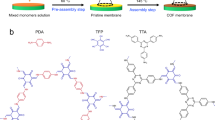
Abstract
Mixed-matrix membranes that combine the merits of polymer and filler materials offer high potential for molecular separations, but precisely engineering the filler phase structure to give full play to the role of filler materials remains challenging. Herein, we explore a reverse-filling approach to fabricate mixed-matrix membranes with continuous and vertically penetrating covalent organic framework channels for CO2 separation. Covalent organic framework nanosheets as building blocks are pre-assembled into a robust and vertically oriented covalent organic framework scaffold via ice templating method, with the subsequent polyimide filling into the scaffold. The scaffold inherits the intrinsic CO2-philic pore structure of nanosheets, which serves as fast and selective CO2 transport channels in the membrane. The resulting membrane exhibits high CO2 permeability of 972 Barrer and CO2/CH4 selectivity of 58, along with long-term stability and scale-up capability. This approach may stimulate the thinking about how to design advanced mixed-matrix membranes.
Similar content being viewed by others
Introduction
Membrane-based technology offers an energy-efficient candidate for diverse molecular separations than traditional separation processes (e.g., adsorption and cryogenic distillation)1. Conventional polymeric membranes with excellent processability are the dominant form in practical applications, but suffering from an intrinsic trade-off relationship between permeability and selectivity2. Mixed-matrix membranes (MMMs), which incorporate favorable fillers into polymeric membranes to optimize the molecular transport channels, have emerged as a class of promising membrane materials to surpass the upper bound limit3,4,5,6,7. Up to now, most MMMs are fabricated through a normal filling approach that fillers are added into the polymer solution and then cast on a flat substrate to evaporate the solvent. This approach is easy to operate and appropriate for various types of filler materials. However, the dispersed fillers are often randomly distributed in the polymer matrix during the solvent evaporation process. The mass transport channels separated by the less permeable and selective polymer often lead to limited performance improvement. Therefore, extensive efforts have been devoted towards high filler loading8,9,10,11,12,13, single-crystal fillers14,15,16,17 and phase separated polymer18,19 to improve the filler connectivity.
To precisely engineer the filler phase structure and give full play to the role of filler materials, we explore a reverse-filling approach to fabricate MMMs in this work. Fillers are beforehand assembled into a robust filler scaffold, followed by filling the polymer matrix into the scaffold. This approach can not only create continuous and penetrating transport channels to reduce mass transfer resistance and thus yield significant performance improvement, but also utilize moderate filler loading to ensure good processing and mechanical properties. In addition, if the filler scaffold possesses a certain orientation along the mass transfer direction, there would be an opportunity to further accelerate the molecular transport throughout the membrane20. As a proof-of-concept, we select polyimide as polymer phase and covalent organic framework (COF) as filler phase. Polyimides are a class of conventional membrane materials that widely used for CO2 separation but faced with low permeability and moderate selectivity21. COFs as an emerging branch of crystalline porous filler materials have the following advantages. Firstly, the designable skeleton and well-defined pore structure can provide abundant molecular transport channels with tailor-made physicochemical properties22,23. Secondly, all organic properties can enable excellent compatibility with polymer to avoid nonselective polymer-filler interfacial void24. Thirdly, COFs can generate different anisotropic morphologies with large external surface area to intensify the connection between fillers25.
Specifically, COF nanosheets as building blocks are pre-assembled into a robust and vertically oriented COF scaffold via ice templating method. Subsequently, polyimide is reversely filled into the COF scaffold to fabricate MMMs where the COF scaffold penetrates through the polyimide matrix (Fig. 1). The properties of COF scaffold can be precisely optimized by modulating COF nanosheets. The oriented scaffold structure and polyimide-COF compatibility endow membrane with good mechanical properties. More importantly, the oriented COF scaffold inherits the intrinsic CO2-philic pore structure of COF nanosheets and shortens the gas transport distance, which serves as fast and selective CO2 transport channels throughout the membrane. The resulting MMMs exhibit high CO2/CH4 separation performances, long-term stability and scale-up potential.
Results
Construction of COF scaffolds
Anionic TpPa-SO3H COF nanosheets were synthesized based on our previously reported single-solution-phase method (Fig. 2a)26,27,28. The obtained colloidal aqueous solution shows typical Tyndall scattering effect, indicating the stable dispersion of COF nanosheets (Fig. 2b). The uniform lamellar morphology of COF nanosheets was observed by scanning electron microscopy (SEM) (Supplementary Fig. 1). The wrinkled and transparent morphology of COF nanosheets in the transmission electron microscopy (TEM) image indicates their ultrathin feature (Fig. 2c). Atomic force microscopy (AFM) image and dynamic light scattering (DLS) result display that the COF nanosheets possess a uniform thickness of around 2.5 nm and a lateral size of around 3.4 μm (Fig. 2d and Supplementary Fig. 2). Solid-state 13C cross-polarization nuclear magnetic resonance (NMR) spectrum exhibits two characteristic signals at 183 ppm (C=O) and 147 ppm (C-N), confirming the target keto-form chemical structure (Supplementary Fig. 3). Powder X-ray diffraction (PXRD) pattern of COF nanosheets reveals two main diffraction peaks, which agrees well with the simulated pattern with an eclipsed stacking model (Fig. 2e). High-resolution TEM (HRTEM) analysis demonstrates the polycrystalline structure of COF nanosheets (Supplementary Fig. 4).
a Chemical structure of TpPa-SO3H COF. b Photographic image of TpPa-SO3H COF nanosheets as a colloidal aqueous suspension. c TEM image of TpPa-SO3H COF nanosheets. d AFM image of TpPa-SO3H COF nanosheets. e Experimental and simulated PXRD patterns of TpPa-SO3H COF nanosheets. f Schematic illustration of unidirectional freezing process for the fabrication of Ori-COF scaffold. g Surface SEM image of Ori-COF10 scaffold. h, i Cross-sectional SEM images of Ori-COF10 scaffold and its wall at high magnification. j Schematic illustration of non-directional freezing process for the fabrication of Ran-COF scaffold. k Surface SEM image of Ran-COF10 scaffold. l, m Cross-sectional SEM images of Ran-COF10 scaffold and its wall at high magnification.
The synthetic route of oriented COF (Ori-COF) scaffold by ice templating method is illustrated in Fig. 2f. Initially, the COF nanosheet suspension was cast on a flat substrate and frozen driven by a temperature gradient along the vertical direction of casting solution. The water solidification resulted in the phase separation of the aqueous nanosheet suspension. The COF nanosheets were rejected from the forming ice and spontaneously accumulated and gradually templated between the vertically growing ice crystals. The freezing process was readily accessible under 10 s. Subsequent cryodesiccation removed the ice templates to obtain the Ori-COF scaffold. The surface morphology of Ori-COF10 scaffold with the density of 10 mg cm−3 exhibits an ordered honeycomb-like cellular structure (Fig. 2g). Cross-sectional SEM image manifests that the COF nanosheets are assembled into vertical walls of COF scaffold as expected (Fig. 2h), which possess a thickness of around 200–350 nm (Fig. 2i) and spacing of around 20 μm (Supplementary Table 1). No interlayer defects are observed in the COF wall due to the sufficient van der Waals force between COF nanosheets.
Once the casting suspension was instantaneously frozen without temperature gradient applied (Fig. 2j), the disordered ice crystals templated the TpPa-SO3H COF nanosheets into random COF (Ran-COF) scaffold with irregular and inhomogeneous cellular structure (Fig. 2k). The COF walls of Ran-COF scaffold display no orientation in the cross-sectional view (Fig. 2l). The smaller COF wall thickness of around 100–200 nm indicates that the slow and directional freezing process promotes the more concentrated assembly of COF nanosheets29 (Fig. 2m). Photographic images manifest that the Ori-COF scaffold possesses more uniform morphology and higher transparency due to its long-range ordered structure with vertical orientation (Supplementary Fig. 5). We immersed the Ori-COF scaffold into several common solvents, which maintains the structure integrity for at least 7 days (Supplementary Fig. 6). The thermal degradation temperature of the Ori-COF scaffold in the thermogravimetric analysis (TGA) curve is determined up to 260 °C (Supplementary Fig. 7), verifying the good thermal and solvent stability for the post-fabrication. We also constructed both oriented and random cationic EBCOF:Br COF scaffolds30,31 to demonstrate the universality of ice templating method for various COF nanosheets (Supplementary Fig. 8).
Modulation of COF scaffolds
By tailoring the concentration of COF nanosheet suspension, the Ori-COF scaffold density can be well manipulated. The COF wall spacing can be reduced to around 10 μm when the scaffold density increases to 15 mg cm−3 (Fig. 3a and Supplementary Table 1). We further concentrated the nanosheet suspension and increased the scaffold density up to 20 mg cm−3, and the Ori-COF20 scaffold is no longer continuous in the vertical direction (Supplementary Fig. 9). It reveals that the growing ice crystals encounter large resistance by excessive COF nanosheets, and the low water content is insufficient to form complete ice templates and thus continuous COF walls. In addition, the corresponding nanosheet suspension with high viscosity gelled in a short time, implying its poor processability and availability. Free-standing Ori-COF scaffold is also available by increasing the casting suspension volume (Supplementary Fig. 10).
a Cross-sectional SEM images of Ori-COF scaffolds with the density of 10, 12, and 15 mg cm−3. b Schematic illustration of the functionalized Ori-PCOF scaffold. c Surface SEM image of Ori-P0.3COF15 scaffold. d Cross-sectional SEM image of Ori-P0.3COF15 scaffold. e Enlarged views of XPS spectra of deconvoluted N 1s of Ori-COF15 and Ori-P0.3COF15 scaffolds. f MD simulations of the calculated non-bonded interaction energy between TpPa-SO3H COF and PEGDA. g Isosteric heats of adsorption for CO2 of Ori-COF15 and Ori-P0.3COF15 scaffolds.
The abundant ionic sites on the TpPa-SO3H skeleton endow COF scaffold with further structure functionalization32. To utilize the COF scaffold as filler phase for target CO2 separation, we introduced CO2-philic poly(ethyleneglycol) diamines (PEGDA) into the TpPa-SO3H COF nanosheet suspension to prepare functionalized Ori-COF (Ori-PCOF) scaffolds (Fig. 3b and Supplementary Fig. 11). The Ori-PCOF scaffold maintains the highly aligned structure (Fig. 3c,d), while the COF wall spacing is decreased to around 5 μm and its thickness is expanded to around 400 nm (Supplementary Fig. 12 and Supplementary Table 1). Fourier transform infrared spectroscopy (FTIR) and solid-state 13C NMR spectra of Ori-PCOF scaffolds exhibit characteristic signals of PEGDA, confirming the successful functionalization (Supplementary Fig. 13). The deconvoluted N 1s spectra of X-ray photoelectron spectroscopy (XPS) analyses reveal the increased intensity of N-H peak (~401.7 eV) after the introduction of amino groups in PEGDA (Fig. 3e). Based on the integration of the peak areas of N-H and C-N33, the functionalization degree (molar ratio of additional amino groups to sulfonic acid groups) of the Ori-P0.3COF15 scaffold is assessed to be around 30%. Moreover, the S 2p peak of the Ori-P0.3COF15 scaffold shifts to lower binding energy compared with that of Ori-COF15 scaffold (Supplementary Fig. 14), suggesting the interactions between TpPa-SO3H COF nanosheet and PEGDA chain. We further performed all-atom molecular dynamics (MD) simulations to elucidate the potential interactions (Fig. 3f). After calculating the non-bonded interaction energies, the results show that the electrostatic interaction between the sulfonic acid groups of TpPa-SO3H COF and amino groups of PEGDA dominates the assembly behavior under aqueous environment. When the functionalization degree of the Ori-P0.5COF15 scaffold increases to 50%, the COF walls are still vertical but slightly twisted due to the massive addition of flexible PEGDA chains (Supplementary Fig. 15).
Gas adsorption-desorption isotherm measurements were performed to characterize the micropore structure of COF scaffold. The pore size distribution curve of Ori-COF15 scaffold shows a main peak position around 1.4 nm derived from the intrinsic micropores of TpPa-SO3H COF nanosheets (Supplementary Fig. 16). After functionalization, the Ori-P0.3COF15 scaffold generates partial ultramicropores with size of about 0.6 nm, suggesting that the sheltering effect by PEGDA chains narrows the COF pore size. In addition, the introduction of dipolar alkoxy groups provides preferential interactions with the quadrupolar CO2 molecules34. Correspondingly, the Ori-P0.3COF15 scaffold exhibits a higher isosteric heat of adsorption for CO2 (25–30 kJ mol−1) than that of Ori-COF15 scaffold (20–25 kJ mol−1), revealing the improved CO2 affinity (Fig. 3g and Supplementary Fig. 17). The above modulation strategies are also applicable to Ran-COF scaffolds for subsequent membrane fabrication (Supplementary Fig. 18). Therefore, we achieve the modulation of COF scaffolds with favorable physicochemical properties for CO2 separation while maintaining the porous structure.
Preparation of mixed-matrix COF membranes
Infiltration of freeze-cast structures with a secondary phase allows to fabricate functional composite materials35,36. As shown in Fig. 4a, the 6FDA-DAM polyimide solution was reversely filled into the space between the COF walls, until the whole scaffold was embedded in the polymer matrix by controlling the amount of polymer. The FTIR spectra of MMMs display strong characteristic bands arising from the C=O (carbonyl) and C-N (imide) stretching of 6FDA-DAM (Supplementary Fig. 19). Compared with the smooth and homogeneous surface of pristine 6FDA-DAM membrane (Supplementary Fig. 20), the COF scaffold can be clearly discerned from the 6FDA-DAM matrix by the hexagonal structure on the MMM surface (Fig. 4b). AFM image shows that the membrane surface roughness is 24.9 nm on average, implying the almost complete filling taking into account the hundred-micron membrane thickness (Fig. 4c). Significantly, the COF scaffold is well preserved in the MMM benefitting from its highly robust and stable structure (Fig. 4d and Supplementary Fig. 21). Focused ion beam scanning electron microscopy (FIB-SEM) was utilized to observe the cross-sectional morphology on the MMM surface (Supplementary Fig. 22), which shows an inevitable but ultrathin polyimide layer (~70 nm) deposited on the top surface of COF scaffold. In addition, the MMM exhibits good compatibility between COF and polymer without apparent interfacial voids observed, due to the strong interactions and similar thermal expansivity of two types of organic materials (Fig. 4e). HRTEM analysis proves that the COF scaffold in the polymer matrix inherits the polycrystalline structure of COF nanosheets (Fig. 4f and Supplementary Fig. 23), and thus the COF walls with isotropic porous structure can serve as molecular transport channels throughout the membrane. Elemental mapping images manifest the approximatively complementary distributions of sulfur and fluorine elements, demonstrating the polymer chains rarely penetrate through the COF walls and maintain their intrinsic COF pore structure (Fig. 4g and Supplementary Fig. 24). TGA curves indicate good thermal stability of MMMs (Supplementary Fig. 25), and the COF loading was calculated according to the weight loss of MMMs and corresponding components (Fig. 4h and Supplementary Table 2).
a Schematic illustration of reverse-filling process to fabricate Ori-COF MMMs. b Surface SEM image of Ori-COF15 MMM. c AFM image of the top surface of Ori-COF15 MMM. d Cross-sectional SEM image of Ori-COF15 MMM. e TEM image of ultrathin section of Ori-P0.3COF15 MMM. f HRTEM image and corresponding FFT image of the COF wall in Ori-P0.3COF15 MMM. g EDS elemental mapping of ultrathin section of Ori-P0.3COF15 MMM. h COF loading in the MMMs calculated by TGA results. i DSC curves of pristine 6FDA-DAM membrane and MMMs. j Young’s moduli of pristine 6FDA-DAM membrane and MMMs calculated by stress-strain curves. Error bars represent standard deviations for three measurements.
To gain insight into the packing state of polymer chains, we first examined the XRD patterns of the MMMs (Supplementary Fig. 26). The broad diffraction peak at 2θ = 10–18° reflects the bulk polymer interchain spacing that agrees well with that of pristine 6FDA-DAM membrane, verifying the introduction of COF scaffolds hardly affects the packing state of bulk polymer matrix. Differential scanning calorimetry (DSC) analysis was performed to examine the polymer chains around the polymer-COF interface (Fig. 4i). The slightly higher glass transition temperature (Tg) of MMMs manifests that the strong interactions between two components restrict the mobility of polymer chains at the interface, but the impact is relatively small. Compared with Ran-COF MMMs, the Ori-COF MMMs generate less rigid interfacial regions due to the more concentrated assembly of COF nanosheets, and the introduction of flexible PEGDA chains further reduces this effect in the Ori-PCOF MMMs. Mechanical properties of the MMMs were also evaluated by stress-strain curves (Fig. 4j). It can be found that the Ori-COF MMMs exhibited higher Young’s modulus and deformation resistance than those of Ran-COF MMMs. This phenomenon extensively exists in various tough natural materials37,38,39, such as nacres and tendons, which can be attributed to the internal anisotropic and ordered structures.
Gas separation performances
To elucidate the potential of MMMs for efficient CO2 separation, we assessed their separation performances for CO2/CH4 mixed gas (volume fraction ratio: 30/70 vol%) as shown in Fig. 5a and Supplementary Fig. 27. Pristine 6FDA-DAM membrane exhibits a CO2 permeability of 457 Barrer and a CO2/CH4 selectivity of 29. In regard to MMMs, the CO2 permeability gradually increases with the scaffold density due to more introduced transport channels provided by the COF walls. Excessive scaffold density leads to an obvious decrease in CO2 permeability due to the discontinuous scaffold structure (Supplementary Fig. 28). More importantly, the CO2 permeability of Ori-COF MMM exhibits more significant improvement compared with its Ran-COF counterpart, which exceeds 900 Barrer with the scaffold density of 15 mg cm−3. We deconvoluted the CO2 and CH4 permeability into sorption coefficient (solubility, Si) and diffusion coefficient (diffusivity, Di) according to the solution-diffusion model (Fig. 5b and Supplementary Table 3). The structure difference between the Ori-COF and Ran-COF scaffolds rarely affects the gas sorption coefficient of their corresponding MMMs. In stark contrast, the Ori-COF MMM exhibits more significant enhancement in gas diffusion coefficient than that of Ran-COF MMM. The results manifest that the vertically oriented COF scaffold provides shorter transport channels for fast permeation (Fig. 5c). In addition, the Ori-COF MMMs maintain the CO2/CH4 selectivity of pristine 6FDA-DAM membrane despite the large COF micropore size (1.4 nm), which is attributed to the polyimide skin layer. Due to the relatively denser polymer chain packing according to the DSC analysis, the Ran-COF MMMs exhibit slightly higher CO2/CH4 selectivity compared with their Ori-COF counterparts. We further evaluated the separation performances of MMMs with functionalized COF scaffolds (Fig. 5d). Likewise, the Ori-PCOF MMMs display obvious superiority in CO2 permeability irrespective of the functionalization degree compared with those of Ran-PCOF MMMs. Surprisingly, the Ori-P0.3COF15 MMM exhibits a substantial improvement of 102% in CO2/CH4 selectivity and a slight increase in CO2 permeability after the functionalization of COF scaffold. We deconvoluted the CO2/CH4 selectivity into sorption selectivity and diffusion selectivity for both Ori-COF15 and Ori-P0.3COF15 MMMs, which exhibit a marked concurrent enhancement (Fig. 5e). The PEG units as CO2-philic nanodomains enhance the CO2 affinity and thus CO2 solubility, and the narrowed COF channels increase the diffusion difference between CO2 and CH4 molecules and strengthen the molecular sieving effect. Excessive functionalization results in the COF pore blocking and thus a sharp decrease in CO2 permeability.
a Mixed CO2/CH4 (30/70 vol %) separation performances of pristine 6FDA-DAM membrane, Ran-COF and Ori-COF MMMs with different scaffold densities at 298 K and 0.2 MPa. b Sorption coefficient and diffusion coefficient of Ran-COF15 and Ori-COF15 MMMs. c Schematic illustration of gas transport in the MMMs with two types of COF scaffolds. d Mixed CO2/CH4 (30/70 vol %) separation performances of Ori-PCOF15 and Ran-PCOF15 MMMs with different functionalization degrees at 298 K and 0.2 MPa. e CO2/CH4 sorption selectivity and diffusion selectivity of Ori-COF15 and Ori-P0.3COF15 MMMs. f Long-term stability of Ori-P0.3COF15 MMM at 298 K and 0.2 MPa for CO2/CH4 (30/70 vol %) mixed gas. g Photographic image of large-scale Ori-P0.3COF15 MMM and its separation performances at 298 K and 0.2 MPa for CO2/CH4 (30/70 vol %) mixed gas. h Comparison of the CO2/CH4 separation performances of MMMs in this work with representative MMMs in the literature (Supplementary Table 4). Error bars represent standard deviations for three measurements.
The optimal Ori-P0.3COF15 MMM exhibits good long-term stability with no appreciable performance loss for 30 days (Fig. 5f). We further evaluated the mixed CO2/CH4 (50/50 vol%) separation performances of the membranes at high pressures up to 40 bar (Supplementary Fig. 29). The Ori-P0.3COF15 MMM shows no CO2 plasticization pressure point up to 20 bar and lower selectivity loss compared with pristine 6FDA-DAM membrane, which indicates the Ori-COF scaffold as filler phase endows membrane with good plasticization-resistant property. Good COF-polymer compatibility and membrane flexibility contribute to no abrupt selectivity or permeability loss of Ori-P0.3COF15 MMM after bending (Supplementary Fig. 30). Benefiting from the facile and scalable fabrication potential of ice-templating and reverse-filling processes, the Ori-P0.3COF15 MMM can be easily scaled up to 200 cm2 with stable separation performance, demonstrating its high potential for large-scale applications (Fig. 5g). The Ori-P0.3COF15 MMM exhibits comparable CO2/CH4 separation performances with advanced MMMs with porous fillers as shown in Fig. 5h. Its great potential for efficient CO2 separation is also proved by high separation performance for CO2/N2 mixed gas (Supplementary Fig. 31). In addition, the reverse-filling approach is applicable for other polymer and COF materials to achieve membrane performance breakthrough (Supplementary Fig. 32).
Discussion
In summary, we explore a reverse-filling approach to fabricate MMMs with continuous and vertically penetrating COF channels for CO2 separation. COF nanosheets as building blocks are pre-assembled into a robust COF scaffold by ice templating method, followed by filling polyimide into the COF scaffold which penetrates through the polyimide matrix. The orientation of COF scaffold can be modulated by controlling the freezing process. The vertically oriented COF scaffold can shorten the mass transport distance and thus improve the CO2 permeability of the MMMs. Besides, the physicochemical properties of COF scaffold can be further modulated by modifying COF nanosheets. The vertically oriented COF scaffold with narrowed COF pore size and enhanced CO2 affinity leads to the improvement of CO2/CH4 selectivity of the MMMs. Accordingly, the structural advantages of COF scaffold endow membrane with high CO2 permeability of 972 Barrer, CO2/CH4 selectivity of 58 and good long-term stability. Combined with its scalability and universality, our reverse-filling approach provides a platform for the rational design of filler phase structure in the MMMs, which may motivate the innovative development of MMMs for diverse molecular separations.
Methods
Materials
4,4′-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA, 99%), diamine 2,4,6-trimethyl-1,3-diaminobenzene (DAM, 96%) and 2,3,5,6-tetra-fluoroterephthalonetrile (TFTPN, 99%) were purchased from Sigma-Aldrich. 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde (TFP, 98%) was purchased from Jilin Chinese Academy of Sciences-Yanshen Technology. 2,5-diaminobenzenesulfonic acid (DABA, 98%) was purchased from TCI. Poly(ethyleneglycol) diamines (PEGDA, Mn=1000) and ethidium bromide (EB, 99%) were purchased from Macklin. Acetic anhydride (99.7%), hydrochloric acid (HCl, 36.5 wt% in water) and trichloromethane (CHCl3, 99.8%) were purchased from Tianjin Jiangtian Chemical Technology Co., Ltd. 3-methyl-pyridine (99.5%), dimethyl sulfoxide (DMSO, 99.9%), N-methylpyrrolidone (NMP, 99.5%), methanol (CH3OH, 99.9%), potassium carbonate (K2CO3, 99.5%), dichloromethane (CH2Cl2, 99.5%) and N,N-dimethylformamide (DMF, 99.9%) were purchased from Aladdin. 5,5′,6,6′-tetrahydroxy-3,3,3′,3′-tetramethyl-1,1′-spirobisindane (TTSBI, 97%) was purchased from Alfa Aesar and purified by the recrystallization with CH3OH and CH2Cl2. Deionized (DI) water used in all experiments was prepared by a Milli-Q ultrapure unit.
Preparation of the TpPa-SO3H COF nanosheets
The TpPa-SO3H COF nanosheets were prepared through a single-solution-phase method. Typically, TFP (0.5 mmol, 105.1 mg) and DABA (0.75 mmol, 141.2 mg) were separately dissolved in DMSO (5 mL) and mixed under gentle stirring. Then, the mixture maintained stable at room temperature for 24 h. The resulting dark red solution was dialyzed in DI water (2 L) for 2 days using a dialysis membrane tube with a molecular weight cut-off (MWCO) of 30,000. The TpPa-SO3H COF nanosheets were obtained as a dark red aqueous colloidal suspension, which can be further diluted or concentrated by rotary evaporation at 50 °C to obtain various concentrations.
Preparation of 6FDA-DAM polyimide
6FDA (0.02 mol, 8.9 g) and DAM (0.02 mol, 3.0 g) were dried under vacuum for 12 h at 110 °C and 60 °C, respectively. Then, the monomers were added into anhydrous NMP (50 mL), and vigorously stirred under N2 atmosphere at 0 °C for 24 h to produce a yellow solution. Then, acetic anhydride (15 mL) and 3-methylpyridine (2 mL) were added into the solution under N2 atmosphere at ambient temperature for 24 h. The resulting polyimide was precipitated and washed with methanol several times, and further dried at 200 °C under vacuum for 24 h to obtain a white polymer. The number-average molecular weight (Mn) of polyimide was assessed to be 126,000 Daltons with the polydispersity index (PDI) of 1.56.
Preparation of TpPa-SO3H COF scaffolds
To prepare the Ori-COF scaffold, a certain amount of TpPa-SO3H COF nanosheet suspension was blade-cast on a quartz glass plate with a blade gap of 200 μm to form a wet film. Then, the bottom of quartz glass plate was carefully inserted into the liquid nitrogen, ensuring that the casting suspension did not contact with the liquid nitrogen. The frozen suspension attached on the quartz glass plate was subsequently freeze-dried at −60 °C and 0.011 mbar for 12 h to remove the ice templates. The obtained scaffold was denoted as Ori-COFx, where x represents the COF scaffold density. For the Ran-COFx scaffold, the casting suspension on the substrate was directly immersed into the liquid nitrogen. The remaining synthetic procedures were the same as mentioned above. For the functionalized COF scaffolds, a certain amount of PEGDA was added into the TpPa-SO3H COF nanosheet suspension and stirred at room temperature for 5 min to obtain a homogeneous suspension. The mass ratios of PEGDA and TpPa-SO3H COF were determined to 1:2 and 1:1, respectively. The remaining synthetic procedures were the same as mentioned above. The obtained scaffolds were denoted as Ori-PyCOFx and Ran-PyCOFx, where y represents the functionalization degree of the COF scaffold calculated by XPS results. For the free-standing COF scaffolds, the COF nanosheet suspension can be blade-cast on an aluminum plate, which is automatically peeled off after freeze-dried.
Preparation of mixed-matrix COF membranes
6FDA-DAM polyimide was dissolved into anhydrous DMF to obtain a homogenous polyimide solution (8 wt%). Then, a certain amount of polyimide solution was applied to the COF scaffold and then slowly dried at 80 °C for 24 h, making sure both top and bottom of COF scaffold were well embedded in the polyimide matrix. The resulting membrane was further dried under vacuum at 200 °C for 24 h and cooled down to room temperature naturally for further characterization and measurement. Pristine 6FDA-DAM membrane without COF scaffold embedded was also prepared using same conditions as mentioned above.
Gas separation performance test
Gas separation performances of the membranes were evaluated using a laboratory-made permeation apparatus based on the constant pressure/variable-volume method. CO2/CH4 (30/70 vol%) mixed gas was utilized as feed gas at an absolute pressure of 2 bar, while pure N2 was applied as sweep gas at a flow rate of 20 mL (STP) min−1 controlled by using a mass flow meter. The gas composition of the permeate side was detected using a gas chromatography (Shimadzu GC2030). At least three samples of each type of membrane were fabricated and tested until the system maintained stable to determine experimental reproducibility. The gas permeability of the membrane was calculated by Eq. (1):
where Pi represents the permeability coefficient of gas “i” (Barrer, 1 Barrer = 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1), Qi (cm3 (STP) s−1) represent the volumetric flow of gas “i”, Δpi (cmHg) represents the partial pressure difference of gas “i” between feed side and permeate side, A (cm2) and l (cm) represent the effective area and average thickness of the membrane, respectively.
The mixed gas selectivity αi/j was calculated by Eq. (2):
The single-gas permeabilities P (Barrer) of the membranes were evaluated using a Labthink VAC-V2 apparatus based on the constant-volume/variable-pressure method. The diffusivity coefficient D (cm2 s−1) was calculated by the time-lag method using the following Eq. (3):
where θ is the time-lag of the permeability measurement.
The solubility coefficient S (cm3 (STP) cm−3 cmHg−1) was calculated by the ratio of gas permeability to diffusivity coefficient as the following Eq. (4):
Molecular dynamics (MD) simulation
The MD simulation was performed by the Material Studio software. The structures of TpPa-SO3H and PEGDA were optimized using the COMPASS force field and the non-bonded interaction energies were calculated based on a Dreiding force field. The non-bonded interactions include van der Waals forces (EvdW), hydrogen bonds (EH-bond) and electrostatic interactions (EElectrostatic). The EvdW and EH-bond are described by the Lennard-Jones (12-6) and Lennard-Jones (12-10) potential, respectively. The EElectrostatic is described by a screened (distance-dependent) Coulombic term and atomic monopoles. The non-bonded interaction energy ΔE between TpPa-SO3H and PEGDA was calculated by the following Eq. (5):
Where ETpPa-SO3H@PEDGA is the non-bonded energy of TpPa-SO3H and PEGDA system, ETpPa-SO3H and EPEDGA is the non-bonded energy of TpPa-SO3H and PEGDA, respectively.
Data availability
All data supporting the findings of this study are available within the article and the Supplementary Information file, or available from the corresponding authors upon request. Source data are provided with this paper.
References
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M. & Freeman, B. D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017).
Chuah, C. Y. et al. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 118, 8655–8769 (2018).
Ghalei, B. et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2, 17086 (2017).
Rodenas, T. et al. Metal-organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 14, 48–55 (2015).
Cheng, Y. et al. Highly efficient CO2 capture by mixed matrix membranes containing three-dimensional covalent organic framework fillers. J. Mater. Chem. A 7, 4549–4560 (2019).
Wu, J., Liang, C. Z., Naderi, A. & Chung, T.-S. Tunable supramolecular cavities molecularly homogenized in polymer membranes for ultraefficient precombustion CO2 capture. Adv. Mater. 34, e2105156 (2022).
Datta, S. J. et al. Rational design of mixed-matrix metal-organic framework membranes for molecular separations. Science 376, 1080–1087 (2022).
Tan, X. et al. Truly combining the advantages of polymeric and zeolite membranes for gas separations. Science 378, 1189–1194 (2022).
Su, Y. et al. Uniformly distributed mixed matrix membranes via a solution processable strategy for propylene/propane separation. Angew. Chem. Int. Ed. 63, e202316093 (2024).
Knebel, A. et al. Solution processable metal-organic frameworks for mixed matrix membranes using porous liquids. Nat. Mater. 19, 1346–1353 (2020).
Chen, G. et al. Solid-solvent processing of ultrathin, highly loaded mixed-matrix membrane for gas separation. Science 381, 1350–1356 (2023).
Hardian, R. et al. Design of mixed-matrix MOF membranes with asymmetric filler density and intrinsic MOF/polymer compatibility for enhanced molecular sieving. Adv. Mater. 36, 2314206 (2024).
Kiesow, I. et al. Bicontinuous zeolite polymer composite membranes prepared via float casting. J. Am. Chem. Soc. 135, 4380–4388 (2013).
Ma, X., Wu, X., Caro, J. & Huang, A. Polymer composite membrane with penetrating ZIF-7 sheets displays high hydrogen permselectivity. Angew. Chem. Int. Ed. 58, 16156–16160 (2019).
Song, S. et al. Weakly pressure-dependent molecular sieving of propylene/propane mixtures through mixed matrix membrane with ZIF-8 direct-through channels. J. Membr. Sci. 648, 120366 (2022).
Wang, B. et al. Unobstructed ultrathin gas transport channels in composite membranes by interfacial self-assembly. Adv. Mater. 32, 1907701 (2020).
Li, C. et al. Establishing gas transport highways in MOF-based mixed matrix membranes. Sci. Adv. 9, eadf5087 (2023).
Qi, A., Li, C., Evans, J. D., Zhao, Y. & Li, T. Self-sorting of interfacial compatibility in MOF-based mixed matrix membranes. Angew. Chem. Int. Ed. 63, e202400474 (2024).
Qiao, Z. et al. A highly permeable aligned montmorillonite mixed-matrix membrane for CO2 separation. Angew. Chem. Int. Ed. 55, 9321–9325 (2016).
Yi, S., Ghanem, B., Liu, Y., Pinnau, I. & Koros, W. J. Ultraselective glassy polymer membranes with unprecedented performance for energy-efficient sour gas separation. Sci. Adv. 5, eaaw5459 (2019).
Guo, Z. et al. Missing-linker defects in covalent organic framework membranes for efficient CO2 separation. Angew. Chem. Int. Ed. 61, e202210466 (2022).
Liu, R. et al. Covalent organic frameworks: an ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 50, 120–242 (2021).
Knebel, A. & Caro, J. Metal–organic frameworks and covalent organic frameworks as disruptive membrane materials for energy-efficient gas separation. Nat. Nanotechnol. 17, 911–923 (2022).
Shao, G., Hanaor, D. A. H., Shen, X. & Gurlo, A. Freeze casting: from low-dimensional building blocks to aligned porous structures-a review of novel materials, methods, and applications. Adv. Mater. 32, e1907176 (2020).
Huang, T. et al. Single solution-phase synthesis of charged covalent organic framework nanosheets with high volume yield. Angew. Chem. Int. Ed. 62, e202209306 (2023).
Chandra, S. et al. Interplaying intrinsic and extrinsic proton conductivities in covalent organic frameworks. Chem. Mater. 28, 1489–1494 (2016).
Peng, Y. et. al, Mechanoassisted synthesis of sulfonated covalent organic frameworks with high intrinsic proton conductivity. ACS Appl. Mater. Interfaces 8, 18505–18512 (2016).
Nishihara, H., Mukai, S. R., Yamashita, D. & Tamon, H. Ordered macroporous silica by ice templating. Chem. Mater. 17, 683–689 (2005).
Ma, H. et al. Cationic covalent organic frameworks: a simple platform of anionic exchange for porosity tuning and proton conduction. J Am. Chem. Soc. 138, 5897–5903 (2016).
Mal, A. et al. Supramolecular reassembly of self-exfoliated ionic covalent organic nanosheets for label-free detection of double-stranded DNA. Angew. Chem. Int. Ed. 57, 8443–8447 (2018).
Guo, Z. et al. Oil-water-oil triphase synthesis of ionic covalent organic framework nanosheets. Angew. Chem. Int. Ed. 60, 27078–27085 (2021).
Zhao, S. et al. Hydrophilicity gradient in covalent organic frameworks for membrane distillation. Nat. Mater. 20, 1551–1558 (2021).
Wang, S. et al. Graphene oxide membranes with heterogeneous nanodomains for efficient CO2 separations. Angew. Chem. Int. Ed. 56, 14246–14251 (2017).
Cao, L. et al. Graphene oxide‐based solid electrolytes with 3D prepercolating pathways for efficient proton transport. Adv. Funct. Mater. 28, 1804944 (2018).
Zhang, W. H. et al. Graphene oxide membranes with stable porous structure for ultrafast water transport. Nat. Nanotechnol. 16, 337–343 (2021).
Hua, M. et al. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 590, 594–599 (2021).
Wegst, U. G., Bai, H., Saiz, E., Tomsia, A. P. & Ritchie, R. O. Bioinspired structural materials. Nat. Mater. 14, 23–36 (2015).
Pan, Y. et al. Structural characteristics and physical properties of lotus fibers obtained from Nelumbo nucifera petioles. Carbohydr. Polym. 85, 188–195 (2011).
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (U20B2023 by Z.J., 22208238 by Y.R.), National Key R&D Program of China (2022YFB3805200 by Z.J.), and the Program of Introducing Talents of Discipline to Universities (No. BP0618007 by Z.J.).
Author information
Authors and Affiliations
Contributions
Z.G., W.L., H.W., and Z.J. conceived the idea and designed the research. J.S., H.W., and Z.J. supervised the research. Z.G. and W.L. carried out the experiment. X.M. performed partial characterization. L.C., S.S., Y.R., T.H., and Y.L. provided constructive suggestions for results and discussion. Z.G., H.W., and Z.J. co-wrote the paper. All authors participated actively in the discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors decare no competing interests.
Peer review
Peer review information
Nature Communications thanks Himadri Sekhar Sasmal, Tai-Shung Chung and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, Z., Li, W., Wu, H. et al. Reverse filling approach to mixed matrix covalent organic framework membranes for gas separation. Nat Commun 16, 3617 (2025). https://doi.org/10.1038/s41467-025-56770-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56770-5