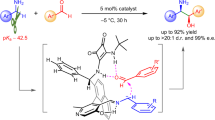
Abstract
Selective functionalization of ubiquitous C-H bonds in organic molecules provides a straightforward and efficient approach to construct complex molecules with fewer synthetic steps and high atom economy, thus promoting more sustainable and economical chemical synthesis. A formidable challenge in the field is to increase the turnover numbers (TONs) for catalytic C-H functionalization reactions reported in the literature (generally <10,000) to reasonably high levels to reduce the cost of the reaction. Another challenge is the selective functionalization of less reactive primary C(sp3)-H bonds compared to other types of more reactive C-H bonds. We now demonstrate an efficient iridium porphyrin-catalysed asymmetric carbene insertion into primary N-adjacent C(sp3)-H bond of N-methyl indoline and N-methyl aniline derivatives. Using chiral iridium porphyrin as a catalyst, chiral β-amino acid derivatives have been obtained with very high yields and excellent ee values (up to 99%), and TONs as high as 84,000 to 1,380,000. The reaction can be readily performed on a 100 g scale while retaining its high efficiency and selectivity. We also show that this iridium catalysis can efficiently access oligomers and polymers of β-amino acid derivatives via stepwise C-H insertion, demonstrating its potential applications in materials science via C-H bond functionalization reactions.
Similar content being viewed by others
Introduction
Efficient and highly selective catalytic chemical transformation reactions are the ‘Holy Grail’ of organic synthesis and play a pivotal role in modern society1,2,3. These reactions are crucial for efficiently producing valuable compounds with minimal waste, driving sustainable practices in the modern chemical industry. For example, transition metal-catalysed asymmetric hydrogenation reactions have revolutionised modern synthetic chemistry and have had far-reaching impacts4,5. This powerful synthetic approach enables stereo-selective reduction of double bonds in starting materials via hydrogenation, resulting in efficient production of value-added products. The large-scale industrial application of asymmetric hydrogenation is attributed to its exceptional attributes, including high product turnover number (TON), which is usually hundreds of thousands to millions, coupled with excellent stereo-selectivity, often in excess of 95% enantiomeric excess (ee) and high atom-economy6,7,8,9,10,11,12. Catalytic C-H bond functionalization (Fig. 1a) via C-H bond activation13,14,15,16,17,18,19 or C-H bond insertion20,21,22,23,24 provides a direct route to convert C-H bonds into new chemical bonds. This makes it possible to install valuable functional groups and complex moieties in target molecules. Given the ubiquity of C-H bonds in organic molecules, this method provides a direct and efficient way to construct complex molecules with fewer synthetic steps and higher atom economy, thus facilitating more sustainable and economical chemical synthesis. In this regard, catalytic C-H bond functionalization has garnered widespread attention and there has been much effort devoted to its application in late-stage modification of complex molecules14,25,26,27. Transition-metal-catalysed asymmetric carbene insertions into C-H bonds with site- and stereo-selectivity offer an attractive and direct approach to this field. It is worth noting that most of the selective carbene C-H insertion reactions reported so far rely on dirhodium catalysis, through the combination of different chiral rhodium catalysts and carbene precursors. On the other hand, there are few examples of using other transition metal catalysts14,20,21,22,23.
Despite significant advances in catalytic C-H functionalization, there are still some key challenges that need to be resolved before further large-scale applications. A particularly pressing issue in this area is how to improve the relatively low product TON in reported examples28,29,30,31,32,33,34,35 (generally <10,000; 98,000 in one example36) to reasonable high levels to reduce reaction costs while minimising the impact of catalyst contamination on product. The second challenge is to selectively functionalise specific C-H bonds in target molecules that have multiple C-H bonds of similar reactivity28,37, as well as primary C(sp3)-H bonds that are less reactive relative to other more reactive C-H bonds38,39.
Natural enzymes have a remarkable ability to catalyse C-H functionalization of specific substrates under mild conditions, with high activity and selectivity40,41. However, the difficulty in obtaining natural enzymes in large quantities means that they are generally expensive and not widely available42,43,44. In addition, the substrate scope of enzymatic reactions is often limited, and the catalytic results are sensitive to multiple factors such as pH and temperature. To address these challenges, various bio-mimetic metal complexes have been developed to catalyse C-H functionalization. Although the complexes reported so far are easier to prepare and modify than natural enzymes, their activity and selectivity are often difficult to match the efficiency of natural enzymes. Therefore, the development of robust metal catalysts that can efficiently and selectively catalyse C-H functionalization over a wide range of substrates is beneficial for unlocking the practical and large-scale application of C-H functionalization reactions in precision organic synthesis. Previously, we demonstrated that chiral iridium porphyrins can catalyse asymmetric intermolecular carbene insertions into O-adjacent secondary C-H bonds in tetrahydrofuran using donor-acceptor diazo compounds as carbene precursors45,46 (Fig. 1a, inset). Here, we report an efficient iridium porphyrin-catalysed asymmetric carbene insertion into primary N-adjacent C(sp3)-H bonds of N-methyl indoline and N-methyl aniline derivatives. Using chiral iridium porphyrin as a catalyst, chiral β-amino acid derivatives were obtained with very high yields and excellent ee values, with product TONs ranging from 84,000 to 1,380,000 (Fig. 1b). Notably, the reaction can be easily carried out on a 100 g scale while retaining its high efficiency and selectivity. In addition, this iridium catalysis can efficiently produce oligomers and polymers of β-amino acid derivatives through stepwise C-H insertion, demonstrating its potential application in materials science through C-H bond functionalization reactions.
Results and discussion
Carbene insertion into C(sp3)-H bonds of N-methyl indolines
Iridium porphyrin catalysis was initiated by exploring the catalytic carbene insertion of N-methyl indoline 1a, which has three types of C-H bonds susceptible to carbene insertion: N-adjacent primary and secondary C(sp3)-H bonds, as well as the aryl C(sp2)-H bonds. Notably, under common conditions, primary C-H insertion is preferred among these options (Supplementary Tables 1 and 2). Using the donor-acceptor diazo compound 2a as the carbene precursor, rapid screening studies show that iridium porphyrin complexes can selectively afford the primary C-H insertion product 3a (Supplementary Table 1). Encouraged by this result, chiral iridium porphyrin complexes were used as catalysts in the reaction. To our delight, 0.5 mol% of Ir(D4-Por)(CO)Cl proved sufficient to carry out the reaction and the desired primary C(sp3)-H insertion product 3a was obtained in 70% NMR yield and 97% ee, and the double insertion product 6 was obtained in 16% NMR yield within 24 h (Supplementary Table 2, entry 1). As expected, Ir(D4-Por)Me with an axial methyl group, showed significantly higher activity due to the strong trans effect of the methyl group, resulting in the reaction being completed within 5 min; the NMR yield of 3a was 67%, although the ee value of 90% is slightly lower (Supplementary Table 2, entry 2). Interestingly, we serendipitously discovered that a simple modification of the separation procedure in the preparation of the iridium porphyrin catalyst could significantly improve the catalytic reaction (with Ir(D4-Por)(CO)Cl catalyst), thereby reducing the reaction time from 24 h to 2 h (Supplementary Table 2, entry 3). The ‘activated Ir(D4-Por)(CO)Cl’ was compared with normal Ir(D4-Por)(CO)Cl by various analytical methods, and no significant difference was found between the two. This suggests that the ‘activated Ir(D4-Por)(CO)Cl’ may contain trace amounts of ‘highly active iridium porphyrin species’ that may be the ‘actual catalyst’ responsible for the observed efficient and selective catalysis of the C-H insertion reactions. In view of our previous findings that axial ligands may have a significant effect on the catalytic activity of metalloporphyrin-catalysed reactions45,46, and the literature reports that iridium complexes with axial methyl47,48,49,50,51,52 or aryl ligands53 are highly active catalysts for carbene transfer reactions, we hypothesised that trace amounts of ‘highly reactive iridium porphyrin species’ may be formed during the preparation of Ir(D4-Por)(CO)Cl in 1,2,4-trichlorobenzene. To test our hypothesis, we successfully synthesised the axially arylated iridium porphyrin Ir(D4-Por)(diClPh) (diClPh = 3,4-dichlorophenyl) and confirmed its structure by X-ray single crystal analysis. As expected, the complex showed high catalytic activity in promoting C-H insertion reactions to produce 3a in moderate yields and excellent enantioselectivity (Supplementary Table 2, entries 4–5). Notably, the NMR yield of 3a increased to 83% by reducing the amount of diazo compound used to suppress the formation of the double insertion product (Supplementary Table 2, entry 6). In addition, the reaction showed excellent tolerance to a variety of common organic solvents, affording 3a in moderate-to-good yields with exceptional enantioselectivity (Supplementary Table 2, entries 7–15). Among the solvents examined, cyclohexane and chloroform performed the best in terms of product yield and enantioselectivity. Strikingly, optimisation of the catalytic reaction by reducing the amount of catalyst (Supplementary Table 2, entries 16–22) showed that, with the use of 0.0004 mol% (4 ppm) Ir(D4-Por)(diClPh) catalyst in cyclohexane at 40 °C and acetic acid as additive, 70% isolated yield, 96% ee and a high product TON of 175,000 were obtained for 3a despite the longer reaction time (Supplementary Table 2, entry 22). The high activity of Ir(D4-Por)(diClPh) is attributable to the strong trans effect of a phenyl group, coupled with introduction of two electron-withdrawing chloro-substituents on the phenyl group, which enhances the electrophilicity of the carbene group. Moreover, the large steric hindrance of diClPh combined with the chiral pocket structure of D4-Por may better protect the catalytic centre and greatly improve the stability of the catalyst. We speculate that this interplay of electronic and steric effects enables Ir(D4-Por)(diClPh) to efficiently catalyse asymmetric C-H insertion reactions even at very low catalyst loading.
Under optimised reaction conditions, the asymmetric C-H insertion reaction of various N-methyl indoline derivatives and donor-acceptor diazo compounds was investigated; the corresponding products are shown in Fig. 2. Highly efficient and selective primary C-H insertion of various substituted N-methyl indoline derivatives was achieved; the corresponding insertion products were obtained with good-to-excellent isolated yields (66–93%), excellent enantioselectivities (90–96% ee), and very high product TONs (88,000–330,000) (Figs. 2, 3a–g). Interestingly, this reaction is sensitive to the steric hindrance of N-methyl indoline and no primary C-H bond insertion reaction was detected for 7-bromo substituted N-methyl indoline 1 h (Figs. 2, 3h). This reaction tolerates a range of different substituted donor-acceptor diazo compounds, and provides the corresponding insertion products (3i-3m) in moderate-to-good yields (60–90%) with excellent enantioselectivities (93–96%) and high TON from 60,000 to 226,000. Similar steric hindrance sensitivity was also observed when o-bromo substituted diazo compounds were used as the carbene source, and the desired insertion product 3n was not detected in the reaction. N-Methyl tetrahydroquinoline also reacts to form 3o, with a yield of 82%, an ee value of 94% and a TON of up to 205,000. The reaction of N-methyl benzomorpholine was also successful, providing the desired insertion product 3p in 84% yield and 89% ee with a TON of 84,000.
Carbene insertion into C(sp3)-H bonds of N-methyl anilines
Subsequently, the reaction was extended to various N-methyl aniline derivatives using the donor-acceptor diazo compound 2a as carbene source, and the results are summarised in Fig. 3. Compared to N-methyl indoline, N,N-dimethyl aniline showed higher reactivity; product 8a was formed in 85% yield and 90% ee with a TON of 428,000. Fluoro substituted N,N-dimethyl aniline derivatives showed high reactivity in the reaction, and the corresponding insertion products 8b-8d were obtained in good yields (60–83%), with excellent enantioselectivity (93–99% ee) and high product TONs (600,000–1,380,000). To our knowledge, this is the highest TON reported to date for a C-H insertion reaction. N,N-Dimethyl aniline with Cl, Br, methyl or ester group on the phenyl ring was also found to be a suitable substrate in the reaction, and the corresponding primary C-H insertion products 8e-8i were obtained in high yields (81–94%), excellent enantioselectivity (89–94% ee) and high TON of 207,000–236,000. N-Ethyl, benzyl or ethyl acetates were also well tolerated in the reaction and afforded 8j-8l with high efficiency and selectivity. Interestingly, when 7k with a benzyl substituent was used as substrate, the more reactive secondary benzylic C-H bond remained intact, and the desired N-methyl insertion product 8k was obtained in 80% yield and 95% ee; this further demonstrated the high selectivity of this iridium catalysis. The reaction can be extended to N,N-dimethyl aminonaphthalene and affords 8m and 8n in moderate-to-good yields and good-to-high enantioselectivities. It is worth noting that methyl substituted diazo compounds, such as methyl 2-(4-bromophenyl)-2-diazoacetate, are also suitable for this reaction; compound 8o was obtained with a yield of 80% and an ee of 89% with a TON of 267,000. These values for compound 8o are lower than those for 8a obtained using the 2,2,2-trichloroethyl substituted counterpart 2a. This is consistent with previous report that 2,2,2-trichloroethyl aryldiazo-acetates outperform their methyl counterparts in metal-catalysed carbene C–H insertion reactions54.
We also explored the reactivity of several amine-substituted pyridine compounds, including dimethylaminopyridine (DMAP) and its derivatives. Using 4-DMAP or its N-oxide as substrate, no desired insertion products 8p or 8q were detected (Supplementary Fig. 1). This is attributed to the coordination of the pyridine or N-oxide groups to the catalyst Ir(D4-Por)(diClPh), which prevents the formation of the corresponding metal-carbene intermediate required for the C-H insertion reaction. We then used sterically hindered 4- or 2-DMAP derivatives as substrates to mitigate the pyridine coordination; the corresponding reactions gave C-H insertion products 8r and 8s with 80% ee (40% yield) and 94% ee (69% yield), respectively, despite the higher catalyst loading (1 mol%) (Supplementary Fig. 1).
The Ir(D4-Por)(diClPh)-catalysed highly enantioselective primary C(sp3)‒H insertion reaction of N-methyl indolines and N-methyl anilines (Figs. 2 and 3) is significantly different from the non-porphyrin metal-catalysed reaction between N,N-dialkyl aniline and diazo compounds reported in the literature; the latter was found to lead to the insertion of carbene into aryl C(sp2)-H bonds (TONs: <100)55,56,57. For the cytochrome P411 enzyme- or chiral Ir(Por)(CO)(Cl)-catalysed primary C(sp3)‒H insertion of N,N-dialkyl anilines using diazo compounds, the TON was as high as 233040 or <10058, respectively. There is also a report on the metal-catalysed reactions of N,N-dialkyl anilines and N-alkyl indolines with TrocNHTs, leading to the insertion of nitrenes into primary C(sp3)‒H bonds, giving products with TON of <2059.
Large scale reactions
To further demonstrate the high efficiency of this iridium catalysed asymmetric primary C(sp3)-H insertion reaction, two 100-gram scale reactions were performed (Fig. 4a). The reaction of N-methyl indoline 1a and diazo compound 2a can also proceed smoothly in cyclohexane in the presence of 4 ppm (-)Ir(D4-Por)(diClPh) at 40 °C; the desired insertion product was obtained in 96% ee, 71% isolated yield and 177,500 TON. Similarly, p-fluoro-N-dimethylaniline 7b reacted smoothly with 2a in the presence of 1 ppm (-)Ir(D4-Por)(diClPh) to give 8b, with an isolation yield of 90% (equivalent to 900,000 TON) and an enantioselectivity of 93% ee. Notably, the large-scale reaction is easy to perform and maintains high efficiency and selectivity, which is of great value for potential practical applications.
Applications in organic synthesis
The insertion product 3a could be easily converted into other valuable compounds (Fig. 4b). After treatment of DDQ, the corresponding dehydrogenated product 9 was obtained in 88% yield and 94% ee. When compound 3a was treated with excess Zn in acetic acid, chiral acid 10 could be obtained in 68% yield, which was further converted into chiral amides 11 and 12. When 10 was treated with N-hydroxyphthalimide, 13 was obtained in 83% yield. The absolute structure of 13 was determined to be S via single crystal X-ray structure analysis and the structures of the other products were assigned accordingly.
This iridium catalysis can be easily used for late-stage modification of complex molecules (Fig. 4c). When estrone derivative 14 was treated with 2a in the presence of iridium porphyrin catalyst, the corresponding insertion product could be obtained in high yield. Notably, no competitive O‒H insertion products were detected in the reaction, and the absolute structure of the insertion product can be fully controlled by the chiral catalyst to selectively and exclusively provide 15 or 16 with high efficiency (Fig. 4c). Subsequently, dihydroartemisinin derived substrate 17 was also tested in the reaction, and the desired C‒H insertion product 18 was obtained with high efficiency and selectivity, retaining the chemically sensitive peroxide bridge motif (Supplementary Fig. 2).
Application in polymerisation reactions
Inspired by the high efficiency and selectivity of chiral iridium porphyrin in catalysing asymmetric carbene insertion of N-methyl anilines, we set out to try polymerisation reaction via sequential stepwise intermolecular carbene insertion of C‒H bonds, which is a challenging task. Although the participation of carbenes in polymerisation reactions through X‒H insertion (X = N, O) has been reported, to the best of our knowledge, the corresponding C‒H insertion examples have not been reported. This may be attributed to the high barrier for C‒H bond activation/insertion. In this regard, compound 19 bearing both the donor-acceptor diazo and N,N-dimethyl aniline moieties was designed and synthesised. To our delight, when 19 was treated with an iridium porphyrin catalyst, the polymerisation reaction proceeded smoothly to produce oligomers or polymers of amino acid ester derivatives under mild conditions (Supplementary Fig. 3). When cyclohexane was used as a solvent, 20 (probably due to its low solubility in cyclohexane) was obtained as an oligomer through which up to 7 units were linked. Strikingly, the use of Ir(TMP)(diClPh) as a catalyst in DCM gave 20 with a Mn of 8375; using (-)Ir(D4-Por)(diClPh) as a catalyst, higher Mn values such as 17242 were obtained (Fig. 5a). This may be due to the larger steric hindrance of the D4-Por structure, which leads to higher selectivity of the corresponding iridium carbene intermediate. Subsequently, the polymerisation reaction of the bisdiazo compound 21 with N,N-dimethyl aniline 7a in the presence of iridium catalyst was studied. The reaction proceeded smoothly to afford polymer 22 with a Mn of 51390 (Fig. 5b) via a double C-H insertion mode. These results demonstrated that by combining robust and selective iridium catalyst with carefully designed substrates, C-H insertion reactions can serve as powerful tools for polymerisation reactions, opening another door for the development of synthetic methods for polymer chemistry.
Mechanistic studies
To elucidate the reaction mechanism, deuterated N-methyl indoline 23 was employed as the substrate; the isolated yield of corresponding deuterated product 24 was 68% and the ee was 96% (Supplementary Fig. 4a). When equal amounts of 1a and 23 were reacted with 2a in the presence of (-)Ir(D4-Por)(diClPh), a mixture of 3a and 24 was with a kH/kD value of 3.54 (Supplementary Fig. 4b), thus indicating that the insertion step can be rate-determining step. When 5 equivalents of TEMPO were added to the reaction mixture, 3a could still be obtained in 61% yield and 96% ee (Supplementary Fig. 4c), so the radical pathway may not be involved in the reaction.
DFT calculations
DFT calculations were performed to give an energy landscape of the catalytic reaction, providing more insight into the unusual selectivity and the results are summarised in Fig. 6. Using the donor-acceptor diazo compound 2a as the carbene source, an Ir-carbene intermediate (Int2) with axial diClPh group was formed. The Gibbs free energy of activation (ΔG⧧) is 14.6 kcal/mol, and the relative Gibbs free energy of Int2 is -21.6 kcal/mol. Int2 exists as a singlet Ir-carbene, and the energy of its corresponding triplet Ir-carbene is 13.3 kcal/mol higher. Using N-methyl indoline 1a as substrate, the calculation results show that primary C(sp3)-H can be easily activated by Int2 with a barrier of 5.3 kcal/mol (TS2) in a concerted insertion manner, giving 3a as the major product. For the generation of its enantiomer, the calculated ΔG⧧ is 8.1 kcal/mol (TS2'), which is higher. Based on the transition state energy difference (2.8 kcal/mol), the calculated ee is 98%, which is consistent with the experimental results. The transition states for the formation of minor products 4 and 5 (Supplementary Table 2) were also calculated. For other more feasible aromatic C(sp2)−H bond activations to give 4, the electrophilic addition mechanism was examined and the calculated transition state energy (4-TS, Fig. 6c) was 7.2 kcal/mol. However, the 2 °C-H insertion is the least favourable, with a ΔG⧧ of 8.6 kcal/mol (5-TS, Fig. 6c).
a Energy profiles of the catalytic pathway. b Non-covalent interaction (NCI) analysis of TS2. c Geometrical analysis of attractive and repulsive interactions in transition states that determine the selectivity. H···H distance (<2.4 Å) between the C-H of substrate (1a) and D4-Por ligand in the space-filling diagrams are shown to quantitatively reveal the steric repulsion interactions.
To better understand the energy differences of these transition states that determine the enantioselectivity and regioselectivity of the reaction, non-covalent interaction (NCI) analysis was further performed. As shown in Fig. 6, the transition state involves attractive π···π, C-H···O and C-H···π interactions as well as steric repulsion. The most favourable primary C-H activation involves (i) a strong π···π interaction between the phenyl group of the carbenoid and the aromatic ring of the indoline (3.69 Å), (ii) C-H···O (2.12 Å) interaction between the methyl group of 1a and the carbonyl oxygen of carbenoid, and (iii) C-H···π (2.28 Å) interaction between the methyl group of 1a and the porphyrin macrocycle. Meanwhile, the steric hindrance between 1a and D4-Por was determined to be minimal, as revealed by the longer H···H distances (2.12 Å and 2.30 Å). While for TS2', general weak attractive interactions were calculated (π···π interaction 4.28 Å, C-H···O 2.36 Å and C-H···π 2.52 Å). In addition, the H···H distances in TS2' are 1.94 Å and 2.19 Å, respectively, so there is a larger steric repulsion. Therefore, both strong attractive interactions and weak repulsive steric hindrance contribute to the high enantioselectivity. For aromatic C-H activation, although the π···π distance is calculated to be 3.37 Å due to the electrophilic addition nature of the transition state, the displacement (twist) between the carbene phenyl and aromatic indoline rings reduces the overlap. Therefore, the π···π interaction is still weak. For the generation of 5, the H···H distances in the DFT-optimised transition state structure are relatively close, 2.16 Å, 2.20 Å and 2.25 Å, respectively, which determines the steric repulsion caused by these three sites, showing that the 2° C-H insertion is the most sterically unfavourable. Overall, the primary C-H activation TS2 is the most stable through attractive interactions and fits spatially into the catalytic pocket yielding the most prevalent product 3a. The presence of multiple attractive NCIs synergistically lowers the activation barrier for C-H activation, making the reaction feasible and selective under mild conditions while also ensuring a high product turnover number for the catalyst.
In summary, we have developed an efficient and practical iridium porphyrin-catalysed asymmetric intermolecular primary C(sp3)-H bond insertion reaction for a variety of N-methyl indoline and N-methyl aniline derivatives. Using ppm-level loading (-)Ir(D4-Por)(diClPh) as the catalyst and a donor-acceptor diazo compound as the carbene source, the corresponding insertion product was obtained with high yield and excellent enantioselectivity. The product TON of 1,380,000 represents the highest TON reported to date in asymmetric C-H insertion reactions. Importantly, the insertion reaction could be easily performed on a 100-gram scale while maintaining high selectivity and efficiency. In addition, we also successfully demonstrated the promising examples of donor-acceptor diazo compound polymerisation to synthesise poly-β-aminoester through stepwise carbene insertion into primary C(sp3)-H bonds. This result may hold promise for the development of functional polymers with a variety of applications. Overall, our results represent a significant advance in the field of C-H bond functionalization and highlight the potential of iridium porphyrin catalysis for scalable and selective synthesis of valuable chiral compounds and for expanding the scope of C-H insertion reactions in materials science.
Methods
General procedure for carbene C–H insertion of indoline and donor-acceptor diazo compounds
Under argon protection, 1 (0.2 mmol), acetic acid (0.06 mmol, 0.3 equiv) and catalyst (4–10 ppm) were dissolved in anhydrous cyclohexane (1 mL) in a Schlenk tube, and the diazo compound 2 (0.22 mmol, 1.1 equiv.) was added into the Schlenk tube in one portion. The tube was placed in a pre-heated 40 °C oil bath and the mixture was stirred and monitored by TLC until complete consumption of 1. The solvent was removed under reduced pressure. The desired product 3 was purified by silica gel flash column chromatography using ethyl acetate/petroleum ether (1:300 v/v) as the eluent.
General procedure for carbene C–H insertion of N-methyl aniline and donor-acceptor diazo compounds
To a solution of 7 (0.2 mmol), acetic acid (0.06 mmol, 0.3 equiv.) and catalyst (1–10 ppm) in anhydrous cyclohexane (1 mL) was added diazo compound 2 (0.22 mmol) under argon atmosphere. The reaction vessel was placed in a pre-heated 60 °C oil bath; the mixture was then stirred. The reaction was monitored by TLC until complete consumption of 7. Solvent was removed under reduced pressure. The desired product 8 was purified by silica gel flash column chromatography using ethyl acetate/petroleum ether (1:300 v/v) as eluent.
Data availability
The authors declare that the data supporting the findings of this study, including synthetic procedures, characterisation data, further details of computational studies and NMR spectra, are available within the article and the Supplementary Information file, or from the corresponding authors upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 1976736 (for catalyst Ir(D4-Por)(diClPh)) and CCDC2045506 (for compound 13). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Mulzer, J. Basic Principles of Asymmetric Synthesis. In Comprehensive Asymmetric Catalysis (eds Jacobsen, E. N., Pfaltz, A. & Yamamoto, H.) 33–100 (Springer, 1999).
Sethi, M. K., Chakraborty, P. & Shukla, R. Biocatalysis-A Greener Alternative in Synthetic Chemistry. In Biocatalysis: An Industrial Perspective (eds de Gonzalo, G. & de María, P. D.) 44–76 (RSC, 2018).
Beller, M. et al. Cross Coupling Reactions. In Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Four Volumes (eds Cornils, B., Herrmann, W. A., Beller, M. & Paciello, R.) 411–464 (Wiley-VCH Verlag GmbH & Co. KGaA, 2018).
Magano, J. & Dunetz, J. R. Large-scale carbonyl reductions in the pharmaceutical industry. Org. Process Res. Dev. 16, 1156–1184 (2012).
Arai, N. & Ohkuma, T. Design of molecular catalysts for achievement of high turnover number in homogeneous hydrogenation. Chem. Rec. 12, 284–289 (2012).
Wen, J., Wang, F. & Zhang, X. Asymmetric hydrogenation catalyzed by first-row transition metal complexes. Chem. Soc. Rev. 50, 3211–3237 (2021).
Xie, J. H., Liu, X. Y., Xie, J. B., Wang, L. X. & Zhou, Q. L. An additional coordination group leads to extremely efficient chiral iridium catalysts for asymmetric hydrogenation of ketones. Angew. Chem. Int. Ed. 50, 7329–7332 (2011).
Yin, C. et al. A 13-million turnover-number anionic Ir-catalyst for a selective industrial route to chiral nicotine. Nat. Commun. 14, 3718 (2023).
Doucet, H. et al. trans-[RuCl2(phosphane)2(1,2-diamine)] and chiral trans-[RuCl2(diphosphane)(1,2-diamine)]: shelf-stable precatalysts for the rapid, productive, and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 37, 1703–1707 (1998).
Ohkuma, T., Ooka, H., Hashiguchi, S., Ikariya, T. & Noyori, R. Practical enantioselective hydrogenation of aromatic ketones. J. Am. Chem. Soc. 117, 2675–2676 (1995).
Zhao, B., Han, Z. & Ding, K. The N-H functional group in organometallic catalysis. Angew. Chem. Int. Ed. 52, 4744–4788 (2013).
Zhang, L., Tang, Y., Han, Z. & Ding, K. Lutidine-based chiral pincer manganese catalysts for enantioselective hydrogenation of ketones. Angew. Chem. Int. Ed. 58, 4973–4977 (2019).
Docherty, J. H. et al. Transition-metal-catalyzed C-H bond activation for the formation of C-C bonds in complex molecules. Chem. Rev. 123, 7692–7760 (2023).
Liu, C.-X. et al. Rhodium-catalyzed asymmetric C-H functionalization reactions. Chem. Rev. 123, 10079–10134 (2023).
Lucas, E. L. et al. Palladium-catalyzed enantioselective β-C(sp3)-H activation reactions of aliphatic acids: a retrosynthetic surrogate for enolate alkylation and conjugate addition. Acc. Chem. Res. 55, 537–550 (2022).
Zhang, Z., Chen, P. & Liu, G. Copper-catalyzed radical relay in C(sp3)-H functionalization. Chem. Soc. Rev. 51, 1640–1658 (2022).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Zhu, Y. et al. Functionality-directed regio- and enantio-selective olefinic C-H functionalization of aryl alkenes. Chem. Rec. 23, e202300012 (2023).
de Jesus, R., Hiesinger, K. & van Gemmeren, M. Preparative scale applications of C–H activation in medicinal chemistry. Angew. Chem. Int. Ed. 62, e202306659 (2023).
Davies, H. M. L. & Manning, J. R. Catalytic C-H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Davies, H. M. L. & Morton, D. Guiding principles for site selective and stereoselective intermolecular C-H functionalization by donor/acceptor rhodium carbenes. Chem. Soc. Rev. 40, 1857–1869 (2011).
Che, C.-M., Lo, V. K.-Y., Zhou, C.-Y. & Huang, J.-S. Selective functionalisation of saturated C-H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 40, 1950–1975 (2011).
He, Y. et al. Recent advances in transition-metal-catalyzed carbene insertion to C-H bonds. Chem. Soc. Rev. 51, 2759–2852 (2022).
Zhang, Q., Wu, L.-S. & Shi, B.-F. Forging C-heteroatom bonds by transition-metal-catalyzed enantioselective C-H functionalization. Chem 8, 384–413 (2022).
Godula, K. & Sames, D. C-H bond functionalization in complex organic synthesis. Science 312, 67–72 (2006).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C-H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Liao, K., Negretti, S., Musaev, D. G., Bacsa, J. & Davies, H. M. L. Site-selective and stereoselective functionalization of unactivated C-H bonds. Nature 533, 230–234 (2016).
Liao, K. et al. Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds. Nature 551, 609–613 (2017).
Fu, J., Ren, Z., Bacsa, J., Musaev, D. G. & Davies, H. M. L. Desymmetrization of cyclohexanes by site- and stereoselective C-H functionalization. Nature 564, 395–399 (2018).
Gensch, T., James, M. J., Dalton, T. & Glorius, F. Increasing catalyst efficiency in C-H activation catalysis. Angew. Chem. Int. Ed. 57, 2296–2306 (2018).
Salazar, C. A. et al. Tailored quinones support high-turnover Pd catalysts for oxidative C-H arylation with O2. Science 370, 1454–1460 (2020).
Das, S., Incarvito, C. D., Crabtree, R. H. & Brudvig, G. W. Molecular recognition in the selective oxygenation of saturated C-H bonds by a dimanganese catalyst. Science 312, 1941–1943 (2006).
Jia, C. et al. Efficient activation of aromatic C-H bonds for addition to C-C multiple bonds. Science 287, 1992–1995 (2000).
Zbieg, J. R., Yamaguchi, E., McInturff, E. L. & Krische, M. J. Enantioselective C-H crotylation of primary alcohols via hydrohydroxyalkylation of butadiene. Science 336, 324–327 (2012).
Tsutsui, H. et al. Dirhodium(II) tetrakis N-tetrafluorophthaloyl-(S)-tert-leucinate]: an exceptionally effective Rh(II) catalyst for enantiotopically selective aromatic C-H insertions of diazo ketoesters. Tetrahedron Asymmetry 14, 817–821 (2003).
Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C-H functionalization. Nat. Rev. Chem. 3, 347–360 (2019).
Thu, H.-Y. et al. Highly selective metal catalysts for intermolecular carbenoid insertion into primary C-H bonds and enantioselective C-C bond formation. Angew. Chem. Int. Ed. 47, 9747–9751 (2008).
Liao, K. et al. Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C-H bonds. Nat. Chem. 10, 1048–1055 (2018).
Zhang, R. K. et al. Enzymatic assembly of carbon-carbon bonds via iron-catalysed sp3 C-H functionalization. Nature 565, 67–72 (2019).
Dydio, P. et al. An artificial metalloenzyme with the kinetics of native enzymes. Science 354, 102–106 (2016).
Greule, A., Stok, J. E., De Voss, J. J. & Cryle, M. J. Unrivalled diversity: the many roles and reactions of bacterial cytochromes P450 in secondary metabolism. Nat. Prod. Rep. 35, 757–791 (2018).
Lukowski, A. L. et al. C-H hydroxylation in paralytic shellfish toxin biosynthesis. J. Am. Chem. Soc. 140, 11863–11869 (2018).
Yang, Y. & Arnold, F. H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 54, 1209–1225 (2021).
Wang, J.-C. et al. Highly enantioselective intermolecular carbene insertion to C-H and Si-H bonds catalyzed by a chiral iridium(III) complex of a D4-symmetric Halterman porphyrin ligand. Chem. Commun. 48, 4299–4301 (2012).
Wang, J. C., Zhang, Y., Xu, Z.-J., Lo, V. K.-Y. & Che, C.-M. Enantioselective intramolecular carbene C-H insertion catalyzed by a chiral iridium(III) complex of D4-symmetric porphyrin ligand. ACS Catal. 3, 1144–1148 (2013).
Liu, Z. et al. Assembly and evolution of artificial metalloenzymes within E. coli Nissle 1917 for enantioselective and site-selective functionalization of C-H and C=C bonds. J. Am. Chem. Soc. 144, 883–890 (2022).
Anding, B. J., Dairo, T. O. & Woo, L. K. Reactivity comparison of primary aromatic amines and thiols in E-H Inertion reactions with diazoacetates catalyzed by iridium(III) tetratolylporphyrin. Organometallics 36, 1842–1847 (2017).
Dairo, T. O. & Woo, L. K. Scope and mechanism of iridium porphyrin-catalyzed S-H insertion reactions between thiols and diazo esters. Organometallics 36, 927–934 (2017).
Anding, B. J., Ellern, A. & Woo, L. K. Olefin cyclopropanation catalyzed by iridium(III) porphyrin complexes. Organometallics 31, 3628–3635 (2012).
Balhara, R., Chatterjee, R. & Jindal, G. A computional approach to understand the role of metals and axial ligands in artificial heme enzyme catalyzed C-H insertion. Phys. Chem. Chem. Phys. 23, 9500–9511 (2021).
Key, H. M., Dydio, P., Clark, D. S. & Hartwig, J. F. Abiological catalysis by artificial haem proteins containing noble metals in place of iron. Nature 534, 534–537 (2016).
Suematsu, H. & Katsuki, T. Iridium(III) catalyzed diastereo- and enantioselective C-H bond functionalization. J. Am. Chem. Soc. 131, 14218–14219 (2009).
Guptill, D. M. & Davies, H. M. L. 2,2,2-Trichloroethyl aryldiazoacetates as robust reagents for the enantioselective C-H functionalization of methyl ethers. J. Am. Chem. Soc. 136, 17718–17721 (2014).
Xu, B., Li, M.-L., Zuo, X.-D., Zhu, S.-F. & Zhou, Q.-L. Catalytic asymmetric arylation of α-aryl-α-diazoacetates with aniline derivatives. J. Am. Chem. Soc. 137, 8700–8703 (2015).
Zhu, D.-X., Xia, H., Liu, J.-G., Chung, L. W. & Xu, M.-H. Regiospecific and enantioselective arylvinylcarbene insertion of a C-H bond of aniline derivatives enabled by a Rh(I)-diene catalyst. J. Am. Chem. Soc. 143, 2608–2619 (2021).
Li, Z. et al. Construction of C-C axial chirality via asymmetric carbene insertion into arene C-H bonds. Angew. Chem. Int. Ed. 60, 25714–25718 (2021).
Yuan, S., Li, S.-Y., Zhao, X.-M., Lin, Y.-Z. & Zheng, S.-C. Enantioselective alkylation of primary C(sp3)−H bonds in N‑methyl tertiary amine enabled by iridium complex of axially chiral β‑aryl porphyrins. J. Am. Chem. Soc. 147, 51–56 (2025).
Chen, G., Arai, K., Morisaki, K., Kawabata, T. & Ueda, Y. Dirhodium-catalyzed chemo- and site-selective C-H amidation of N,N-dialkylanilines. Synlett 32, 728–732 (2021).
Acknowledgements
This work is supported by the Hong Kong Research Grants Council General Research Fund (17302020, (C.-M.C.)), the National Key R&D Program of China (2017YFE0190100, (C.-M.C.)), the National Natural Science Foundation of China (NSFC 91856203, (C.-M.C.), 21472216, (Z.-J.X.) and 52173014, (G.-L.L.)), and the CAS-Croucher Funding Scheme for Joint Laboratories (C.-M.C.).
Author information
Authors and Affiliations
Contributions
C.-M.C. conceived the project and directed the research. Z.-J.X. designed the experimental studies. Z.-R.L., K.Z., Y.-J.W., and Z.-J.X. carried out the reactions. L.-L.W. performed DFT calculations. G.-L.L. carried out the GPC and corresponding analysis of polymer. H.-Y.W. carried out the mass spectrum analysis. X.-L.W. carried out the HPLC analysis of the chiral products. K.-H.L. carried out the X-ray crystal structure analysis. C.-M.C. and Z.-J.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jun-Fang Gong, Xiao-Wei Liang and Tatsuya Uchida for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, ZR., Zhan, K., Wang, YJ. et al. Iridium porphyrin-catalysed asymmetric carbene insertion into primary N-adjacent C–H bonds with TON over 1000000. Nat Commun 16, 3311 (2025). https://doi.org/10.1038/s41467-025-58316-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58316-1