signalling in tertiary lymphoid structures and immunotherapy. Cell Mol. Immunol. 14, 809–818 (2017).
Chang, H. F. et al. Identification of distinct cytotoxic granules as the origin of supramolecular attack particles in T lymphocytes. Nat. Commun. 13, 1029 (2022).
Eberlein, J. et al. Chemokine signatures of pathogen-specific T cells I: effector T cells. J. Immunol. 205, 2169–2187 (2020).
Davenport, B. et al. Chemokine signatures of pathogen-specific T cells II: memory T cells in acute and chronic infection. J. Immunol. 205, 2188–2206 (2020).
Galeano Nino, J. L. et al. Cytotoxic T cells swarm by homotypic chemokine signalling. Elife 9, e56554 (2020).
Cannons, J. L. et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 167, 1313–1324 (2001).
Otano, I. et al. CD137 (4-1BB) costimulation of CD8(+) T cells is more potent when provided in cis than in trans with respect to CD3-TCR stimulation. Nat. Commun. 12, 7296 (2021).
Cho, E. et al. 4-1BB-4-1BBL cis-interaction contributes to the survival of self-reactive CD8(+) T cell. Cell Mol. Immunol. 20, 1077–1080 (2023).
Montemurro, A. et al. NetTCR-2.0 enables accurate prediction of TCR-peptide binding by using paired TCRalpha and beta sequence data. Commun. Biol. 4, 1060 (2021).
Yarmarkovich, M. et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature 599, 477–484 (2021).
Carlsson, B., Forsberg, O., Bengtsson, M., Totterman, T. H. & Essand, M. Characterization of human prostate and breast cancer cell lines for experimental T cell-based immunotherapy. Prostate 67, 389–395 (2007).
Moya, L. et al. Characterisation of cell lines derived from prostate cancer patients with localised disease. Prostate Cancer Prostatic Dis. 26, 614–624 (2023).
Tsai, H. F., Trubelja, A., Shen, A. Q. & Bao, G. Tumour-on-a-chip: microfluidic models of tumour morphology, growth and microenvironment. J. R. Soc. Interface 14, 20170137 (2017).
Wolfl, M. et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 110, 201–210 (2007).
Parkhurst, M. et al. Isolation of T cell receptors specifically reactive with mutated tumor-associated antigens from tumor-infiltrating lymphocytes based on CD137 expression. Clin. Cancer Res. 23, 2491–2505 (2017).
Chokkalingam, V. et al. Probing cellular heterogeneity in cytokine-secreting immune cells using droplet-based microfluidics. Lab Chip 13, 4740–4744 (2013).
Tiemeijer, B. M., Descamps, L., Hulleman, J., Sleeboom, J. J. F. & Tel, J. A microfluidic approach for probing heterogeneity in cytotoxic T cells by cell pairing in hydrogel droplets. Micromachines (Basel) 13, 1910 (2022).
Segaliny, A. I. et al. Functional TCR T cell screening using single-cell droplet microfluidics. Lab Chip 18, 3733–3749 (2018).
Wang, S. et al. High-throughput functional screening of antigen-specific T cells based on droplet microfluidics at a single-cell level. Anal. Chem. 94, 918–926 (2022).
Li, H. M. et al. TCRbeta repertoire of CD4+ and CD8+ T cells is distinct in richness, distribution, and CDR3 amino acid composition. J. Leukoc. Biol. 99, 505–513 (2016).
Qi, Q. et al. Diversity and clonal selection in the human T cell repertoire. Proc. Natl Acad. Sci. USA 111, 13139–13144 (2014).
Robins, H. S. et al. Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci. Transl. Med. 2, 47ra64 (2010).
Guy, C. S. et al. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat. Immunol. 14, 262–270 (2013).
Shah, K., Al-Haidari, A., Sun, J. & Kazi, J. U. T cell receptor (TCR) signaling in health and disease. Signal Transduct. Target Ther. 6, 412 (2021).
Kalergis, A. M. et al. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat. Immunol. 2, 229–234 (2001).
Dushek, O. et al. Antigen potency and maximal efficacy reveal a mechanism of efficient T cell activation. Sci. Signal 4, ra39 (2011).
Corse, E., Gottschalk, R. A., Krogsgaard, M. & Allison, J. P. Attenuated T cell responses to a high-potency ligand in vivo. PLoS Biol. 8, e1000481 (2010).
Lever, M., Maini, P. K., van der Merwe, P. A. & Dushek, O. Phenotypic models of T cell activation. Nat. Rev. Immunol. 14, 619–629 (2014).
Limozin, L. et al. TCR-pMHC kinetics under force in a cell-free system show no intrinsic catch bond, but a minimal encounter duration before binding. Proc. Natl Acad. Sci. USA 116, 16943–16948 (2019).
Lin, J. J. Y. et al. Mapping the stochastic sequence of individual ligand-receptor binding events to cellular activation: T cells act on the rare events. Sci. Signal 12, eaat8715 (2019).
Tario, J. D. Jr et al. Dextramer reagents are effective tools for quantifying CMV antigen-specific T cells from peripheral blood samples. Cytom. B Clin. Cytom. 88, 6–20 (2015).
Allard, M., Hebeisen, M. & Rufer, N. Assessing T cell receptor affinity and avidity against tumor antigens. (eds. Zitvogel, L., Kroemer, G.) Oncoimmunology. https://doi.org/10.1007/978-3-319-62431-0_40 (Springer, Cham, 2018).
You, M. et al. DNA probes for monitoring dynamic and transient molecular encounters on live cell membranes. Nat. Nanotechnol. 12, 453–459 (2017).
Boshtam, M., Asgary, S., Kouhpayeh, S., Shariati, L. & Khanahmad, H. Aptamers against pro- and anti-inflammatory cytokines: a review. Inflammation 40, 340–349 (2017).
Kim, J. et al. Recent advances in aptasensor for cytokine detection: a review. Sensors (Basel) 21, 8491 (2021).
Shyer, J. A., Flavell, R. A. & Bailis, W. Metabolic signaling in T cells. Cell Res 30, 649–659 (2020).
Heintzman, D. R., Fisher, E. L. & Rathmell, J. C. Microenvironmental influences on T cell immunity in cancer and inflammation. Cell Mol. Immunol. 19, 316–326 (2022).
Siwei, L. Codes for microfluidic parameter simulation, droplet counting and particle encapsulation estimation. Zenodo https://doi.org/10.5281/zenodo.13905305 (2024).
Shugay, M. et al. VDJtools: unifying post-analysis of T cell receptor repertoires. PLoS Comput Biol. 11, e1004503 (2015).
Acknowledgements
This work was supported by National Institutes of Health grant R01GM121827 (L.L.), W.W. Smith Charitable Trust award C2004 (L.L.) and the Children’s Hospital of Philadelphia Research Institute Cell & Gene Therapy Collaborative Seed Grant (L.L.). We thank Dr. John M. Maris, Quinlen F. Marshall and Dr. Erin Runbeck for helpful technical support and generous sharing of equipment, T2 cells, Jurkat cells, DMF5 Jurkat cells and SK-N-AS cells; Dr. Owen N. Witte and Dr. Pavlo A. Nesterenko for sharing HLA-A2+CMV+GFP+ PC3 cells; Miss Sara Akbar for technical assistance. This work was carried out in part at the Flow Cytometry Core Laboratory at Children’s Hospital of Philadelphia, and the Singh Center for Nanotechnology, which is supported by the NSF National Nanotechnology Coordinated Infrastructure Program under grant NNCI-2025608.
Author information
Authors and Affiliations
Contributions
S.L. and L.L. conceived the study, designed the research, and developed the methodology. S.L. and A.N. performed the experiments and generated the data. S.L. analyzed the data. S.L. and L.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
S.L. and L.L. are named inventors on a patent application (application number: PCT/US2023/024722, in process) filed by The Children’s Hospital of Philadelphia covering the ATLAS-seq method. The other author declares no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, S., Notaro, A. & Lin, L. ATLAS-seq: a microfluidic single-cell TCR screen for antigen-reactive TCRs. Nat Commun 16, 216 (2025). https://doi.org/10.1038/s41467-024-54675-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-54675-3